Lucian Predescu1,2, Adrian Mereuta1,2, Carmen Beladan1,2, Aura Popa1, Andrei Iosifescu1, Alina Paunescu1, Carmen Ginghina1,2, Dan Deleanu1
1 “Prof. Dr. C. C. Iliescu” Emergency Institute for Cadiovascular Diseases, Bucharest
2 „Carol Davila” University of Medicine and Pharmacy, Bucharest
Abstract: Intramural hematoma of the ascending aorta has a poor prognosis if it is not promptly diagnosed and treated. Even with surgical correction, postoperative management is feared because of its complicated course. We present a case of a 58 years old male patient admitted with intramural hematoma of the ascending aorta complicated with signifi cant pericardial effusion. The patient was treated by surgery (the ascending aorta and aortic hemi-arch was replaced with a collagen-sealed Dacron prostheses). Early after surgery the patient developed acute myocardial infarction. Emergent coronary angiography demonstrated a pronounced spasm of the distal portion of the right coronary artery, apparently related to compression of this vessel by a chest drain tube. The pericardial drainage tube was extracted and intracoronary nitroglycerine was administrated with the disappearance of coronary stenosis and normalizing of coronary blood flow. This case is particular because it draws attention to the fact that mechanical compression of coronary artery by pericardial drainage tube may trigger an exaggerated vasomotor response, leading to severe coronary artery spasms and to the importance of correct placement of drainage tubes in the pericardium. Keywords: acute coronary syndrome, postoperative myocardial infarction, acute aortic syndrome, intramural hematoma, coronary artery spasm
INTRODUCTION
Perioperative myocardial infarction (PMI) is one of the most important predictors of short- and long-term morbidity and mortality associated with cardio-vascular surgery. The etiology of PMI after ascending aorta surgery can be multifactorial. Among other conditions, perioperative coronary spasm is a potentially life-threatening complication of cardiac surgery. Most published
cases occurred following coronary artery bypass surgery and aortic surgery, its occurrence after valve replacement is uncommon1.
CASE REPORT
We report the case of a 58 years old male patient admitted to our clinic with severe anterior chest pain radiated to the back and syncope at pain onset. The patient had no similar episodes of chest pain and his past medical history was unremarkable. When the patient arrived in our clinic he was at 3 hours from symptoms onset. Clinical examination revealed muffled heart sounds, distended neck veins, blood pressure = 100/50 mmHg, heart rate = 120 bpmin, oxygen saturation level of 95% without oxygen supplementation, no focal neurological deficits. The electrocardiogram showed sinus tachycardia, heart rate = 120 bpmin, without ST segment or T wave changes. Blood tests at presentation revealed elevated levels of D-dimer, normal troponin and lactic acid level, stage 3 chronic kidney disease (glomerular fi ltration rate = 50 ml/min). Transthoracic echocardiography revealed a concentric left ventricular hypertrophy, normal left ventricular systolic function without regional or global wall motion abnormalities, left ventricular ejection fraction of 60%, mild mitral and tricuspid valve regurgitation, tricuspid aortic valve with moderate aortic regurgitation due to a leaflet coaptation defect, important ascending aortic dilatation (51 mm), intramural hematoma of the ascending aorta and aortic arch without evidence of intimal flap, moderate pericardial effusion with right atrium collapse. After initial evaluation the patient was diagnosed with acute aortic syndrome due to intramural hematoma with fissuration into the pericardial space superimposed on an ascending aortic aneurysm. The patient was transferred to the intensive care unit, where the preoperative preparation protocol was instituted. The diagnostic was confirmed by thoracic computer tomography (Figure 1) and transesophageal echocardiography certified the absence of the intimal flap, the integrity of aortic valve, the absence of extension of
intramural hematoma to the Valsalva sinuses and the absence of detectable blood flow in the intramural hematoma. The patient was rapidly transferred to the operating room. The surgery was made under mild hypothermia, extracorporeal circulation and selective bilateral antegrade cerebral perfusion. The hemopericardium was evacuated and the ascending aorta and aortic hemiarch
was replaced with a number 26 collagen-sealed Dacron prostheses with gluing of the two cylinders of the dissected aorta.
