Sebastian Onciul1,2, Radu Nicolaescu1, Lucian Predescu3, Razvan Capsa1,4, Radu Vatasescu1,2
1 „Carol Davila” University of Medicine and Pharmacy, Bucharest, Romania
2 Department of Cardiology, Clinical Emergency Hospital, Bucharest, Romania
3 Department of Interventional Cardiology, „CC Iliescu” Emergency Institute for Cardiovascular Diseases, Bucharest, Romania
4 Laboratory of Radiology and Medical Imaging, Fundeni Clinical Institute, Bucharest, Romania
Abstract: Due to recent technical developments, magnetic resonance imaging (MRI) has become one of the most attrac-tive imaging modalities for a multitude of pathologies, offering unique information with the potential to modify diagnosis and treatment strategies in a cost-effective manner. Cardiovascular magnetic resonance (CMR) in particular has dramatically evolved to a reliable diagnostic tool bringing unique information for virtually all cardiovascular pathologies1. Patients with coronary stents, prosthetic heart valves or pacemakers and others cardiac implanted electronic devices (CIEDs) have been historically denied the opportunity to undergo clinically indicated MRI scans. As the population ages, it has been suggested that 50% to 75% of patients with CIEDs will have a clinical indication for MRI examination in their lifetimes2,3. As we are fa-cing a growing need for MRI scans in the large population of patients with implanted cardiac devices, it is crucial to accurately inform healthcare professionals about the actually low risk of performing MRI in these patients. As a rule of thumb, virtually all patients with coronary stents, prosthetic heart valves, annuloplasty rings and MR-conditional CIEDs can safely undergo MRI scanning at 1.5 tesla, and some of them at 3 tesla3-5. Moreover, recent studies have led the scientific societies to allow MRI scanning even for patients with non-conditional CIEDs under special circumstances.
This review aims to inform physicians of all specialties on how to safely indicate clinical MRI in patients with implanted car-diovascular devices based on an evidence-based approach.
Keywords: magnetic resonance imaging, safety, stents, prosthetic heart valves, cardiac implanted electronic devices.
VERY BASIC MRI PHYSICS
MRI is an imaging method relying on the nuclear mag-netism properties of the atoms. These properties can be used for detecting compounds with an odd number of nuclear particles (nucleons). In living organisms, the atoms with magnetic properties are: 1H, 13C, 19F, 31P, 23Na. From these compounds, only 1H (the proton) is generally responsible for playing the major role in MRI. Therefore, MRI might be considered as proton imaging6.
In order to perform an MRI examination, several events should be considered. First, the patient is po-sitioned in a high-intensity, static and homogenous magnetic field (the magnet itself). Then, a short radio-frequency (RF) wave is transmitted from the scanner to the patient, producing a radio-magnetic transfer of energy7. This energy transfer will result in levels of magnetization that are different in each tissue and organ of the body according to their intrinsic physi-cal properties. Following this radiofrequency impulse, the tissues of the patient will generate a radio signal, „returning” the energy received during the RF impul-se towards the scanner (by use of specific receiver coils). During this stage of the process (called relaxa-tion), each tissue and organ will gradually return to the previous balanced state, but this process will be accomplished at different times and levels througho-ut the body. The scanner is detecting this latter rela-xation signal that is subsequently used for producing the images7. Therefore, in MRI, unlike in other imaging modalities, the very body of the patient represents the origin of energy required for data processing and ulti-mately for image reconstruction.
In order to spatially locate the anatomical structu-res of the body, the system uses specially tailored an-cillary variable magnetic fields, called gradients. There are several types of gradients, each serving different purposes, the most widely used being the slice-coding gradients (perpendicular to the slices to be acquired) and the phase and readout (frequency-encoding) gradients, used for the spatial location of structures wi-thin one slice6,7.
From these basic principles, multiple types of acqui-sitions, sequences and applications have been deve-loped, each having special indications and features, according to the required area of examination, patient condition and specific study items and methods8.
To summarize, in order to obtain MR images, the following are necessary: the static magnetic field (1.5 tesla, 3 tesla, etc.), the RF waves and the gradient magnetic fields. All these can interfere with ferromagnetic objects in the body.
