Adrian Linte1, Cristina Căldăraru2, Dana Constantinescu2, Călin Popa3, Theodor Cebotaru3, Flaviu Şteiu3, Maria Alexandrescu4, Ana Fruntelată2, Şerban Bălănescu1
1 Department of Angiography and Endovascular Interventions, Monza Hospital, Bucharest
2 Department of Cardiology, Monza Hospital, Bucharest
3 Department of Cardiovascular Surgery, Monza Hospital, Bucharest
4 Department of Radiology, Monza Hospital, Bucharest
Contact address:
Dr. Adrian Linte, Department of Angiography and Endovascular Interventions, Monza Hospital – Cardiovascular Centre 27, Tony Bulandra Street,
Sector 2, Bucharest, Romania.
E-mail: adrian.linte@spitalulmonza.ro
Abstract: Surgical replacement of the native aortic valve with either a biological or a mechanical valve has been the “gold standard” treatment for severe symptomatic aortic stenosis for a long time. However, during the last ten years transcutaneous aortic valve replacement (TAVR) has emerged as an alternative to classic surgery, especially for patients considered to be inoperable or at high surgical risk. TAVR, developed to avoid the high morbi-mortality and complications associated with classic surgical intervention, has its own complications which need to be known in order to prevent them properly. We present the case of a 63 year-old man with symptomatic severe aortic stenosis deemed to be inoperable because of severe depression of left and right ventricular systolic function, which benefited from TAVR with a “valve in valve” procedure because of significant paravalvular aortic regurgitation after the insertion of the first percutaneously implanted valve.
CASE REPORT
Mr. G.C., a 63 year-old gentleman, was referred to our hospital by his cardiologist to be evaluated for correction of his severe aortic stenosis symptomatic by dyspnea at rest and repeated syncope. Beside valvular heart disease, he was an ex-smoker with chronic obstructive pulmonary disease (Gold III stage), had a history of dyslipidemia and had a diagnosis of previous anterior myocardial infarction. Two vessel coronary artery disease (40% stenosis of mid left anterior descending artery and chronic total occlusion of distal right coronary artery) was previously diagnosed by coronary angiography in another hospital. He was in permanent atrial fibrillation under chronic efficient oral anticoagulation with acenocumarol. At presentation he was in NYHA class IV (with dyspnea at rest, orthopnea and severe cough on decubitus); he had atrial fibrillation with a fast ventricular rate (110/min) and a blood pressure of 100/60 mmHg. He had bi-basal wet pulmonary rales, normal jugular pulse and bilateral pretibial edema. His cardiac auscultation revealed an intense pancardiac mid-systolic murmur radiating to the anterior neck area.
His rest ECG showed atrial fibrillation with a fast ventricular response, LV hypertrophy and mixed ST-T wave changes (Figure 1).
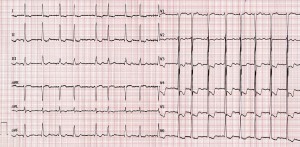
Figure 1. ECG at presentation: atrial fibrillation with fast ventricular rate, LV hypertrophy and mixed ST-T wave changes.
Echocardiography examination at admission revealed severe degenerative aortic stenosis with a mean transvalvular gradient of approximately 37-38 mmHg (in the context of severely depressed left ventricular systolic function) and a valve area calculated by continuity equation of 0.78 cm2 (at a body surface area of 2.06 m2). The left ventricle was mildly dilated (136 ml) with severely thickened walls (left ventricular septum and posterior wall of 16 mm) and a severely depressed systolic function (left ventricular ejection fraction – LVEF – of 20-25%) (Figure 2). There was a grade III/IV mitral regurgitation that we interpreted as both degenerative and functional. The pulmonary systolic arterial pressure was initially estimated at 68 mmHg (under-evaluated because of the severe dysfunction of the right ventricle, with a TAPSE of only 10 mm).
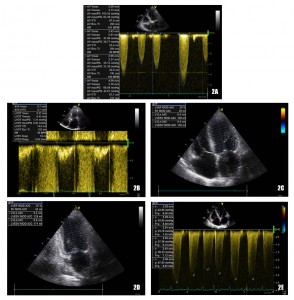
Figure 2. Echocardiography at presentation: severe aortic stenosis (Figure 2A and 2B) with severely depressed LVEF (Figure 2C and 2D) and severe elevation of systolic pulmonary arterial pressure (Figure 2E).
The chest X-ray showed an enlarged cardiac silhouette and changes of the pulmonary areas characteristic for elevated pulmonary venous pressure.
