Elena Ene1, Karin Nentwich1, Philipp Halbfaß1, Kai Sonne1, Arthur Berkovitz2, Thomas Deneke1,3
1 Cardiology Clinic II with interventional electrophysiology, Cardiovascular Center Bad Neustadt, Germany
2 Sacre Coeur Hospital, Montreal, Canada
3 Ruhr- University Bochum, Germany
Abstract: Ventricular tachycardias (VTs) remain worldwide the leading cause of sudden cardiac death. The therapy with implantable cardio defibrillator (ICD) has reduced the mortality due to VTs, but recurrent ICD therapies are associated with increased mortality and might have a major negative impact on the patient. Taking into account the results of multi-center studies the current ESC guidelines recommend an early interventional treatment of recurrent VTs in patients with ischemic cardiomyopathy or electrical storm1. Still the same studies report a VT recurrence rate after catheter ablation between 46%-70%2,3. This aspect can be explained by insufficient mapping during the index procedure partially related to the mapping technology, but also intramyocardial or epicardial localized anatomical substrates may be responsible. Some struc-tural heart diseases as non – ischemic dilative cardiomyopathy (NIDCM), arrhythmogenic right ventricular cardiomyopathy (ARVCM) and Chagas disease show at least in the early phases a broader epicardial than endocardial substrate and therefore a combined ablative approach (epi-/endocardial) if not exclusively epicardial should be planned. Cardiac imaging techniques (cardiac computer tomographie with late enhancement – cardio CT LE or cardiac magnetic resonance with LE – cardio MRI LE) play a crucial role in the identification of anatomical substrate distribution and therefore are of tremendous help in planning the VT – ablation approach.
Epicardial VT ablation is a highly complex procedure and therefore should only be performed in experienced centers and by experienced operators. In our experience in such a center complications are rare and the midterm VT free survival can be as high as 75%.
Keywords: epicardial access, ventricular tachycardia ablation, 3D cardiac mapping, anatomical substrate identification, success rate
INTRODUCTION
Cardiovascular diseases are one of the leading cause of mortality and ventricular arrhythmias are on top of all the main cause for sudden cardiac deaths.
The emerging therapies such as primary percutane-ous transcatheter coronary angioplasty (PTCA), phar-macological therapy or ICD implantation have impro-ved the survival rate, but recurrent appropriate ICD therapies also lead to increased mortality. In this re-gard and based on the results of multiple multicenter studies current ESC guidelines recommend an early referral of patients with multiple ICD therapies but also in those with electrical storm1. In depth analysis of these studies indicates a VT recurrence rates between 40-75%2,3. strongly depending on the underlying struc-tural heart disease (higher in NIDCM) and ablation success during the fi rst procedure defi ned as VT non – inducibility and/or complete substrate modifi cation. The natural course of different structural heart di-sease entities (almost all cases of Chagas Disease and ARVCM, the majority of NIDCM and ICM after big an-terior wall infarction or more frequently after inferior wall infarction) is characterized by a larger epicardial than endocardial scar distribution. In many patients fa-ilure of endocardial ablation is due to a not-targeted epicardial substrate and in 50% of these an epicardial VT ablation leads to effective arrhythmia stabilization during long term4.
Therefore the substrate characterization and its distribution (transmural, intramural respectively epi-cardial) using cardiac imaging techniques plays a cen-tral role in planning an VT ablations in these patients (Figure 1). Most of the patients presenting with VTs have already implanted ICD and this is the most im-portant limiting factor in choosing the cardiac imaging techniques (specifically magnetic resonance imaging, MRI). While for patients with ICM a CT+LE identi fies the anatomical scar quite accurate (Figure 1), this te-chnique has limitations when applied to patients with NIDCM. The gold standard for substrate characteri-zation remains cardiac MRI with excellent tissue diffe-rentiation. The anatomical identifi ed scar represents the premise for the reentry mechanism of VTs in pati-ents with structural heart diseases and shows a good correlation in terms of localization with the electrical identified substrate using a 3D electroanatomical cardiac mapping5.
Indication for epicardial mapping and ablation Depending on the underlying structural heart disease but also on the scar distribution documented before the ablation procedure using cardiac imaging, an epi-cardial ablation can be planned as first line approach or as a secondary one after a failed endocardial VT ablation.