MRI IN PATIENTS WITH CORONARY AND PERIPHERAL STENTS
There used to be a historical excessive precaution among radiologists in performing MRI in patients with implanted coronary or peripheral stents, especially when closer to the implantation date. Most coronary and peripheral stents are made of 316L stainless ste-el or nitinol (Nickel-Titanium alloy) and exhibit non-ferromagnetic or weakly ferromagnetic properties. The forces caused by the magnetic field on the implan-ted stent are proportional to its length and mass, but they seem to be insufficient to provoke any shifting even in the case of weakly ferromagnetic stents11,5.
There was a consensus that suggested to wait aro-und 4 to 8 weeks after stent implantation before per-forming an MRI. The reason behind this recommenda-tion was the additional anchoring of the stent to vessel wall due to endothelization, process that take longer in patients with drug eluting stents. However, there are studies that showed no differences in terms of all-cau-se deaths, myocardial infarction, and revascularization in the 30 days after MRI examination between patients who had undergone MRI soon after stent implantation and those who had waited 4 to 8 weeks20,21. There is only one case report of a patient with ostial left main lesion treated with a stent that was partially retracted in the aorta that was completely dislodged after MRI, performed 2 weeks after stent implantation22. So, we must bear in mind that a short stent at an aorto-ostial location, especially a drug-eluting stent with delayed endothelial coverage, and a short time of MRI after stenting may be high-risk features for an adverse effect in a strong magnetic field22.
Other clinical trials demonstrated the safety of per-forming a 1.5 T MRI within 1 to 14 days after stents implantation23,24. Similar evidences came from ex vivo studies that showed the safety of 3 tesla MRI perfor-med early after stent implantation25.
The following guidelines apply to using MRI in all patients with coronary artery stents (including two or more overlapped stents):
1.Patients with all commercially available coronary artery stents (including drug-eluting and non-drug eluting or bare metal stents) can be scanned at 1.5 tesla or 3 tesla, regardless of the value of the spatial gradient magnetic field.
2.Patients with all commercially available coronary artery stents can undergo MRI immediately after placement of these implants.
AORTIC STENT GRAFTS AND LEFT ATRIAL APPENDAGE OCCLUSION DEVICES
Most endovascular aortic stent grafts, but not all, are made from nonferromagnetic or weakly ferromagne-tic materials and several studies have documented the safety of MRI after aortic stent grafts implantation26,27. Only the Zenith AAA endovascular graft (Cook) is considered unsafe for 1.5 tesla MRI. The only problem with aortic stent grafts are the increased number of artifacts induced by the metallic components of stent grafts.
Regarding the cardiac closure and occluder devices, at least one left atrial appendage occlusion device, the Watchman left atrial appendage device (Atritech, Inc), can be safely scanned at 3 tesla5.
MRI IN PATIENTS WITH PROSTHETIC HEART VALVES
Several studies have documented the safety of MRI in patients with prosthetic heart valves and annuloplasty rings9,10. No case of a patient incident or injury related to the presence of a heart valve prosthesis or annu-loplasty ring in association with an MR examination were reported in the literature5.
The materials used for manufacturing prosthetic heart valves include metals, polymers, and carbons11,12. The metals used are Titanium, alloys of Cobalt and Chromium, and alloys of Nickel, Molybdenum, and Tungsten, as well as Aluminum and Vanadium11. How-ever, it was demonstrated that the forces exerted on these valves and rings by the MRI scanner are less than the forces exerted by gravity and considerably less than those exerted by the beating heart and re-sultant pulsatile blood flow5,6,14. Moreover, no case of movement of the mobile parts of valves caused by a magnetic field greater than 1.5 tesla has ever been re-ported11,12.
The heating effect also is not a hazard, as heat from the area involved is dissipated by flowing blood15,12,5.
Similarly, sternal wires do not produce any signi-ficant heating effects and their metallic artifacts are usually minor permitting optimal interpretation of the images.
As such, there is no evidence to justify withholding a cardiac or extracardiac MR study at 1.5 tesla just due to the presence of a prosthetic heart valve. Most of the prosthetic heart valves are also safe at 3 tesla, but testing is less widely available5.