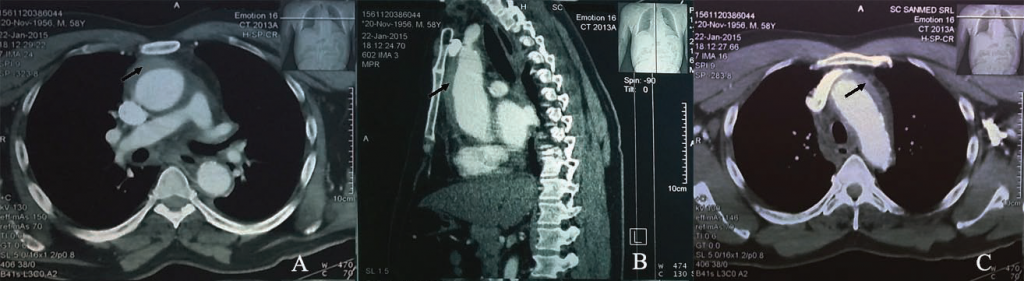
Figure 1. Thoracic computer tomography: Circumferential intramural hematoma (black arrow) of ascending aorta (A – transverse plane, B – sagital plane) and aortic arch (C – transverse plane).
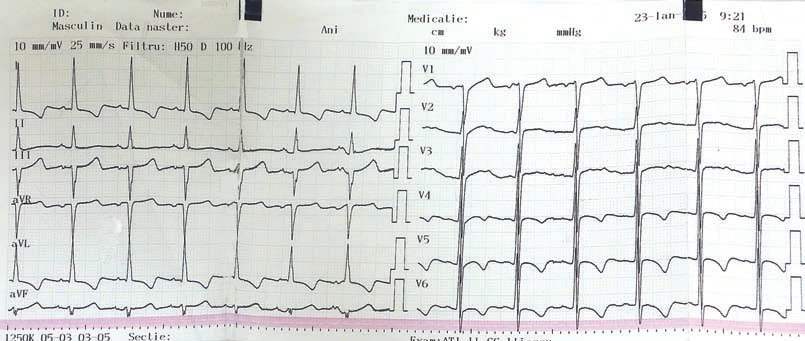
Figure 2. Postoperative electrocardiogram: Sinus rhythm, heart rate = 80 bpmin, negative T waves in V3-V6, D1 and aVL.
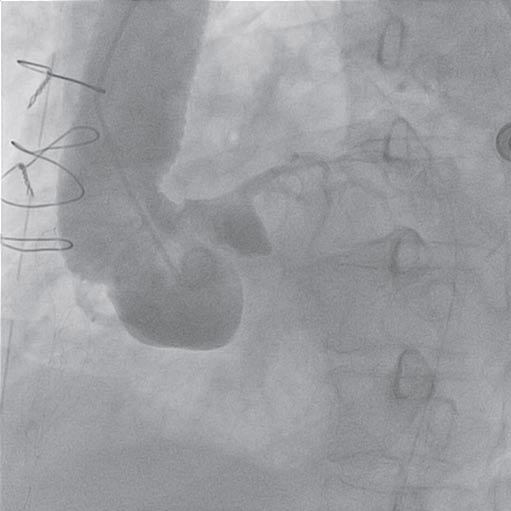
Figure 3. Aortography: ascending aortic prostheses without any sign of intimal flap, mild aortic regurgitation.
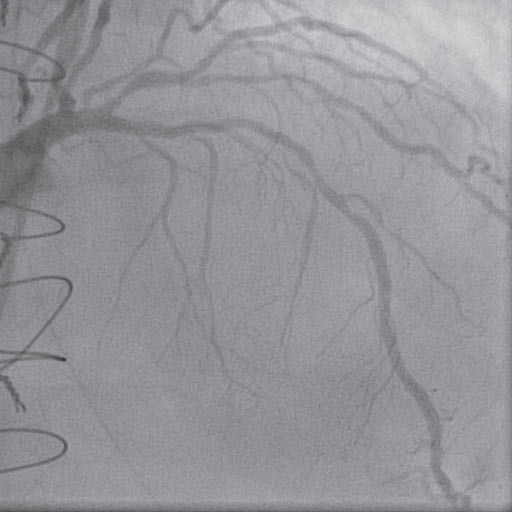
Figure 4. Coronary angiogram – left coronary artery (RAO 10° CRA 40°): atheromatous plaque on left anterior descending artery.
