Viorel Nicolae1, Ayman Elkahlout1, Razvan Constantin Serban1,2
1 Emergency Institute for Cardiovascular Diseases and Transplantation, Tirgu Mures, Romania
2 University of Medicine and Pharmacy of Tirgu Mures, Romania
Abstract: Introduction – Acute aortic dissection (AAD) is associated with high morbi-mortality and with potentially fatal complications. Surgery is the “gold standard” for Stanford A dissections, whereas thoracic endovascular aortic repair (TEVAR) has emerged as a less invasive alternative for complicated Stanford B dissections. The best approach to uncomplicated Stanford B aortic dissections remains controversial. Case presentation – We present the case of a 42-year-old hypertensive male hospitalized for AAD extended from the aortic arch to the left common iliac artery. Two stent grafts were implanted from the ostium of the left common carotid artery, almost reaching distally the origin of the coeliac trunk. Follow-up CT angiography performed 6 weeks later showed persistent compression of the true lumen by the false lumen at the level of the coeliac trunk, and a bare-metal stent was implanted distally to the second stent graft. Six months later, CT angiography showed well-seated grafts and stent, false lumen thrombosis, good diameter of the aortic true lumen, and no signs of progression of the aortic dissection distally to the abdominal aorta stent. Conclusion – Management of uncomplicated Stanford B dissections is very challenging. New devices and combination of bare-metal stents and stent grafts might offer good long-term results.
Keywords: acute aortic dissection, Stanford B, thoracic endovascular aortic repair
INTRODUCTION
Acute aortic dissection (AAD) is part of the acute aortic syndrome. This condition affects 3 to 4 in every 100,000 individuals every year1, is associated with high morbidity and mortality, and has potentially fatal complications2. Regardless of the dissection site, presentation may be with one of AADs’ major complications such as aortic rupture, intractable pain, loss of consciousness3, severe bleeding and shock, or organ malperfusion, all of which are associated with poor prognosis. The classification of AADs is based on the anatomic location of the dissection, the two most widely used classifications in clinical practice being DeBakey and Stanford4. Classification of AADs has direct clinical implications in AAD management. Surgery continues to represent the “gold standard” therapy for Stanford A dissections. Thoracic endovascular aortic repair (TEVAR) has emerged as a less invasive alternative to surgery for complicated Stanford B dissections5 and has been associated with improved early mortality and morbidity rates in this group of patients6. Meanwhile, the best approach to uncomplicated Stanford B aortic dissections remains a matter of debate. While close surveillance and blood pressure control may provide good short-term results, long-term prognosis may be less favorable7; optimal medical therapy (OMT) has been associated with disease progression towards complicated dissection or aneurysm formation in up to 40% of patients8. Whether a more invasive strategy is able to improve long-term prognosis in this high-risk population remains to be elucidated.
CASE PRESENTATION
We present the case of a 42-year-old male, obese, smoker, with a medical history of grade III arterial hypertension, with hypertriglyceridemia, sleep apnea, and chronic bronchitis. The patient presented at his local hospital with severe and prolonged acute chest pain. Computed tomography (CT) angiography revealed acute dissection of the aortic arch extending all the way to the left common iliac artery (Figure 1). The patient was immediately transferred to our tertiary center for further evaluation and management. Physical examination at admission showed increased body mass index (35.9 kg/m2); blood pressure was 160/80 mmHg and heart rate was 86 bpm. Heart and pulmonary examinations were within normal limits and there was no sign of organ malperfusion. The ECG revealed normal sinus rhythm and signs of left ventricular (LV) hypertrophy. Laboratory tests demonstrated very high triglycerides level (850 mg/dl), normal full blood count and liver tests, and an estimated glomerular filtration rate of 84.5 ml/min/1.73m2. Transthoracic echocardiography revealed LV hypertrophy with preserved LV systolic function (LV ejection fraction 60%) and type I diastolic dysfunction, without other significant abnormalities. During hospital stay, achievement of proper blood pressure control proved very difficult, even with high doses of blood pressure-lowering drugs associating five therapeutic classes. Although the patient had a EuroSCORE II of only 1.31, TEVAR was preferred over surgery, which was considered to be associated with higher risk of morbidity and mortality according to local expertise.