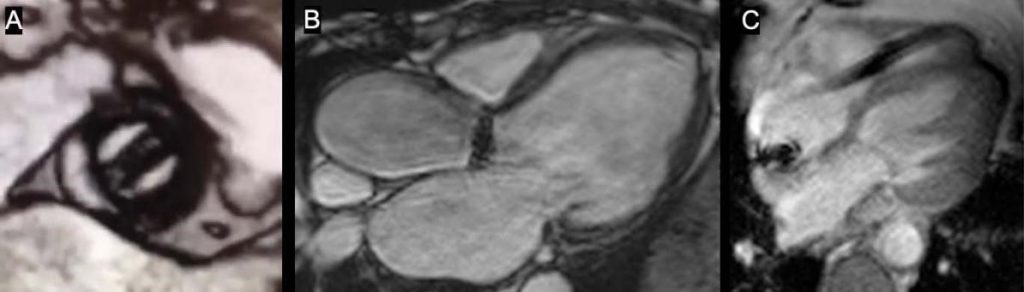
Figure 1. Cardiac MRI in patients with implanted cardiac devices. With careful adjustments, no signifi cant metal artifacts are seen. A. Cine balanced-SSFP in a patient with bileaflet metallic prosthesis in mitral position. B. Cine balanced-SSFP in a patient with bileaflet metallic prosthesis in aortic position. C. Spoiled gradient-echo cine image in a patient with implanted cardiac defi brillator (ICD).
METALLIC ARTIFACTS FROM THE PROSTHETIC VALVES
Various models of prosthetic valves may induce diffe-rent degrees of metallic artifacts, depending on the amount of contained ferromagnetic material. Howe-ver, in most instances accurate and interpretable ima-ges may be obtained (Figure 1 A and B).
Bioprosthetic heart valves are composed primarily of nonmetallic materials (usually porcine tissue or bo-vine pericardium) but may contain small amounts of metal (used for scaffolding rings)5. As such, they are also prone to producing metallic artifacts obscuring not only the valve but also the adjacent structures. On contrary, stentless bioprosthetic valves do not cause any artefacts as they do not contain any metal.
In case of severe metal artefacts, specific MRI sequences with less susceptibility to artifacts may be employed resulting in better image quality (Figure 1).
MRI IN PATIENTS WITH CARDIAC IMPLANTED ELECTRONIC DEVICES (CIEDS)
Terminology
The designation MR Safe requires there be no hazard in any MR environment. For example, plastic objects are MR safe16. On contrary, the designation MR Unsa-fe refers to an object that is known to pose hazards in all MR environments.
However, no CIED has an MR Safe designation, most of them being labelled as MR conditional. The term MR conditional refers to any device for which a specifi ed MRI environment with specified conditions of use does not pose a known hazard16. Field conditi-ons that defi ne the MRI environment can include the region of imaging, static magnetic field strength, spa-tial gradient, time-varying magnetic field (dB/dt), radi-ofrequency (RF) fields, and specific absorption rate. Additional conditions might be required, including the use of specific leads and generator combinations, as well as MRI mode programming of the CIED system16.
MR non-conditional – This includes MR conditional generators that have been combined with noncondi-tional leads or MR conditional systems implanted in patients that do not meet all specified conditions of use, such as patients with abandoned leads.
POTENTIAL HAZARDS WITH MRI SCANNING OF PATIENTS WITH NON-MRI CONDITIONAL CARDIAC IMPLANTED ELECTRONIC DEVICES
There is no risk of CIED generator or leads dislodge-ment due to magnetic field forces. The CIED genera-tor is confined in the subcutaneous tissues and leads do not contain any significant ferromagnetic materials to cause movement in a magnetic field16. It has been demonstrated that pacemakers released after 1995 have very low magnetic force values, even lower than the gravity of the earth (the measured acceleration <9.81 N/kg)17.
However, there are some potential interactions between CIEDs and electromagnetic interference from MRI including the following:
Gradient magnetic field-induced electrical cur-rents that could lead to myocardial capture and potentially lead to atrial or ventricular arrhythmias
RF energy pulses or rapidly changing magnetic field gradients might cause oversensing that can lead to inappropriate inhibition of demand pacing and possibly asystole in a pacing-dependent pati-ent, or induction of therapies such as inappropri-ate shocks in a patient with an implantable cardioverter defibrillator (ICD).
RF fields can lead to electrodes heating and sub-sequent myocardial thermal injury at the lead-tissue interface resulting in detrimental changes in pacing properties (small changes in lead sensing, impedances, and capture thresholds immediately after the MRI)16,18,19.
Electrical reset: High-energy electromagnetic in-terference can lead rarely to electrical or power-on reset, a backup demand mode.