On the other hand some entities of structural heart diseases are indicative of a much more extensive epi-cardial than endocardial scar as anatomical substrate of VTs: almost 100% of patients with ARVCM (actu-ally the scarring process begin in this particular case epicardially) and those with Chagas disease (still very rarely encountered in Europe, but most often in So-uth America), 80% of patients with NIDCM but also around 25% of patients with ICM – mostly those with transmural posterior wall infarction respectively with big anterior wall myocardial infarction. In this latter category of patients an epicardial approach should rather be considered after a failed or partially success-ful endocardial ablation.
An epicardial origin should be assumed based on some specifi c ECG criteria6,7 (Figure 2), but these cri-teria are valid only for patients with NIDCM; much more than that, on one hand 1/3 of patients in whom a successful endocardial ablation was documented pro-ved at least 1 epi – ECG criteria and on the other hand only 30% of patients with a successful epicardial ablati-on meet the epi – ECG criteria. Still the ECG aspect of clinical VT remains important in patients with NIDCM who might prove also an extensive septal mid – myo-cardial substrate. In patients where ECG-morphology suggests a septal origin of VT, an epicardial approach may be less helpful and should only be considered if complete endo- and epicardial substrate modification is the ablation endpoint. In this regard, the standard of care in our EP Lab is to perform primarily an endo-cardial VT ablation in patients with NIDCM and septal VTs (i.e. usually a left bundle branch type with inferior QRS Axis).
Endocardial unipolar voltage mapping might offer reliable evidences on existence of epicardial scar and its distribution. Thus in patients with ARVCM an up-per cut off limit of 5.5 mV used during the endocardial substrate mapping identifi es epicardial scar area8. The same result have been described when using an upper cut off limit of 8.7 mV in patient with NIDCM9.
Nevertheless, the VT ablation approach (if isolated endocardial or combined epi-/endocardial) depends also on the pre – defined ablation end point i.e. non – inducibility of any VT and/or complete substrate modification. The later one may be the only achievable goal in patients with clinically documented sustained VT which are non – inducible during the ablation procedure.
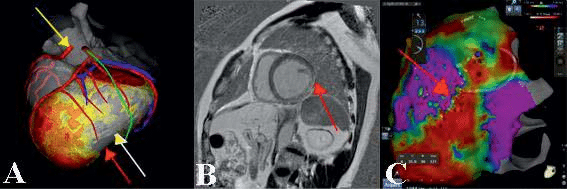
Figure 1. Role of cardiac imaging for substrate characterization and planning of VT ablation strategy – 1A – Epicardial involvement after anterior wall myocardial infarction (red arrow – LV epicardium, white arrow – LV endocardium); in the same patient epicardial course of left phrenic nerv in green and coronary artery in red – abnormal origin of right coronary artery superior to the left sinus of Valsalva; 1B – cardiac MRI showing intramyocardial and epicardial late enhancement localized in the postero-lateral LV in a patient with history of myocarditis (red arrow); 1C – in the same patient epicardial substrate mapping showing a corresponding area of electrical scar (red area), catheter ablation positioned at borderline zone where the clinical VT was epicardialy successfully ablated.
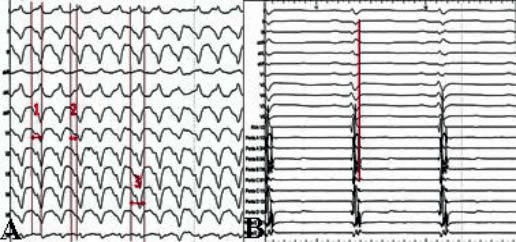
Figure 2. ECG criteria suggesting an epicardial origin of VT in a patient with ARVCM: 2A – (1) intrinsecoid deflection 100 ms (i.e. > 85 ms); (2) pseudodelta wave 70 ms (i.e. > 35 ms); (3) QRS duration 200 ms (i.e. > 120 ms); 2B – in the same patient substrate mapping using a multipolar mapping catheter showing „early” late potentials corresponding on surface ECG to the epsilon wave in lead V1 and II – III as expression of delayed local epicardial activation.