The patient was asymptomatic during hospital stay and TEVAR was performed 4 days later, after thorou-gh planning of the procedure. In order to facilitate the use of transesophageal echocardiography (TOE), the intervention was performed under general anesthesia. A lumbar catheter was implanted for monitoring cerebrospinal fluid pressure. Arterial access was gained percutaneously on both groins. On the right-hand side, two ProGlide suture-mediated percutaneous closure systems (Abbot Vascular; Santa Clara, CA) were de-ployed to allow percutaneous closure of the puncture site at the end of the procedure. A 6 French sheath was inserted into the left femoral artery to allow contrast injection via a pigtail catheter positioned in the ascending aorta using TOE guidance to discriminate between the false and the true lumen. After confirming adequate positioning of the wire in the true lumen of the aorta, the first stent graft was implanted from the ostium of the left common carotid artery, completely covering the left subclavian artery. A second graft was positioned distally to the first one with approximately 3 cm of overlapping, almost reaching distally the origin of the coeliac trunk (Figure 2). Due to the high risk of rupture in the setting of AAD, no post-dilation was performed. Hemostasis was achieved via the two Pro-Glide closure systems on the right-hand side and a 6 French Angio-Seal (St Jude Medical; Minnetonka, MN) on the left-hand side. Post-procedural clinical course was uneventful. Control CT angiography performed one week later showed false lumen thrombosis at the level of the grafts. At the level of the abdominal aorta, the dissection was extended, as previously noted, to the left common iliac artery. Although abdominal and pelvic organs were well perfused, the true lumen of the aorta was significantly compressed by the intramural hematoma, with a diameter of only 8 mm (Figure 3). The patient was discharged one week later with well-controlled blood pressure and was prescribed 3 months of double antiplatelet therapy. The first follow-up CT angiography, performed 6 weeks after discharge, showed well-seated thoracic aorta endograft and no further progression of the dissection was noted. Due to persistent compression of the true lumen by the false lumen at the level of the coeliac trunk, implantation of a bare-metal stent distally to the second stent graft was decided in an attempt to ensure a larger aortic true lumen (Figure 4). Follow-up CT angiography performed 6 months later showed well-seated thoracic and abdominal aorta grafts and stent, false lumen thrombosis, good (20 mm) diameter of the aortic true lumen, and persistence of the aortic dissection distally to the abdominal aorta stent with extension to the left common iliac artery, but without any signs of progression (Figure 5).
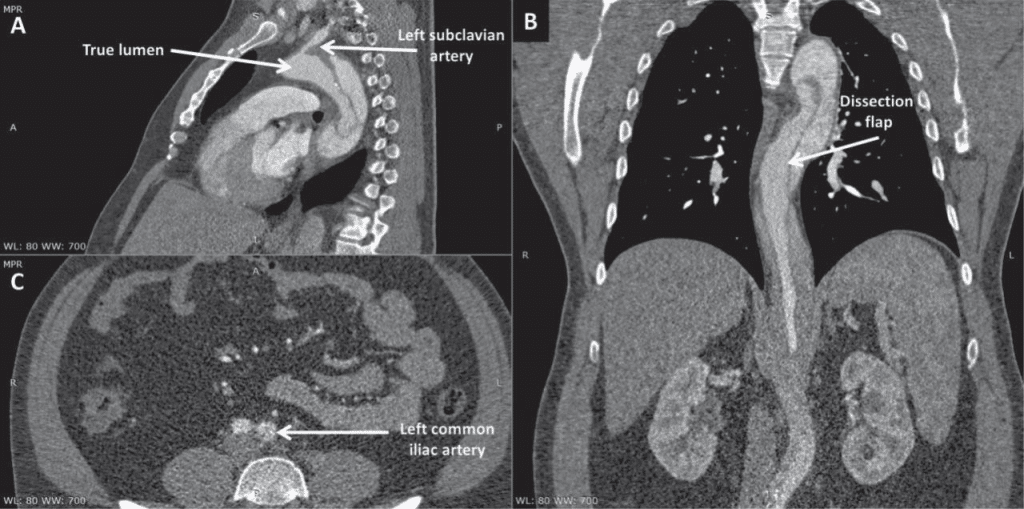
Figure 1. Computed tomography angiography scans in sagittal (A), coronal (B), and axial (C) view at admission showing aortic dissection extended from the aortic arch to the left common iliac artery.
