Bogdan Enache1,2, Gabriel Decebal Latcu1, Sok-Sithikun Bun1, Ahmed Mostafa Wedn1, Nadir Saoudi1
1 Department of Cardiology, Princess Grace Hospital, Monaco
2 Department of Cardiology, “Victor Babes” University of Medicine and Pharmacy, Timisoara, Romania
Abstract: Introduction – Ultra-high resolution electroanatomical mapping is a new tool for understanding in detail the mechanisms of atrial arrhythmias, whether focal or reentrant, and their accurate activation. The highlights of the new Rhythmia system (Boston Scientifi c) are its mapping catheter (Orion), the high-density mapping and the high-quality algorithm behind the automatic point annotation. Case report – A 73-year old patient, with a prior persistent atrial fibrillation ablation, who developed an atypical atrial fl utter. The Rhythmia system was used to map the atrial fl utter showing a circuit involving gaps in the previous ablation line around the pulmonary veins which lead to a successful termination with ablation. A second tachycardia was induced through programmed atrial stimulation. A new map revealed a reentrant circuit around a small scar which again was successfully ablated. Following sinus rhythm resumption, no other tachycardia (including atrial fibrillation) was inducible any more, even with “aggressive” pacing protocols, under isoprenaline challenge.
Keywords: ultra-high resolution electroanatomical mapping, activation mapping, atrial tachycardia.
INTRODUCTION
The advent of ultra-high resolution electroanatomical mapping has brought on a new era in the understanding of arrhythmias and arrhythmia circuits1, be they macroreentrant or focal2 (through microreentry or automaticity). This advanced mapping allows for the accurate identification of the substrate (scar quantification3) and of the precise localization of the critical isthmus4 or focal source in the case of atrial tachycardias5- 7. We present a case of an atypical atrial fl utter after atrial fibrillation (AF) ablation mapped with the Boston Scientific RhythmiaTM system which lead to a precise and successful ablation of two successive reentrant tachycardias. The RhythmiaTM system includes a workstation and an amplifier. It’s most