The routine blood count showed mild leukocytosis (12.800/µl), normal hemoglobin and platelets; a low serum sodium was found (126 mEq/dl), but the serum potassium concentration was normal. The NTproBNP was markedly increased (10.855 pg/dl) in agreement with the severe LV dysfunction. The rest of the blood tests were within normal ranges (serum creatinine, with a calculated eGFR of 90,3 ml/min, liver enzymes, blood glucose, the lipid panel, thyroid function (TSH) and CRP).
A slight improvement in his general condition was obtained after a carefully tailored initial medical therapy with a loop diuretic, antialdosteronic diuretic and digitalis PO; he was maintained on an initially low dose of selective beta-1 blocker, which he was already on when arriving to our hospital; he was also given a bronchodilator and a statin at regular doses.
The patient was considered to have a high operative risk associated with classic surgical intervention for replacement of the aortic valve in the presence of severe systolic dysfunction of both left and right ventricle associated with NYHA class IV, the presence of the coronary artery disease and chronic obstructive pulmonary disease: he had a calculated EUROScore of 7%. Therefore the patient was referred for percutaneous transcatheter aortic valve replacement (TAVR) in agreement with the cardiovascular surgeon and the anesthesiologist. The patient was then evaluated for feasibility of transcatheter implantation of a Medtronic CoreValve by transesophageal echocardiography (TEE) and evaluation of the aortic root, aortic arch and iliofemoral axis by the means of both classic angiography and multi-slice CT reconstruction; the coronary angiogram was performed in another hospital a month prior to the admission to our department. The percutaneous procedure was approved by a central Medtronic Europe TAVR core after careful evaluation. Considering that the measurements of the aortic annulus showed a 26,9 mm per 22,7 mm annulus (Figure 3, courtesy of Medtronic Europe) a 29 mm Medtronic CoreValve was considered.
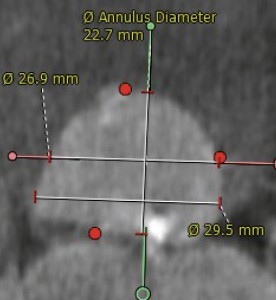
Figure 3. Aortic annulus dimensions on angio-CT (courtesy of Medtronic Europe; measurements standardized for CoreValve implantation as performed by Medtronic Europe with the help of a specialized proctor).
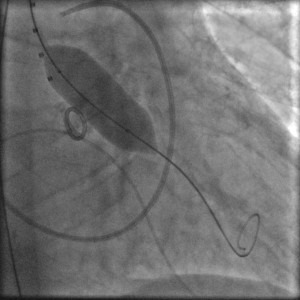
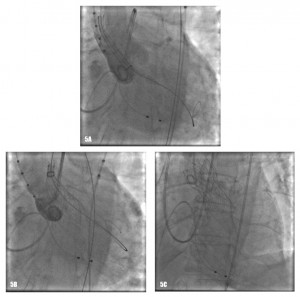
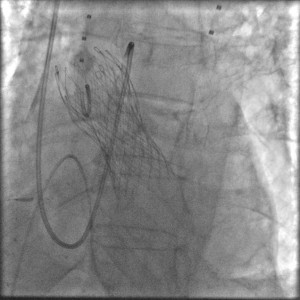
The procedure was performed under general anaesthesia in the cathlab of our hospital. A temporary balloon-tipped pacing wire was placed in the right ventricle via the left common femoral vein for rapid burst pacing during valve predilatation and valve release. A Swan-Ganz catheter was placed in a right pulmonary artery via the right internal jugular vein. A TEE probe was inserted prior to starting the interventional procedure on the angiography table. The right common femoral artery was exposed by direct cut-down by the cardiovascular surgeon. The native aortic valve was predilated with a 20×40 mm NuMed balloon on a 0.035” super-stiff Amplatz guide wire placed at the apex of the left ventricle (Figure 4). After the positioning of the Medtronic CoreValve with the tip in the left ventricular outflow tract just below the annular plane, the first valve was progressively released (Figure 5). The verification of the precise location of the prosthesis release was done by contrast injection on a pigtail catheter in the non-coronary Valsalva sinus and TEE. During the final valve deployment the valve slowly fell in the LV outflow tract a few millimeters below the ideal alignment with the heavily calcified aortic cusps. Both contrast angiography and TEE demonstrated grade III/III paraprosthetic valvular leak (Figure 6). In the presence of the suboptimal result with significant paravalvular aortic regurgitation, after a Heart Team discussion with the cardiovascular surgeons, we considered to implant a second CoreValve as the only treatment option. Therefore, a second 29 mm Medtronic CoreValve was concentrically placed and subsequently released 4-6 mm cranial to the position of the first one (Figure 7). The complete expansion of the second prosthesis was obtained by postdilation with a 28×40 mm NuMed balloon. At the end of the procedure TEE and angiography showed minimal paravalvular regurgitation (Figure 8). No effect of the first prosthesis on the anterior mitral leaflet was observed at echocardiography. A total amount of 100 ml of contrast was given during the procedure. The right common femoral breach was surgically sutured.