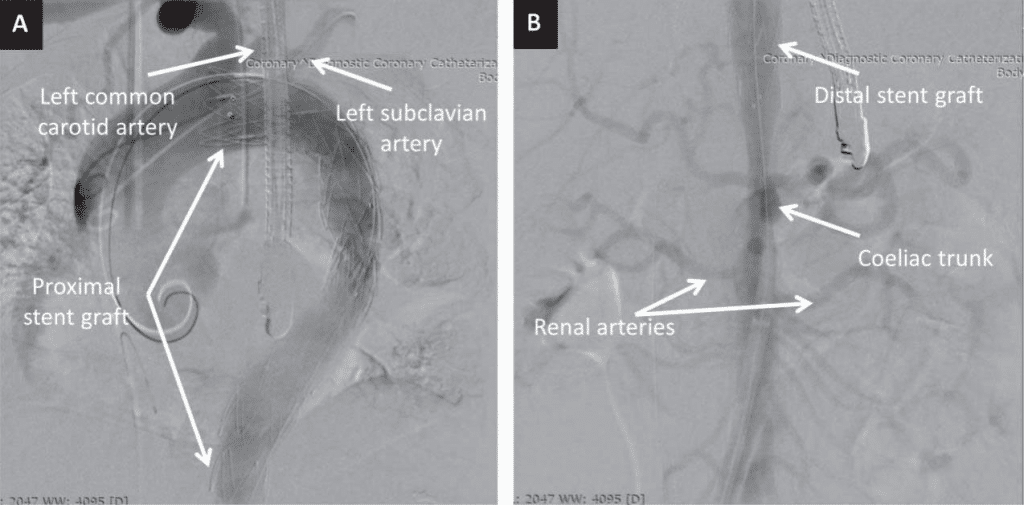
Figure 2. Digital subtraction angiography images in postero-anterior view showing the proximal (A) and the distal (B) stent grafts.
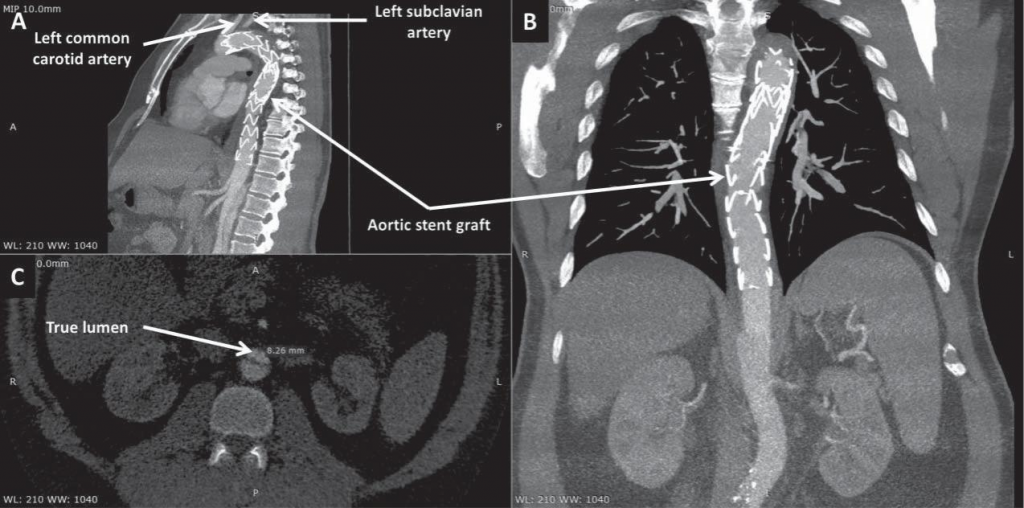
Figure 3. Computed tomography angiography scans in sagittal (A), coronal (B), and axial (C) view at one-week follow-up showing false lumen thrombosis at the level of the grafts. Compression of the true lumen of the aorta by an intramural hematoma, down to a diameter of 8 mm, can be noticed in panel C.
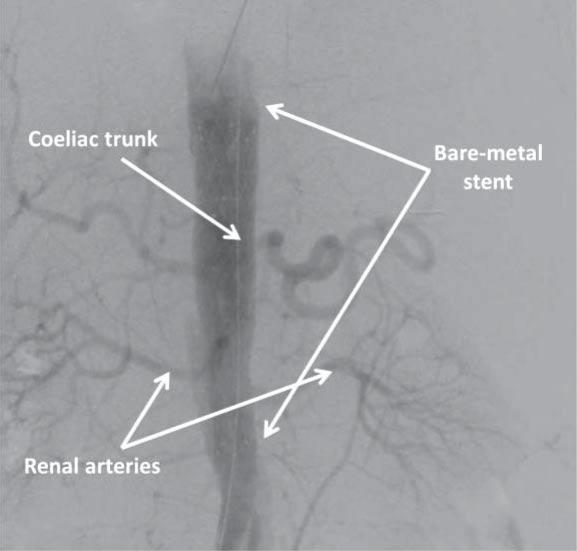
Figure 4. Digital subtraction angiography image in postero-anterior view showing the bare-metal stent covering the coeliac trunk and the renal ar-teries.