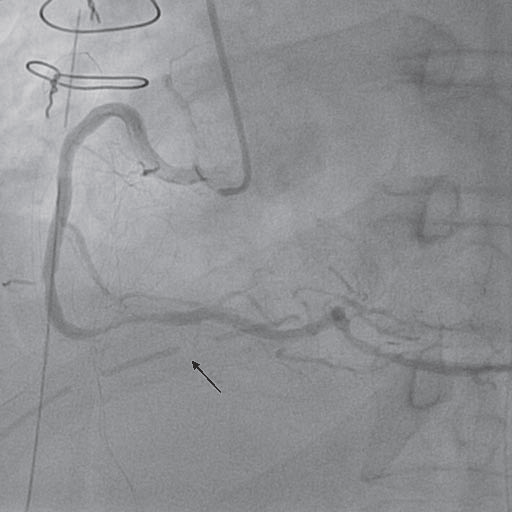
Figure 5. Coronary angiogram – right coronary artery (LAO 30°): severe stenosis in the third segment (compresion of right coronary artery by the pericardial drainage tube – black arrow).
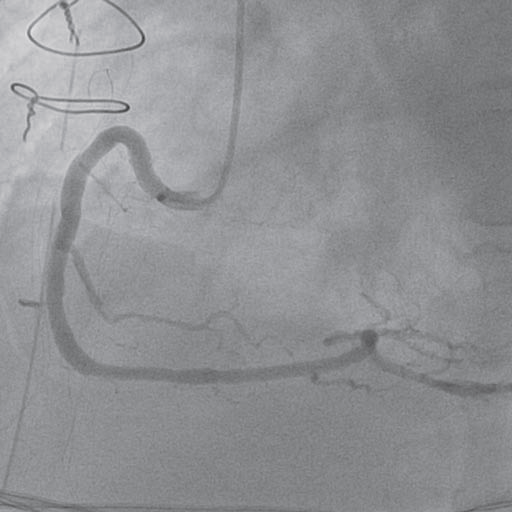
Figure 6. Coronary angiogram – right coronary artery (LAO 30°): disappearance of coronary spasm after pericardial drainage tube removal and intracoronary nitroglicerine administration.
Early after surgery the patient presented an episode of ventricular fibrillation that needed electrical cardioversion. The postoperative electrocardiogram showed sinus rhythm, heart rate = 80 bpmin, negative T waves in V3-V6, D1 and aVL, changes that were not present on preoperative electrocardiogram (Figure 2). Cardiac troponin was increased 10 times the preoperative level.
Taking into account the electrocardiographic changes and the elevation of cardiac enzymes, the diagnosis of perioperative acute coronary syndrome was made. The patient was transferred to the catheterization laboratory. The aortography showed the aortic prostheses without any sign of intimal flap and a mild aortic regurgitation (Figure 3). The coronary angiogram revealed a left coronary artery with atheromatous plaques on the left anterior descending artery (Figure 4) and a severe stenosis in the third segment of the right coronary artery with a TIMI II fl ow (Figure 5).
After coronary angiogram, the heart team decided to revascularize the patient by percutaneous coronary intervention. But a closer look at the lesion on the right coronary artery revealed a close relation of the stenosis on the third segment of right coronary artery with the pericardial drainage tube (Figure 5 – black arrow). At that point, the suspicion of compression of the right coronary artery by the pericardial drainage tube with superimposed coronary spasm was raised. So, the surgeon extracted, in the catheterization laboratory, the pericardial drainage tube and intracoronary
nitroglycerine was administrated. After that the coronary angiogram revealed the disappearance of right coronary compression and spasm, without any other and a TIMI III fl ow (Figure 6). The post procedural electrocardiogram showed normalization of the T waves changes in V3-V6, D1 and aVL (Figure 7). After surgery the evolution of the patient was good, he was extubated the following day, he needed no hemodynamic support and cardiac enzymes normalized in 3 days.