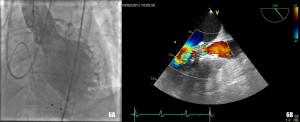
Figure 6. Grade III/III paraprosthetic valve leak, as demonstrated both by contrast angiography (Figure 6A) and TEE (Figure 6B).
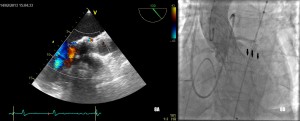
Figure 8. Grade I/III paravalvular leak a demonstrated by TEE (Figure 8A) and contrast angiography (Figure 8B).
The patient’s in-hospital outcome was good, with prompt improvement in NYHA class and ejection fraction: at discharge he was in NYHA class II and with a global LVEF of 35%. No neurological deficits or haemorrhagic complications were observed. He was discharged 10 days after the index procedure with acenocumarol associated with clopidogrel (for one month), digitalis, bisoprololum and furosemide. The six month post-discharge visit showed a patient in NYHA class I with normal function of the aortic bio-prostheses with no residual paravalvular leak and normalization of left ventricular function with a LVEF of 50-53%, the regression of left ventricular wall thickness to normal values (interventricular septum and posterior wall of 11 mm); the calculated pulmonary arterial pressure was within normal ranges (Figure 9). He remained in permanent atrial fibrillation and was chronically anticoagulated with acenocumarol.
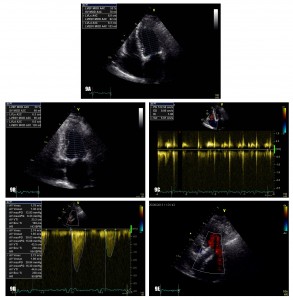
Figure 9. Transthoracic echocardiography at 6 months: normalization of LVEF (Figure 8A and 8B) and systolic pulmonary arterial pressure (Figure 8C) with normal functioning of the aortic prosthesis (Figure 8D) and no sign of paravalvular leak (Figure 8E).
DISCUSSION
With TAVR emerging as a more and more used as a feasible alternative to classic surgical aortic valve substitution in patients considered inoperable or at high surgical risk with severe aortic stenosis, a special attention is currently given to the procedure related complications and their impact on short and long term prognosis. Our case presentation focuses on aortic paravalvular leak (PVL), one of the most important and frequent complication after TAVR. The overall incidence of aortic paravalvular leaks after TAVR is around 70%1, with mild or greater aortic regurgitation being present in 6% to 36% in different studies2-7 and there is strong proof that moderate to severe aortic PVL is associated with a worse prognosis after TAVR7-9. There are three mechanisms implicated in aortic regurgitation: paravalvular, intravalvular and the recently described supra-skirtal (which implies a leakage through the upper part of the valve stent when it is implanted too low, keeping in mind that only the lower part of the valve stent is covered with a skirt)1. By far the most frequent mechanism is the paravalvular leak11, mechanism that was also present in our case.
There are four important risk factors for paravalvular regurgitation: patient–prosthesis mismatch, under-expansion, malposition of the prosthesis or extensive calcification of the aortic valve1, our patient being at an important risk of paravalvular regurgitation because of his extensive bulky asymmetric calcification of the aortic cusps. A patient prosthesis mismatch was avoided by oversizing the prosthesis according to the CoreValve implantation protocol, therefore a 29 mm prosthesis was chosen for a 26,9 mm per 22,7 mm aortic annulus as measured by multi-slice CT. The proper positioning of the valve is of paramount importance, the malposition of the valve (either too high or too low) being a very important determinant of paravalvular leak. There are several ways to correct the problem in case of the malposition: recapturing and repositioning of the prosthesis when it was not fully expanded (a maneuver which implies an important risk of stroke), dragging the prosthesis up a few millimeters using a snare when implanted too low or the technique of valve-in-valve (TAV-in-TAV) for either too high or too low position (procedure implied in less than 5 percent of TAVR cases in literature)11; the post-dilation, although widely used for under-expanded prosthesis in case of important calcifications (30%)11 is not effective in malposition. Our first CoreValve was properly positioned at the level of the aortic annulus, but during deployment, it slided towards the left ventricular outflow tract; with the prosthesis not being fully expanded we tried to reposition it by dragging the whole system to an upper position which resulted in tilting of the valve without proper repositioning and hence the valve was expanded too low in the LV outflow tract. After full deployment, angiography and TEE showed severe paravalvular regurgitation and a decision to implant a second valve for a TAV-in-TAV approach was made. The second 29 mm CoreValve was positioned and released in the proper place after post dilation resulting in trivial paravalvular leak.