important feature is the OrionTM (Boston Scientific) mapping catheter which is a basket catheter with 8 splines and 8 fl at printed electrodes on each spline. With the reduced surface of each electrode (0.4 mm2) and the small inter-electrode space of 2.5 mm, these 64 electrodes are capable of recording extremely low-noise signals. Coupled with a proprietary algorithm for instant accurate automatic electrogram annotation, the system is able to quickly acquire up to several tens of thousands of points8,9.
CASE REPORT
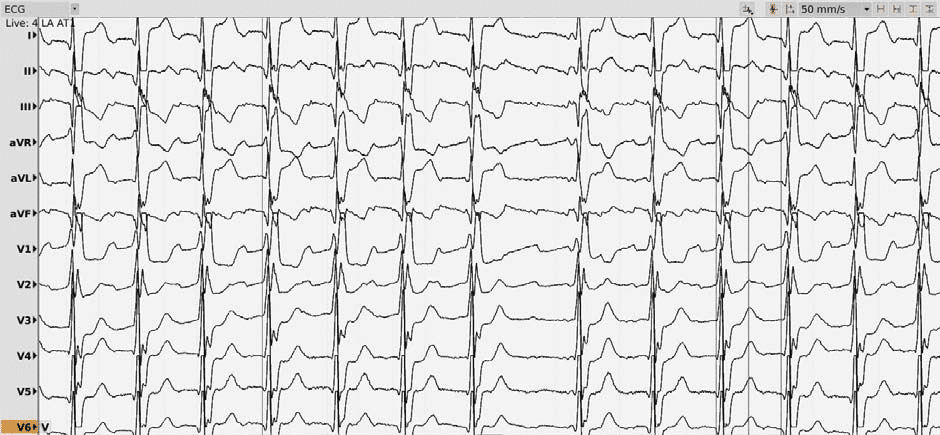
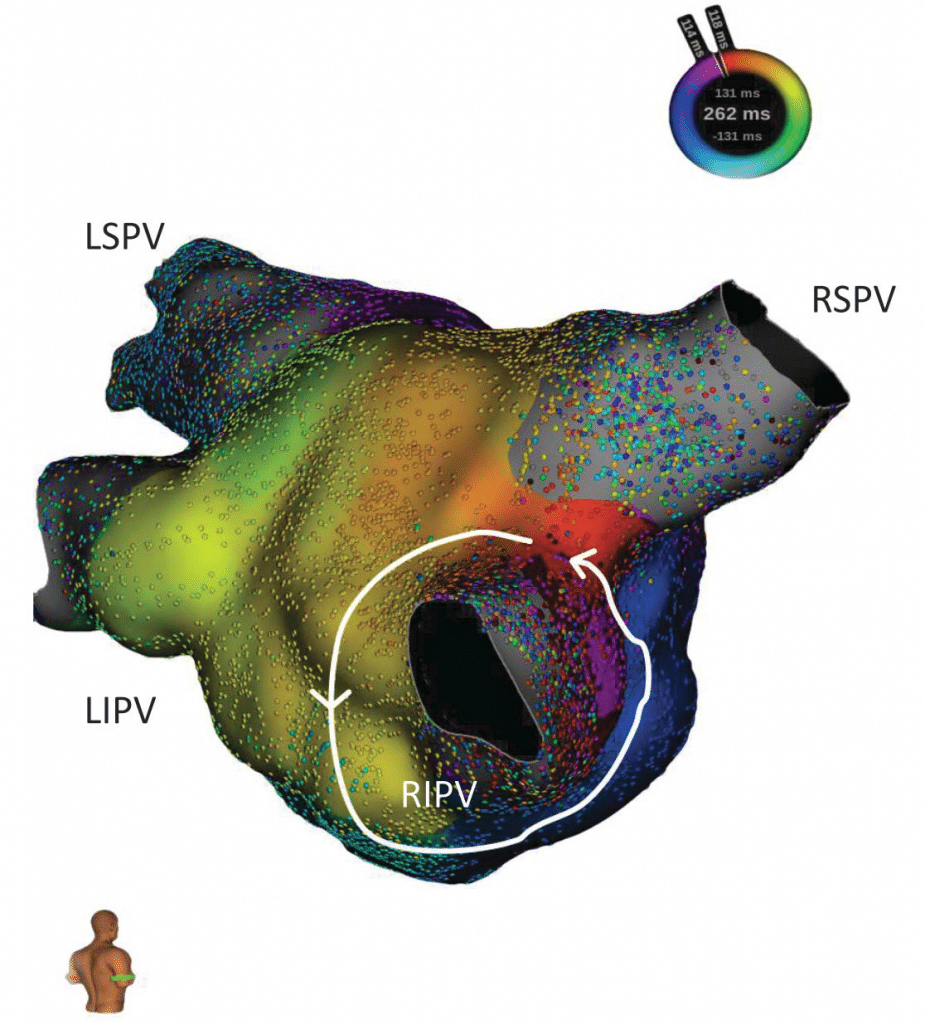
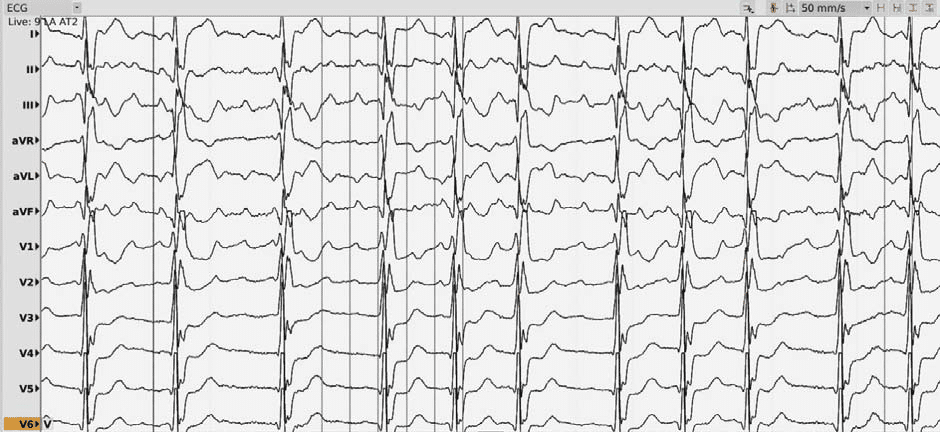
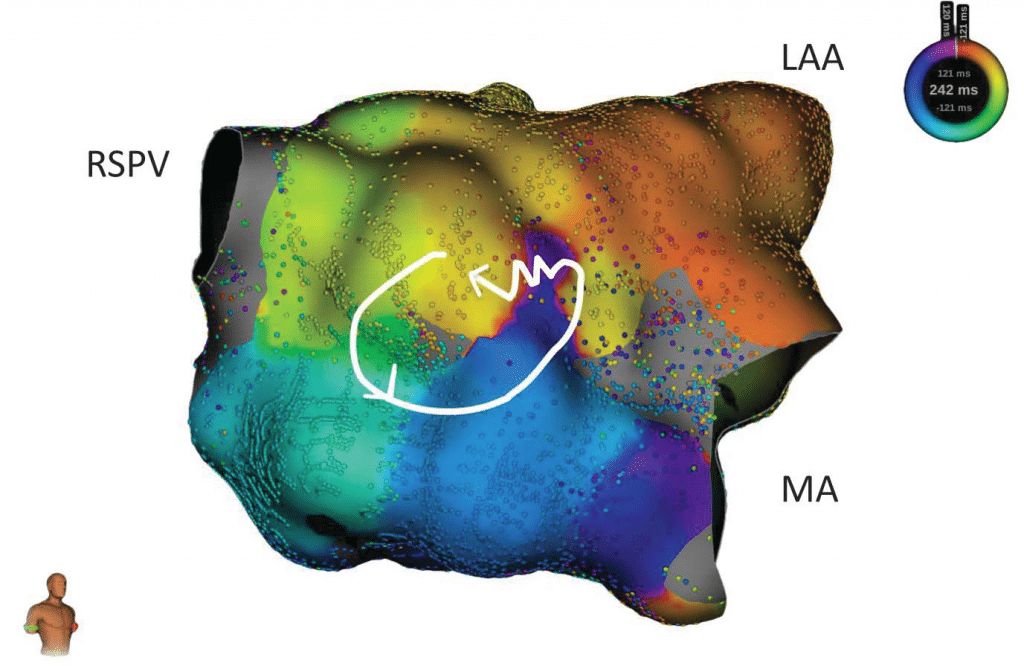
We present the case of a 73-year-old male with a history of myocardial infarction (with stent placement on the right coronary artery and the left marginal coronary artery in 2013) with a left ventricle ejection fraction (LVEF) of 56% and no significant active ischemia on a myocardial perfusion scintigraphy. His cardiovascular risk factors include hypertension (under triple therapy), non-insulin-dependent type 2 diabetes mellitus, obesity (body-mass index of 32 kg/m2) and sleep apnea (with previous non-compliance to the continuous positive airway pressure machine). The patient’s history of AF begins in 2012 with a persistent episode treated by electrical cardioversion. In 2013 the patient underwent in our center (Princess Grace Hospital Monaco) a robotic magnetic navigation radiofrequency (RF) ablation for recurring symptomatic persistent AF which included wide area circumferential pulmonary vein isolation (PVI), a roofline ablation and extensive complex fractionated atrial electrogram ablation of the left atrium with termination to atrial tachycardia and fi nally to sinus rhythm. In the following 3 years, the patient remained in sinus rhythm with signifi cant symptomatic improvement compared to AF. An atrial tachycardia recurrence was documented in 2017 (12-lead ECG of the clinical atrial tachycardia in Figure 1; tachycardia cycle length (TCL) 275 ms); after anti-arrhythmic drug failure and early post-cardioversion recurrence, a new ablation was proposed. Under general anesthesia, using