DISCUSSIONS
Intramural hematoma account for 10-25% of acute aortic syndromes 2. It is diagnosed in the presence of a circular or crescent-shaped thickening of >5 mm of the aortic wall in the absence of detectable blood fl ow3. The involvement of the ascending aorta and aortic arch (Type A) may account for 30% and 10% of cases, respectively, whereas it involves the descending thoracic aorta (Type B) in 60-70% of cases 4. Therapeutic management in acute intramural hematoma should be similar to that for aortic dissection. Emergency surgery is indicated in complicated cases
with pericardial effusion, periaortic hematoma, large aneurysms (maximum aortic diameter ≥50 mm), progressive maximum aortic wall thickness >11 mm, penetrating ulcer or ulcer-like projection secondary to localized dissections in the involved segment or detection of organ ischemia (brain, myocardium, bowels, kidneys, etc), and urgent surgery (<24 hours after diagnosis)
is required in most of Type A intramural hematomas4. Perioperative myocardial infarction (PMI) is one of the most important predictors of short- and longterm morbidity and mortality associated with cardiovascular surgery1. Most (>80%) PMIs occur early after surgery, are asymptomatic, of the non-Q-wave type (60–100%), and are most commonly preceded by STsegment
depression rather than ST-segment elevation5. In most cases, there is pathological and angiographic evidence that the etiology of PMI resembles that in the non-surgical setting6. However, establishing the etiology of PMI after acute aortic syndrome surgery can be a challenging task. There are many causes that can be responsible for the PMI after aortic surgery, like:
rupture of a pre-existing coronary plaque with superimposed thrombosis
embolization of aortic dissection associated thrombus
embolus associated with glue usage7
air embolism
prolonged myocardial oxygen supply-demand imbalance in the presence of stable coronary artery disease
iatrogenic dissection of coronary ostia
coronary artery spasm8,9
coronary artery compression by pericardial drainage tube10
dissection reoccurrence of Valsalva sinuses when only the ascending aorta is replaced with a prosthesis.
Acute coronary syndrome occurs when an unstable or vulnerable plaque undergoes spontaneous rupture, fissuring, or erosion, leading to acute coronary thrombosis, ischemia, and infarction. Perioperative stress can predispose to plaque rupture and myocardial infarction because of the sympathetic induced hemodynamic (tachycardia and hypertension, common in the perioperative
period, may exert shear stress), coronary vasoconstriction, and prothrombotic status (increased platelet reactivity, decreased endogenous anticoagulants and decreased fibrinolysis)1.
Prolonged myocardial oxygen supply-demand imbalance in the presence of stable coronary artery disease can be generated by postoperative hypotension, hypertension, tachycardia, anemia, hypoxemia and hypercarbia11. Other causes of PMI after aortic repair surgery are: embolization of aortic dissection associated thrombus, an embolus associated with glue usage or an embolus
arose from somewhere in the heart. On opening the aorta at the time of surgery, thrombus is sometimes found in the aortic lumen. Embolization of this material into the coronary arteries has been suggested as a possible cause for peri-operative ischemic myocardial dysfunction7. Those embolic event tend to occur early after surgery and they can be the reason for the hemodynamic instability of the patient and for electrocardiographic changes. In our case, the patient had T wave changes early after surgery with an important raise in cardiac troponin. Coronary artery spasm is defined as a reversible coronary stenosis that limits coronary blood flow. Perioperative coronary spasm is a potentially life-threatening complication of cardiovascular surgery. Postoperative
coronary arterial spasm has been associated to coronary artery trauma during surgical manipulation, compression by chest drain tubes, alkalosis, low body temperature and release of vasospastic factors by platelets damage during cardiopulmonary bypass10. The manifestations of spasm range from asymptomatic ST elevation/depression to hypotension and circulatory collapse, related to arrhythmias or myocardial injury. When the coronary spasm is diffuse and severe suggests that this represents a true vasospasm and not only a simple case of focal compression of the vessel
wall. In our case, there was a focal coronary spasm, only in third segment of right coronary artery. Based on this observation we can speculate that the focal trauma due to external compression of the vessel wall by a chest drain tube may have had some influence in the development of spasm. After removal of pericardial drainage tube and intracoronary nitroglycerine administration the coronary spasm ceded with normalization of coronary blood flow.