The excellent six month outcome of the patient with not only excellent clinical status and prosthesis hemodynamics but also with normalization of left ventricular wall thickness and ejection fraction, somewhat in contrast to the reports in the literature11-14, is probably due to the fact that most of the patients undergoing this procedure are older than 75 years with multiple non-cardiac comorbidities and non-valvular heart disease-related comorbidities witch are less likely to improve after the interventional correction of the valvular heart disease.
CONCLUSION
The reported case shows one of most concerning complications after TAVR for severe aortic stenosis in patients at high surgical risk, namely severe paravalvular leak due to the malpositioning of the valve. The treatment option we chosed in this case, deployment of a second valve, proved to be successful in terms of clinical and technical result, both in short and medium term.
Conflict of interests: none declared.
References
1. Stähli BE, Maier W, Corti R, Lüscher TF, Jenni R, Tanner FC. Aortic regurgitation after transcatheter aortic valve implantation: mechanisms and implications. Cardiovasc Diagn Ther 2013;3(1):15-22.
2. Smith CR, Leon MB, Mack MJ et al. Transcatheter versus surgical aortic-valve replacement in high-risk patients. N Engl J Med 2011;364: 2187-98.
3. Yared K, Garcia-Camarero T, Fernandez-Friera L et al. Impact of aortic regurgitation after transcatheter aortic valve implantation: results from the REVIVAL trial. JACC Cardiovasc Imaging 2012;5:469-77.
4. Abdel-Wahab M, Zahn R, Horack M et al. Aortic regurgitation after transcatheter aortic valve implantation: incidence and early outcome. Results from the German transcatheter aortic valve interventions registry. Heart 2011;97:899-906.
5. Rajani R, Kakad M, Khawaja MZ et al. Paravalvular regurgitation one year after transcatheter aortic valve implantation. Catheter Cardiovasc Interv 2010;75:868-72.
6. Yan TD, Cao C, Martens-Nielsen J et al. Transcatheter aortic valve implantation for high-risk patients with severe aortic stenosis: A systematic review. J Thorac Cardiovasc Surg 2010;139:1519-28.
7. Moat NE, Ludman P, de Belder MA et al. Long-term outcomes after transcatheter aortic valve implantation in high-risk patients with severe aortic stenosis: the U.K. TAVI (United Kingdom Transcatheter Aortic Valve Implantation) Registry. J Am Coll Cardiol 2011;58:2130-8.
8. Ozkan A. Low gradient “severe” aortic stenosis with preserved left ventricular ejection fraction. Cardiovasc Diagn Ther 2012;2:19-27.
9. Makkar RR, Fontana GP, Jilaihawi H et al. Transcatheter aortic-valve replacement for inoperable severe aortic stenosis. N Engl J Med 2012;366:1696-704.
10. PCR-EAPCI Percutaneous Interventional Cardiovascular Medicine Textbook. Eeckhout E, Serruys PW, Vahanian A, van Sambeek M, Wijns (eds). PCR Publishing, Toulouse, France. 2012; 3: 79-139.
11. Leon MB, Smith CR, Mack M, et al. Transcatheter aortic-valve implantation for aortic stenosis in patients who cannot undergo surgery. N Engl J Med 2010;363:1597-607.
12. Smith CR, Leon MB, Mack MJ et al. Transcatheter versus surgical aortic-valve replacement in high-risk patients. N Engl J Med 2011; 364:2187-98.
13. Makkar RR, Fontana GP, Jilaihawi H et al. Transcatheter aortic-valve replacement for inoperable severe aortic stenosis. N Engl J Med 2012; 366:1696-704.
14. Kodali SK, Williams MR, Smith CR et al. Two-year outcomes after transcatheter or surgical aortic-valve replacement. N Engl J Med 2012;
366:1686-95.
 This work is licensed under a
This work is licensed under a