an echography-guided triple puncture of the right superficial femoral vein, a decapolar catheter was advanced in the coronary sinus, the OrionTM mapping catheter was advanced through a non-steerable sheath in the right atrium (RA) and later on transseptally in the left atrium (LA) and a TactiCathTM (St. Jude Medical) contact force ablation catheter was advanced through a steerable sheath in the LA transseptally. The double transseptal puncture was made under transesophageal echography guidance after confirmation of the absence of clots in the left atrial appendage. We started with an RA map which showed that the activation of this chamber only covered half of the TCL. (Video 1*). Then the LA was mapped (186 ml, 27549 electrograms, mapping time 17 minutes); the LA anatomy was strictly similar to the reconstructed 3D computer tomography image obtained prior to the procedure (both 3D images were merged). The activation map showed a macroreentry circuit utilizing gaps in the ablation line encircling the right pulmonary veins and turning counter-clockwise around the right inferior pulmonary vein (Figure 2, Video 2). Also, of note, we could observe that the left pulmonary veins and the right superior PV had no potentials and a collision of wavefronts on the LA roof was suggestive of the existence of a complete line of block10. Several ablation points (25-30 W with a 17 cc/min irrigation and an optimized contact) around the left inferior pulmonary vein terminated the tachycardia and there was a return to sinus rhythm. An aggressive programmed atrial stimulation protocol (400 ms drive-cycle length and 3 extrastimuli) initiated a second tachycardia with a cycle length of 242 ms (Figure 3). A new activation map was performed which showed reentry around a small area of scar on the anterior wall (Video 3). An area of slow conduction was observed between the scar and the mitral annulus. Ablation in this area (Figure 4) resulted in the termination of AT2 then a return to sinus rhythm. Afterwards, the patient remained non-inducible with the same aggressive programmed atrial stimulation protocol and with atrial burst pacing in both basal conditions and under isoprenaline.