Techniques of epicardial access
As initially described by Sosa et al. in 199610, the non – surgical percutaneous pericard puncture using a subxyphoidal access became the most utilized pericar-dial access for epicardial ablation of VT. Due to the low associated complications the anteri-or pericardial (Figure 3A, 3B) access became the most used technique and represents the standard approach in our center. Still in some patients, most frequently due to pericardial adhesions, the posterior pericardial puncture represents an alternative but may be associ-ated with a higher complications rate (i.e. accidental liver puncture, injury of posterior interventricular ar-tery) – Figure 3C, 3D. Pericardial adhesions may also be mobilized carefully manipulating the ablation catheter bluntly dissecting adhesions. This technique should only be used in experienced centers and on-site cardio-surgical backup in case of acute epicardial bleeding may be needed. In rare cases (i.e. in patients with history of open heart surgery) a surgical pericardial window may be the only alternative for access into the pericard. The indication for epicardial VT ablation must critically appraise the complications associated with different access routes. Technically the pericardial puncture is usually per-formed using a dedicated non traumatic needle (i.e. Tuhoy needle or micropuncture needle) as described in Figure 3.
After getting access into the pericardial space, a 3D reconstruction with concomitantly registration of bi-polar voltage of local electrograms (3D electroanato-mical mapping) is performed using the same cut-offs for scar defi nition as for endocardial bipolar mapping (0.5 mV – 1,5 mV). As the low – voltage areas and also the regions with double and late potentials may indicate the critical zones of reentry circuits of a VT the identifi cation of these potentials is of paramount importance (especially if only hemodynamically not to-lerated VTs are targeted based on a substrate modifi-cation approach). Special attention must be paid when interpreting signal quality during epicardial substrate mapping as low – amplitude signals do not necessari-ly represent scar areas but may also be normally en-countered in areas with epicardial fat like the inter-ventricular sulci or around the heart valve annuli and epicardial coronary. The regions with real epicardial scars can be identified by electrogram morphology like fragmentation, longer duration and the presence of late potentials11. In a pre – acquired CT or MRI 3D reconstruction not only the pericardial anatomy but also the scar architecture including epicardial conduc-ting channels may be identifi ed and should be recom-mended if available – Figure 1A. Also endocardial the unipolar mapping with a cut off value of 3.7 – 3.9 mV can identify reliably the epicardial regions covered by fat tissue thicker as 1 mm12. Ideally for a high density map a multipoint catheter should be used for quick and effective epicardial substrate mapping (Pentarray, Biosense Webster, Diamond Bar, California; HD-Grid; Abbott) Figure 3B.
Practically every 3D mapping system allows a (semi) automatic 3D electroanatomical mapping using prede-fi ned criteria introduced as filters by the user. In this regard, during the substrate mapping specific attention needs to be drawn to differentiate „early” late poten-tials (defined as a local late electrogram placed before the end of QRS complex) from “true” late potentials (defi ned as a local EGM definitely placed after the end of QRS complex in the reference ECG derivation) as only the later may represent parts of critical isthmus of a VT. However local areas with delayed conduction can also be identified during decremental programmed ventricular stimulation in the so called „decrement evoked potentials” technique (DEEP) which seems to better discriminate potentials crucial for diastolic pathway formation of VTs from other sites displaying late potentials13.
After substrate mapping has been performed, pro-grammed ventricular stimulation is routinely perfor-med in the attempt to induce any clinical VT (inducible VT). If the induced VTs are hemodynamically tolera-ted (maximum 10 to 20% of cases) an activation ma-pping can be performed. In case of hemodynamically not tolerated VT, termination and substrate based ablation is the method of choice. In some cases ex-ternal defi brillation can be ineffective due to modified position of intraprocedurally placed defibrillation pads or fluid and/or air accumulation in the pericardial spa-ce after epicardial access. Fluid or air may serve as an insulation milieu and in these cases internal defi brilla-tion via implanted ICDs is part of standard approach in our EP lab. Based on the morphology of induced VT and considering the scar areas detected during sub-strate mapping, pace mapping may be attempted as a next procedural step to identify exit regions of VTs by correlating 12-lead-ECG-QRS-morphology of VT and pacemap (i.e. automated comparison between the induced VT morphology and stimulated VT morpho-logy). Still this method should be critically view as the morphology of paced QRS greatly depends on the catheter orientation (perpendicular or parallel to the tissue),on stimulation energy – epicardially mostly high output energy is necessary for effective stimulation which must also leads to far field capture – and pro-pagation of electrical impulses. Pace-mapping should rather be used as a tool to narrow down on potential targets rather than reliably predicting effective pointy ablation sites.