The radiofrequency energy generated during MRI scanning creates a temporary decrease in battery voltage, which has typically been reported to re-solve after several weeks18.
PATIENTS WITH NON-MR-CONDITIONAL CIEDS CAN SAFELY UNDERGO MRI SCANNING
The MagnaSafe Registry was designed to prospectively determine the risks associated with MRI among pa-tients with non-MR-conditional CIEDs who undergo nonthoracic MRI at a magnetic field strength of 1.5 tesla18. MRI was performed in 1000 cases in which patients had a pacemaker and in 500 cases in which patients had an ICD. No deaths, lead failures, losses of capture, or ventricular arrhythmias occurred during MRI.
At the end of 2017, another study demonstrated the safety of thoracic and nonthoracic MRI examina-tions in patients with CIEDs19. No long-term clinically significant adverse events were reported among the 2103 thoracic and nonthoracic MRI examinations. In nine MRI examinations (0.4%; 95% confidence inter-val, 0.2 to 0.7), the patient’s device reset to a backup mode. The most common notable change in device parameters (>50% change from baseline) immediately after MRI was a decrease in P-wave amplitude, which occurred in 1% of the patients.
PRACTICAL RECOMMENDATIONS ON MRI SCANNING OF PATIENTS WITH NON-MR-CONDITIONAL CIEDS
Before the MRI
A full understanding of the implanted hardware is mandatory. The MR conditionality for each of the components of the CIEDs system (pulse generator, leads) should be established. Sometimes older non-conditional leads receive MR conditional approval in combination with certain devices after their initial market release16. For up-to-date information on MR conditionality, one can consult company-specific data-bases or other web-based data sources such as www. mrisafety.com (manufacturer independent)16.
The components of the CIEDs systems may be ma-nufactured by different vendors. In such cases, even if each separate component is labeled MR-conditional, the combined hardware from various vendors does not meet the conditional labeling, and the system sho-uld be considered nonconditional.
It is crucial to check the presence of abandoned or fractured leads, extenders or adaptors, lead remnants, surgically implanted epicardial leads, all of which would render the system nonconditional. If a medical history is not available and/or there is any suspicion of the presence of abandoned leads, a chest X-ray may be useful to clarify whether such hardware is present16.
Before the scan, the electrophysiologist must ma-nually switch the CIED to MR settings. However, some of the new devices have the capacity to activate automatically within the MRI magnet.
The non-MR-conditional CIEDs require special programming before the scan by the skilled person-nel. The appropriate device programming will depend on the patient’s characteristics, including pacing de-pendence, but its complexity is beyond the scope of this paper. The 2017 HRS expert consensus statement provides exhaustive recommendations on appropria-te device programming in patients with CIEDs16. As a rule of thumb an asynchronous pacing mode will be chosen for pacing-dependent patients while an inhi-bited pacing mode will be used for patients without pacing dependence. Tachyarrhythmia functions should be disabled. Patients with a CRT device should be pro-grammed to an asynchronous pacing mode with deac-tivation of advanced or adaptive features during the MRI examination, and with a pacing rate that avoids competitive pacing (IIA C)16.
During the MRI scan
ECG and pulse oximetry should be monitored during the MRI scan of patients with both MR conditional and non-conditional systems. Many of the MRI sequences induce signifi cant electrical artifacts, which may render the ECG tracing uninterpretable. However, transcu-taneous pulse oximetry is relatively unaffected during MR sequences, and thus can confirm a change in pulse rate16.
A defibrillator/monitor (with external pacing func-tion) and a manufacturer-specific device programming system should be immediately available16.
For patients who are pacing-dependent with an MR-nonconditional CIED, additional personnel are needed: personnel with the skill to program the CIED, a physician who can establish temporary transvenous pacing (I, B)16.
After the MRI
After completion of the MRI, full device interrogation should be performed and the devices must be repro-grammed to the original settings19.