DISCUSSIONS
Thoracic endovascular aortic repair is a safe, minimally invasive procedure, widely used as an alternative to surgery due to the lower morbidity and mortality rates in selected patient groups9. The procedure was initially used as an alternative to surgery for complicated Stan-ford B dissections, which include evidence of thoracic aorta rupture, organ malperfusion, or rapid expansion of the dissection in the distal aortic arch or in the descending aorta10. Later on, it has been shown that, due to better aortic remodeling process, endovascular re-pair also improves long-term survival in patients with uncomplicated dissections7. The Investigation of Stent Grafts in Aortic Dissection (INSTEAD) trial was the first prospective randomized trial to compare TEVAR with OMT in patients with uncomplicated acute Stan-ford B dissections11. A number of 140 patients were randomly assigned, at least 2 weeks after the index dissection, to elective stent graft placement in addition to OMT or OMT alone. Although the study failed to show a survival benefit of TEVAR over the short-term due to the low number of patients included and lack of statistical power12, TEVAR enhanced the rates of false lumen thrombosis and aortic remodeling11. At 5-year follow-up, TEVAR decreased aortic-related mortality and aortic disease progression13. Additionally, complete false lumen thrombosis was confirmed in 90.6% of cases at 5 years after TEVAR, whilst this was rarely seen in patients treated with OMT. Optimal medical therapy alone was also associated with an expansion of the aortic diameter. These results encourage elective TEVAR in suitable patients for long-term benefit13. Similar results regarding false lumen thrombosis and true lumen expansion in the OMT group were also obtained in a different trial. However, despite these findings, there was also no survival benefit14.
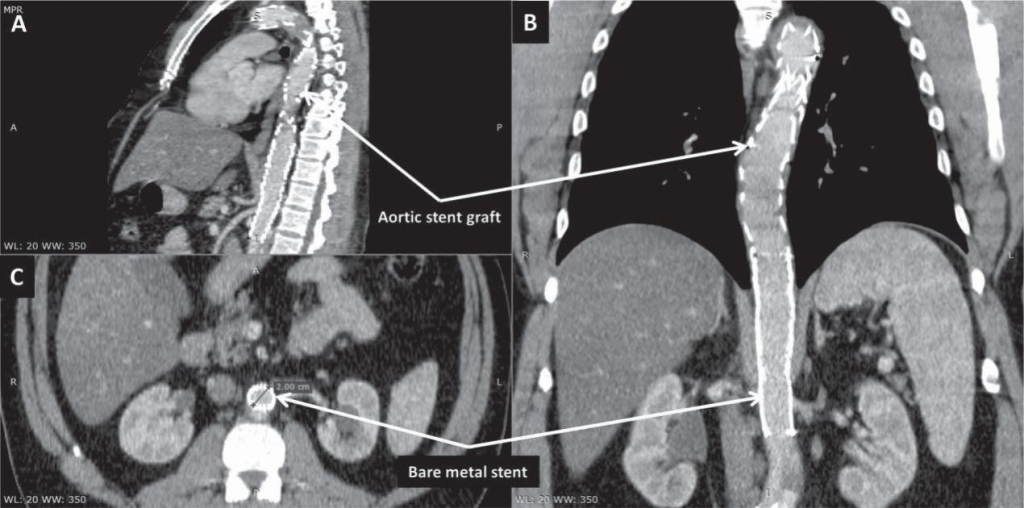
Figure 5. Computed tomography angiography scans in sagittal (A), coronal (B), and axial (C) view at 6-months follow-up showing well-seated thoracic and abdominal aorta grafts and stent, false lumen thrombosis, good (20 mm) diameter of the aortic true lumen, and aortic dissection distally to the abdominal aorta stent, with extension to the left common iliac artery.
The benefit of implanting a bare-metal stent distally to the second stent graft, completely covering the co-eliac trunk, is controversial. Limited data exist regarding its potential benefit. One might hypothesize that this will ensure better false lumen thrombosis and true lumen collapse. Indeed, Mossop et al. reported 6 cases of patients with type B dissections in which stent grafts were used for closure of the proximal (en-try) tear and a bare-metal stent was employed for the residual dissection. Patients with true lumen collapse and no further expansion of the dissection after proximal endograft placement were selected for bare-metal stent implantation. Stent deployment resulted in an immediate increase in the true lumen index (true lumen/total aorta) from 39% to 71%, and average true lumen expansion was 141%. The results were maintained at 3 months and 18 months of follow-up, with no complications related to the bare-metal stent15.