Complete and effective substrate modification in addition to VT non – inducibility has the lowest VT recurrence rates reported14. During catheter ablation special attention needs to be addressed towards ne-arby epicardial structures such the coronary arteries or the left phrenic nerv. The epicardial course of co-ronary arteries can be characterized using real time integration of coronary angiography or pre – acquired CT images (Figure 1A). The course of the phrenic ner-ve may be adequately lined out by maximum output pacing and capture site annotation.
Epicardial VT ablation is a highly complex procedu-re and should be done only in specialized centers ha-ving rapid access to cardiac surgery in case of possible relevant complications.
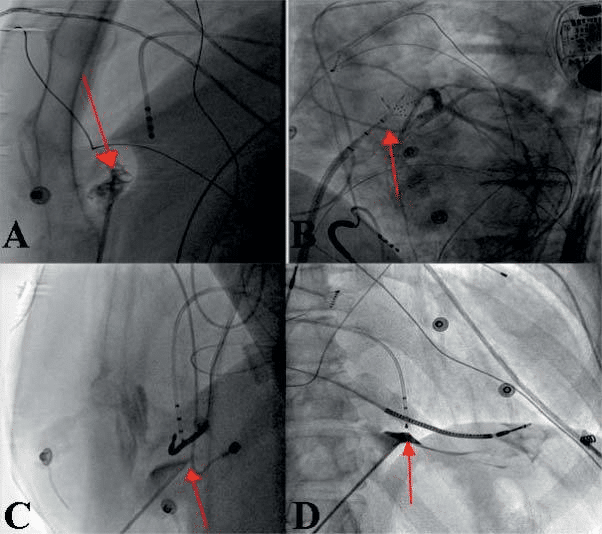
Figure 3. Technique of epicardial puncture – 3A – LAO 90° projection: anterior subxyphoidal transcutaneous epicardial puncture – the puncture needle is oriented to the free wall of RV (here also air bubbles in pericard ) with evidence of pericardial adhesions; 3B – LAO 50° projection: epicardial sheath and multipolar mapping catheter also on the anterior surface of RV (red arrow); 3C – LAO 90° projection: posterior epicardial puncture – puncture needle oriented to the inferior LV wall; 3D – RAO 30° – pericard tenting after contrast dye injection preceding the successful pericardial puncture.
Results of epicardial VT ablation
The type of structural heart disease plays a decisive role not only in planning the strategy for catheter ablation (primary epicardially respectively after a fai-led endocardial procedure) but also influences success rate and VT – free survival after an epicardial ablati-on. Whereas arrhythmia recurrence may be a rele-vant finding during follow-up, mode of death is mostly aggravation of pre-existing heart failure stressing the need for a multimodality approach to VT patients in-cluding heart failure specialty.
Data from a multicenter European study which enrolled only patients with epicardial VT (from these 31% with NIDCM respectively 39% with ICM) ablation showed a VT recurrence rate of 39% in patients with NIDCM at a follow-up of 1.5 years (35% in patients with ICM)15.
Almost all ARVCM patients prove an epicardial sub-strate as the course of fatty degeneration usualy starts from epi- to endocardial. An isolated endocardial abla-tion is associated with a very high recurrence rate However a combined approach (endo- & epicardial ablation) leads to an acute success rate of 90 % and on mid-term follow – up VT recurrence appears to be only 26.8%16. Also in patients with old myocardial infarction and documented epicardial substrate using cardiac imaging a combined endo-/epicardial ablation leads to a recurrence rate of only 12.5 % compared to isolated endocardial ablation associated with a signifi-cantly higher recurrence rate (40.6%)17.
In our experience from 2013 to 2016 more than 110 VT epicardial ablation have been performed from a total of 630 patients with structural heart disease referred VT ablation. From these patients 70% had a NIDCM as structurally heart disease and 30% suffered from ICM (Roos et all. under review). In one half of the included patients an epicardial VT procedure was indicated after a failed endocardial ablation whereas in the remaining patients a primary combined approach (epi-/eondicardial) was used. Of note patients with a combined endo- and epicardial procedure had NIDCM in 58%. 20% of patients were referred for VT ablation due to electrical storm (ES); during follow-up no ES recurrence.