CONCLUSIONS AND IMPLICATIONS FOR CLINICAL PRACTICE
Type A intramural hematoma is a life threatening condition that must be rapidly diagnosed and treated. After aortic surgery, the etiology of postoperative myocardial infarction can be multifactorial. Coronary angiogram can be very useful in defi ning the exact nature of coronary disease and it can be followed in some cases by percutaneous coronary intervention. Early after cardiac surgery, in a patient with postoperative myocardial infarction, when we are dealing with a focal stenosis of a coronary artery we must differentiate between a true atherosclerotic coronary lesion and a coronary spasm. This differentiation is of paramount importance because it can avoid an unnecessary percutaneous coronary intervention with stent in case of a coronary spasm. The interventional cardiologist must pay attention to the proximity of pericardial drainage tube to the coronary artery and he must take into account the possibility of mechanical compression of coronary artery by the pericardial drainage tube. In such cases pericardial drainage tube removal with intracoronary nitroglycerine administration can be a simple solution to eliminate the cause of the perioperative myocardial infarction.
Acknowledgments: n one declared.
Disclosures: The authors declare that there is no conflict of interest.
References
1. Priebe HJ. Perioperative myocardial infarction–aetiology and prevention. Br J Anaesth 2005;95:3-19.
2. Evangelista A, Mukherjee D, Mehta RH, et al. Acute intramural hematoma of the aorta: a mystery in evolution. Circulation 2005;111:1063- 70.
3. von Kodolitsch Y, Csosz SK, Koschyk DH, et al. Intramural hematoma of the aorta: predictors of progression to dissection and rupture. Circulation 2003;107:1158-63.
4. Erbel R, Aboyans V, Boileau C, et al. 2014 ESC Guidelines on the diagnosis and treatment of aortic diseases: Document covering acute and chronic aortic diseases of the thoracic and abdominal aorta of the adult. The Task Force for the Diagnosis and Treatment of Aortic Diseases of the European Society of Cardiology (ESC). Eur. Heart J. 2014;35:2873-926.
5. Landesberg G, Beattie WS, Mosseri M, et al. Perioperative myocardial infarction. Circulation 2009;119:2936-44.
6. Almeida GF, Vegni R, Japiassu AM, et al. Postoperative complications of surgically treated ascending aortic dissection. Rev. bras. ter. intensiva 2011;23:304-11.
7. Hoschtitzky JA, Crawford L, Brack M, Au J. Acute coronary syndrome following repair of aortic dissection. Eur J Cardiothorac Surg: official journal of the European Association for Cardio-thoracic Surgery 2004;26:860-2.
8. Pinho T, Almeida J, Garcia M, Pinho P. Coronary artery spasm following aortic valve replacement. Interact Cardiovasc Thorac Surg 2007;6:387-8.
9. Alizadeh-Ghavidel A, Basiri H, Totonchi Z, et al. A rare presentation of late right coronary artery spasm following aortic valve replacement. ARYA atherosclerosis 2015;11:50-3.
10. Arnould MA, Barbey C, Chassaing S. An unusual right coronary artery obstruction after mitral surgery. Arch Cardiovasc Dis 2013;106:63-5. 11. Landesberg G. The pathophysiology of perioperative myocardial infarction: facts and perspectives. J Cardiothorac Vasc Anesth 2003; 17:90-100.
 This work is licensed under a
This work is licensed under a