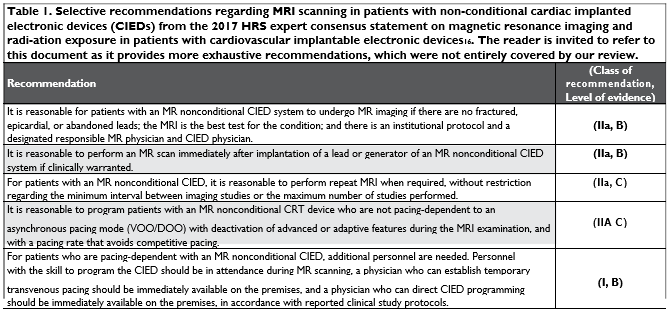
Table 1 shows selective recommendations regar-ding MRI scanning in patients with non-MR-conditio-nal CIEDs from the 2017 HRS expert consensus sta-tement on magnetic resonance imaging and radiation exposure in patients with cardiovascular implantable electronic devices16. The reader is invited to refer to this document as it provides more exhaustive recom-mendations, which were not entirely covered by our review.
METALLIC ARTIFACTS DUE TO CIEDS
Beside the safety issues, CIEDs cause various types of metallic artifacts within MR images, such as ima-ge distortions or signal loss near the device. Artifacts cannot be predicted in advance, however most of the MRI scans in patients with a CIED yield interpretable images. (Figure 1C) The images acquisition can be furt-her optimized by using wideband filtering algorithms16.
MRI SCANS OF PATIENTS WITH IMPLANTABLE LOOP RECORDERS (ILR)
The currently available ILRs are classified as MR condi-tional by their manufacturers for use at both 1.5 tesla and 3 tesla field strengths16. It is recommended that prior to MRI scanning patients that the ILR be eva-luated and that any desired recorded information be removed/ downloaded from the system and cleared after the MRI16.
Conflict of interest: none declared.
List of abbreviations and acronyms
CIED – Cardiac Implantable Electronic Device (e.g. pa-cemaker, implanted cardiac defibrillator) CMR – Cardiovascular Magnetic Resonance
CRT – Cardiac Resynchronisation Therapy ICD – Implantable Cardioverter Defibrillator ILR – Implantable Loop Recorder MR – Magnetic Resonance
MRI – Magnetic Resonance Imaging RF – Radiofrequency
SSFP – steady state free precession TAVR – Transcatheter Aortic Valve Replacement
References
1. S. Onciul and S. Plein, “Cardiovascular magnetic resonance for de-tection of coronary artery disease – a practical approach,” Rom. J. Cardiol., vol. 27, no. 4, pp. 490–498, 2017.
2. R. Kalin and M. S. Stanton, “Current clinical issues for MRI scanning of pacemaker and defibrillator patients.,” Pacing Clin. Electrophysi-ol., vol. 28, no. 4, pp. 326–328, Apr. 2005.
3. C. J. Culbertson and C. A. Gold, “Expanding Access to Magnetic Res-onance Imaging for Patients With Cardiac Rhythm Devices.,” JAMA Neurol., vol. 75, no. 10, pp. 1173–1174, Oct. 2018.
4. T. D. Karamitsos and H. Karvounis, “Magnetic resonance imaging is a safe technique in patients with prosthetic heart valves and coronary stents,” Hell. J. Cardiol., vol. 60, no. 1, pp. 38–39, 2019.
5. L. G. N. et al., “Safety of Magnetic Resonance Imaging in Patients With Cardiovascular Devices,” Circulation, vol. 116, no. 24, pp. 2878–2891, Dec. 2007.
6. J. T. Catherine Westbrook, MRI in Practice, 5th Edition, 5th ed. Wi-ley-Blackwell, 2018.
7. R. C. DALE, B. M., BROWN, M. A. AND SEMELKA, MRI: Basic Prin-ciples and Applications; Fifth Edition. John Wiley & Sons, Inc., 2015.
8. Vivian S. Lee, Cardiovascular MRI: physical principles to practical protocols. Lippincott Williams & Wilkins, Philadelphia, 2006.
9. G. G. Hartnell, L. Spence, L. A. Hughes, M. C. Cohen, R. Saouaf, and
B. Buff, “Safety of MR imaging in patients who have retained metallic materials after cardiac surgery.,” AJR. Am. J. Roentgenol., vol. 168, no. 5, pp. 1157–1159, May 1997.
10. P. A. Randall, L. J. Kohman, E. M. Scalzetti, N. M. Szeverenyi, and D.
M. Panicek, “Magnetic resonance imaging of prosthetic cardiac valves in vitro and in vivo.,” Am. J. Cardiol., vol. 62, no. 13, pp. 973–976, Nov. 1988.