Figure 1. 12-lead ECG of the first Atrial Tachycardia, cycle length of 275 ms.
Figure 2. Postero-septal view of the LA showing the activation sequence during AT1 with an activation around the right inferior pulmonary vein (RIPV).
CONCLUSION
Ultra-high resolution electroanatomical mapping is useful in the precise identification of substrate (previous RF lesions / fibrosis) and tachycardia circuits (in our case, reentry around an anatomical structure and around a scar area). Complete understanding of a tachycardia mechanism is the first step towards successful ablation.
Figure 3. 12-lead ECG of the second Atrial Tachycardia, cycle length of 242 ms.
Figure 4. RAO view of the LA with the activation mapping of the AT2 with an activation around a small area of scar (shown in grey in the center) with a region of slow conduction between the scar and the mitral annulus (wobbly line, which was also the successful ablation site). LAA – left atrial appendage. MA – mitral annulus.
References
1. Pathik B, Lee G, Sacher F, Jais P, Massoullié G, Derval N, et al. New Insights Into an Old Arrhythmia: High-Resolution Mapping Demonstrates Conduction and Substrate Variability in Right Atrial Macro–Re- Entrant Tachycardia. JACC: Clinical Electrophysiology.
2. Saoudi N. A classification of atrial flutter and regular atrial tachycardia according to electrophysiological mechanisms and anatomical bases. A Statement from a Joint Expert Group from the Working Group of Arrhythmias of the European Society of Cardiology and the North American Society of Pacing and Electrophysiology. European Heart Journal. 2001 Jul 15;22(14):1162–82.
3. Anter E, McElderry TH, Contreras-Valdes FM, Li J, Tung P, Leshem E, et al. Evaluation of a novel high-resolution mapping technology for ablation of recurrent scar-related atrial tachycardias. Heart Rhythm. Elsevier; 2016 Oct;13(10):2048–55.
4. Latcu DG, Bun S-S, Viera F, Delassi T, Jamili El M, Amoura Al A, et al. Selection of Critical Isthmus in Scar-Related Atrial Tachycardia Using a New Automated Ultrahigh Resolution Mapping System. Circulation: Arrhythmia and Electrophysiology. American Heart Association, Inc; 2016 Dec 30;10(1):e004510.
5. Rottner L, Metzner A, Ouyang F, Heeger C, Hayashi K, Fink T, et al. Direct Comparison of Point-by-Point and Rapid Ultra-High-Resolution Electroanatomical Mapping in Patients Scheduled for Ablation of Atrial Fibrillation. Journal of Cardiovascular Electrophysiology. 2017; 28(3):289–97.
6. Bun S-S, Latcu DG, Delassi T, Jamili ME, Amoura AA, Saoudi N. Ultra- High-Defi nition Mapping of Atrial Arrhythmias. Circ J. 2016;80(3): 579–86.
7. Schaeffer B, Hoffmann BA, Meyer C, Akbulak RO, Moser J, Jularic M, et al. Characterization, Mapping, and Ablation of Complex Atrial Tachycardia: Initial Experience With a Novel Method of Ultra High-Density 3D Mapping. Journal of Cardiovascular Electrophysiology. 2016;27(10):1139–50.
8. Ptaszek LM, Chalhoub F, Perna F, Beinart R, Barrett CD, Danik SB, et al. Rapid acquisition of high-resolution electroanatomical maps using a novel multielectrode mapping system. J Interv Card Electrophysiol. 2013;36(3):233–42.
9. Nakagawa H, Ikeda A, Sharma T, Lazzara R. Rapid high resolution electroanatomical mapping: evaluation of a new system in a canine atrial linear lesion model. Circulation: Arrhythmia and Electrophysiology. American Heart Association, Inc; 2012 Apr; 5(2):417–24.
10. Anter E, Tschabrunn CM, Contreras-Valdes FM, Li J, Josephson ME. Pulmonary vein isolation using the Rhythmia mapping system: Verification of intracardiac signals using the Orion mini-basket catheter. Heart Rhythm. 2015;12(9):1927–34.
 This work is licensed under a
This work is licensed under a