An acute procedural success (i.e. non – inducibility of any VT) was achieved in 64 % patients. The suc-cessful ablation site was endocardially in almost one third of patients and epicardialy in almost half of the patients. At a follow – up of 1.5 years 25% of patients had a VT recurrence and 10% died. 30day-mortality was low at 2.7% (3 pts). The overall freedom from car-diac death was similar between patients with NIDCM (88%) and ICM (80%).
An acute major complication was documented in 4.4% of cases: one acute but reversible right heart fai-lure due to ablation, one electrical storm necessitating intraprocedural resuscitation, one perforation of right ventricle with the epicardial sheath, one relevant pe-ricardial effusion with resuscication and one phrenic nerve palsy.
CONCLUSION
Epicardial VT ablation in patients with recurrent VTs and structural heart disease known to have epicardial scar should be considered as first line therapy. When performed in high experienced centers epicardial in-strumentation is associated with an event free survival of almost 80% of patients and is of paramount impor-tance as acute rhythm stabilizing therapeutic option in patients presenting with electrical storm. The com-plication rate is low in experienced centers and early referral for an invasive therapy is important.
Conflict of interest: none declared.
References
1. Authors/Task Force Members, Document Reviewers. 2015 ESC Guidelines for the management of patients with ventricular arrhyth-mias and the prevention of sudden cardiac death: The Task Force for the Management of Patients with Ventricular Arrhythmias and the. Priori SG, Blomström-Lundqvist C, Mazzanti A, Blom N, Borg-grefe M, Camm J, Elliott PM, Fitzsimons D, Hatala R, Hindricks G, Kirchhof P, Kjeldsen K, Kuck KH, Hernandez-Madrid A, Nikolaou N, Norekvål TM, Spaulding C, Van Veldhuisen DJ. 36(41):2757-9, s.l.: Eur Heart J, 2015. doi: 10.1093/eurheartj/ehv44.
2. Freedom from recurrent ventricular tachycardia after catheter abla-tion is associated with improved survival in patients with structural heart disease: An International VT Ablation Center Collaborative Group study. Tung R, Vaseghi M, Frankel DS, Vergara P, Di Biase L, Nagashima K, Yu R, Vangala S, Tseng CH, Choi EK, Khurshid S, Patel M, Mathuria N, Nakahara S, Tzou WS, Sauer WH, Vakil K, Tedrow U, Burkhardt JD, Tholakanahalli VN, Saliaris A. 12(9):1997-2007, s.l. : Heart Rhythm, 2015.
3. Catheter ablation of ventricular tachycardia in patients with structural heart disease using cooled radiofrequency energy: results of a pro-spective multicenter study. Cooled RF Multi Center Investigators Group. Calkins H, Epstein A, Packer D, Arria AM, Hummel J, Gilligan DM, Trusso J, Carlson M, Luceri R, Kopelman H, Wilber D, Whar-ton JM, Stevenson W. 35(7):1905-14, s.l.: J Am Coll Cardiol, 2000.
4. Catheter ablation for ventricular tachycardia after failed endocardial ablation: epicardial substrate or inappropriate endocardial ablation? Schmidt B1, Chun KR, Baensch D, Antz M, Koektuerk B, Tilz RR, Metzner A, Ouyang F, Kuck KH. 7(12):1746-52, s.l.: Heart Rhythm, 2010.
5. Correlation between computer tomography-derived scar topogra-phy and critical ablation sites in postinfarction ventricular tachycar-dia. Ghannam M, Cochet H, Jais P, Sermesant M, Patel S, Siontis KC, Morady F, Bogun F. 29(3):438-445, s.l.: J Cardiovasc Electrophysiol., 2018.
6. ECG criteria to identify epicardial ventricular tachycardia in nonisch-emic cardiomyopathy. Vallès E, Bazan V, Marchlinski FE. 3(1):63-71., s.l.: Circ Arrhythm Electrophysiol., 2010.
7. Electrocardiographic recognition of the epicardial origin of ventricu-lar tachycardias. Berruezo A, Mont L, Nava S, Chueca E, Bartholo-may E, Brugada J. 109(15):1842-7, s.l.: Circulation.