11. N. G. Baikoussis, E. Apostolakis, N. A. Papakonstantinou, I. Saran-titis, and D. Dougenis, “Safety of magnetic resonance imaging in pa-tients with implanted cardiac prostheses and metallic cardiovascular electronic devices.,” Ann. Thorac. Surg., vol. 91, no. 6, pp. 2006– 2011, Jun. 2011.
12. E. Giroletti and G. Corbucci, “Cardiac magnetic resonance imaging: patient safety considerations,” Phys. Medica Eur. J. Med. Phys., vol. 21, no. 1, pp. 5–13, Jan. 2005.
13. B. Condon and D. M. Hadley, “Potential MR hazard to patients with metallic heart valves: the Lenz effect.,” J. Magn. Reson. Imaging, vol. 12, no. 1, pp. 171–176, Jul. 2000.
14. R. L. Soulen, T. F. Budinger, and C. B. Higgins, “Magnetic resonance imaging of prosthetic heart valves.,” Radiology, vol. 154, no. 3, pp. 705–707, Mar. 1985.
15. F. Duru, R. Luechinger, M. B. Scheidegger, T. F. Luscher, P. Boesiger, and R. Candinas, “Pacing in magnetic resonance imaging environ-ment: clinical and technical considerations on compatibility.,” Eur. Heart J., vol. 22, no. 2, pp. 113–124, Jan. 2001.
16. J. H. Indik et al., “2017 HRS expert consensus statement on magnetic resonance imaging and radiation exposure in patients with cardio-vascular implantable electronic devices.,” Hear. Rhythm, vol. 14, no. 7, pp. e97–e153, Jul. 2017.
17. R. Luechinger, F. Duru, M. B. Scheidegger, P. Boesiger, and R. Candi-nas, “Force and torque effects of a 1.5-Tesla MRI scanner on cardiac pacemakers and ICDs.,” Pacing Clin. Electrophysiol., vol. 24, no. 2, pp. 199–205, Feb. 2001.
18. R. J. Russo et al., “Assessing the Risks Associated with MRI in Pa-tients with a Pacemaker or Defibrillator,” N. Engl. J. Med., vol. 376, no. 8, pp. 755–764, Feb. 2017.
19. S. Nazarian et al., “Safety of Magnetic Resonance Imaging in Patients with Cardiac Devices,” N. Engl. J. Med., vol. 377, no. 26, pp. 2555– 2564, Dec. 2017.
20. Gerber TC, Fasseas P, Lennon RJ, Valeti VU, Wood CP, Breen JF, et al. Clinical safety of magnetic resonance imaging early after coronary artery stent placement. J Am Coll Cardiol. 2003 Oct;42(7):1295–8.
21. Porto I, Selvanayagam J, Ashar V, Neubauer S, Banning AP. Safety of magnetic resonance imaging one to three days after bare metal and drug-eluting stent implantation. Am J Cardiol. 2005 Aug;96(3):366–8.
22. Parthasarathy H, Saeed O, Marcuzzi D, Cheema AN. MRI-induced stent dislodgment soon after left main coronary artery stenting. Circ Cardiovasc Interv. 2013 Oct;6(5):e58-9.
23. Syed MA, Carlson K, Murphy M, Ingkanisorn WP, Rhoads KL, Arai AE. Long-term safety of cardiac magnetic resonance imaging per-formed in the first few days after bare-metal stent implantation. J Magn Reson Imaging. 2006 Nov;24(5):1056–61.
24. Patel MR, Albert TSE, Kandzari DE, Honeycutt EF, Shaw LK, Sketch MHJ, et al. Acute myocardial infarction: safety of cardiac MR imag-ing after percutaneous revascularization with stents. Radiology. 2006 Sep;240(3):674–80.
25. Shellock FG, Forder JR. Drug eluting coronary stent: in vitro evalu-ation of magnet resonance safety at 3 Tesla. J Cardiovasc Magn Re-son. 2005;7(2):415–9.
26. van der Laan MJ, Bartels LW, Viergever MA, Blankensteijn JD. Com-puted tomography versus magnetic resonance imaging of endoleaks after EVAR. Eur J Vasc Endovasc Surg. 2006 Oct;32(4):361–5.
27. Pitton MB, Schweitzer H, Herber S, Schmiedt W, Neufang A, Kalden P, et al. MRI versus helical CT for endoleak detection after endovas-cular aneurysm repair. AJR Am J Roentgenol. 2005 Nov;185(5):1275– 81.
 This work is licensed under a
This work is licensed under a