In conclusion, management of uncomplicated Stan-ford B dissections is a continuous challenge in medical practice. Percutaneous treatment emerged as an alter-native to surgery with lower morbidity and mortality rates. The results of the first trials are encouraging, but so far there is no mortality benefit of stent graft implantation over medical treatment. New devices and combination of bare-metal stents and stent grafs might offer new alternatives and better long-term results.
Funding: This work was supported by the University of Medicine and Pharmacy of Tîrgu Mureş Research Grant number 15609/6/29.12.2017.
Conflicts of interest: none declared.
References
1. Nathan DP, Xu C, Gorman JH 3rd, Fairman RM, Bavaria JE, Gorman RC, Chandran KB, Jackson BM. Pathogenesis of acute aortic dissec-tion: A finite element stess analysis. Ann Thorac Surg 2011;91: 458-64.
2. De León Ayala IA, Chen YF. Acute aortic dissection: An update.
Kaohsiung J Med Sci 2012;28: 299-305.
3. Radulescu B, Preda S, Valeanu L, Jurcut R, Iliescu VA. Acute aortic dissection – always surprising. Romanian Journal of Cardiology 2016; 26: 479-84.
4. Braverman AC. Acute aortic dissection. Circulation 2010;122: 184-8.
5. Arnáiz-García ME, González-Santos JM, Arnáiz-García AM, Arnáiz J. Endovascular repair or best medical treatment: what is the optimal management of uncomplicated type B acute aortic dissection. J Tho-rac Dis 2017;9: 3458-62.
6. Iranmanesh S, Ricotta S. Current management of acute type B aortic dissection. World J Surg Proced 2015;5: 208-16.
7. Song C, Lu Q, Zhou J, Yu G, Feng X, Zhao Z, Bao J, Feng R, Jing Z. The new indication of TEVAR for uncomplicated type B aortic dis-section. Medicine (Baltimore) 2016;95: e3919.
8. Moulakakis KG, Mylonas SN, Dalainas I, Kakisis J, Kotsis T, Liapis CD. Management of complicated and uncomplicated acute type B dissection. A sistematic review and meta-analysis. Ann Cardiothorac Surg 2014;3: 234-46.
9. Dake MD, Miller DC, Semba CP, Mitchell RS, Walker PJ, Liddell RP. Transluminal placement of endovascular stent grafts for the treatment of descending thoracic aortic aneurysms. N Engl J Med 1994; 331: 1729-34.
10. Sueyoshi E, Onitsuka H, Nagayama H, Sakamoto I, Uetani M. Endo-vascular repair of aortic dissection and intramural hematoma: indica-tions and serial changes. Springerplus 2014;3: 670.
11. Krol E, Panneton JM. Uncomplicated acute type B aortic dissection: selection Guidelines for TEVAR. Ann Vasc Dis 2017;10: 165-9.
12. Nienaber CA, Rousseau H, Eggebrecht H, Kische S, Fattori R, Re-hders TC, Kundt G, Scheinert D, Czerny M, Kleinfeldt T, Zipfel B, Labrousse L, Ince H; INSTEAD Trial. Randomized Comparison of strategies for type B aortic dissection. The INvestigation of STEnt Grafts in aortic DIssection (INSTEAD) trial. Circulation 2009;120: 2519-28.
13. Nienaber CA, Kische S, Rousseau H, Eggebrecht H, Rehders TC, Kundt G, Glass A, Scheinert D, Czerny M, Kleinfeldt T, Zipfel B, Labrousse L, Fattori R, Ince H; INSTEAD-XL trial. Endovascular repair of type B aortic dissection. Long term results of The randomized investigation of stent grafts in aortic dissection trial. Circ Cardiovasc Interv 2013;6: 407-16.
14. Nauta FJ, Trimarchi S, Kamman AV, Moll FL, van Herwaarden JA, Pa-tel HJ, Figueroa CA, Eagle KA, Froehlich JB. Update in the management of type B aortic dissection. Vasc Med 2016;21: 251-63.
15. Mossop P, Nixon I, Oakes J, Devine TJ, McLachlan CS. Immediate “total” aotic true lumen expansion in type A and B acute aortic dis-section after endovascular aortic endografting and GZSD bare stenting. J Thorac Cardiovasc Surg 2007;134: 1360-2.
 This work is licensed under a
This work is licensed under a