8. Endocardial unipolar voltage mapping to identify epicardial substrate in arrhythmogenic right ventricular cardiomyopathy/dysplasia. Polin GM1, Haqqani H, Tzou W, Hutchinson MD, Garcia FC, Callans DJ, Zado ES, Marchlinski FE. 8(1):76-83, s.l.: Heart Rhythm.
9. Endocardial unipolar voltage mapping to detect epicardial ventricu-lar tachycardia substrate in patients with nonischemic left ventricu-lar cardiomyopathy. Hutchinson MD1, Gerstenfeld EP, Desjardins B, Bala R, Riley MP, Garcia FC, Dixit S, Lin D, Tzou WS, Cooper JM, Verdino RJ, Callans DJ, Marchlinski FE. 4(1):49-55, s.l.: Circ Ar-rhythm Electrophysiol, 2011.
10. A new technique to perform epicardial mapping in the electrophysi-ology laboratory. Sosa E, Scanavacca M, d’Avila A, Pilleggi F. s.l.: J Cardiovasc Electrophysiol, 1996, Vols. Vols. 7(6):531-6.
11. Distinguishing epicardial fat from scar: analysis of electrograms us-ing high-density electroanatomic mapping in a novel porcine infarct model. Tung R, Nakahara S, Ramirez R, Lai C, Fishbein MC, Shivku-mar K. s.l.: Heart Rhythm, 2010, Vols. 7(3):389-95.
12. Venlet J, Piers SRD, Kapel GFL, de Riva M, Pauli PFG, van der Geest RJ, Zeppenfeld K. Delineation, Unipolar Endocardial Voltage Map-ping in the Right Ventricle: Optimal Cutoff Values Correcting for Computed Tomography-Derived Epicardial Fat Thickness and Their Clinical Value for Substrate. 10(8). pii: e005175, s.l.: Circ Arrhythm Electrophysiol, 2017.
13. Decrement Evoked Potential Mapping: Basis of a Mechanistic Strat-egy for Ventricular Tachycardia Ablation. Jackson N, Gizurarson S, Viswanathan K, King B, Massé S, Kusha M, Porta-Sanchez A, Jacob JR, Khan F, Das M, Ha AC, Pashaei A, Vigmond E, Downar E, Nanthaku-mar K. 8(6):1433-42, s.l.: Circ Arrhythm Electrophysiol, 2015.
14. Noninducibility and late potential abolition: a novel combined prog-nostic procedural end point for catheter ablation of postinfarction ventricular tachycardia. Silberbauer J, Oloriz T, Maccabelli G, Tsia-chris D, Baratto F, Vergara P, Mizuno H, Bisceglia C, Marzi A, Sora N, Guarracini F, Radinovic A, Cireddu M, Sala S, Gulletta S, Paglino G, Mazzone P, Trevisi N, Della Bella P. 7(3):424-35, s.l.: Circ Ar-rhythm Electrophysiol, 2014.
15. Epicardial ablation for ventricular tachycardia: a European multi-center study. Della Bella P, Brugada J, Zeppenfeld K, Merino J, Neuzil P, Maury P, Maccabelli G, Vergara P, Baratto F, Berruezo A, Wijn-maalen AP. 4(5):653-9, s.l.: Circ Arrhythm Electrophysiol, 2011.
16. Safety, long-term outcomes and predictors of recurrence after first-line combined endoepicardial ventricular tachycardia substrate ablation in arrhythmogenic cardiomyopathy. Impact of arrhythmic sub-strate distribution pattern. A prospective multicentre st. Berruezo A, Acosta J, Fernández-Armenta J, Pedrote A, Barrera A, Arana-Rue-da E, Bodegas AI, Anguera I, Tercedor L, Penela D, Andreu D, Perea RJ, Prat-González S, Mont L. 19(4):607-616, s.l.: Europace, 2017.
17. Infarct transmurality as a criterion for first-line endo-epicardial sub-strate-guided ventricular tachycardia ablation in ischemic cardiomy-opathy. Acosta J, Fernández-Armenta J, Penela D, Andreu D, Borras R, Vassanelli F, Korshunov V, Perea RJ, de Caralt TM, Ortiz JT, Fita G, Sitges M, Brugada J, Mont L, Berruezo A. 13(1):85-95, s.l.: Heart Rhythm, 2016.
 This work is licensed under a
This work is licensed under a