Corneliu Iorgulescu1, Dan-Andrei Radu1, Alexandrina Nastasa1, Georgiana Malaescu1, Stefan Bogdan1, Radu Vatasescu1
1 Department of Cardiology, Clinical Electrophysiology and Pacing Laboratory, Emergency Clinical Hospital, Bucharest, Romania
Abstract: Aims – The aim of the present study was to determine the frequency, characteristics and success rate of device, lead extraction procedures performed during 8 years in our-center. Methods and results – Between 2008 and 2015 2031 patients, mean age 63.4±26.8 years, 60% males were implanted in our center. Explant was defined as the removal of a lead within 1 year of implantation, by simple traction. Beyond 1 year, or via a vascular route not utilized at implant or employing specialized equipment, was termed extraction. Patient characteristics, strategies to remove the devices and periprocedural clinical management were noted for each case, and then outcomes were compared. The overall device removal rate was 1.23% from which 44% were explants and 56% were extractions. The indication for removal was pocket infection in 92% of the cases and endocarditis in 8%. In all the cases that involved leads less than 1 year old, simple traction was enough to completely remove the device. For leads older than 1 year, more complex techniques were necessary: femoral approach (14%), locking stylet (7%) or referral to another center for extraction with laser sheath (21%). After a mean procedure number of 1.2±0.5 there were a total of 3 abandoned leads. Conclusions – Lead extractions represent a signifi cant percent of device removal procedures. Simple traction can lead to success in some patients, but special techniques and training are needed to achieve success in an important number of cases.
Keywords: lead extraction, cardiac devices.
INTRODUCTION
In recent years, the use of implanted devices for cardiac pacing, resynchronization therapy and defibrillation has significantly expanded, increasing the number of device-related complications and, consequently, the need for removal1-4. As known, fibrotic tissue develops over time and entraps the implanted leads in the veins and in the cardiac chambers. Over the last three decades several extraction techniques have evolved including the use of a locking stylet, dilators5-11 and powered sheaths12-20 with reported success rates over 95%. However percutaneous lead removal is still associated with a small but significant procedural failure, morbidity, and mortality associated mainly with lead age, ICD leads, use of laser sheaths and operator’s lack of experience21. The current European practice is to have these procedures performed in specialized centers with high experience, multiple extraction techniques available and on site cardiac surgery22. In Romania such a center was not created. The aim of this study is to report the incidence, characteristics and success rates of device removal procedures in our center during an 8 year period.
METHODS
Population
Between January 2008 and May 2016, all consecutive patients admitted to our institution for cardiac pacing, resynchronization therapy and internal cardiac defibrillator (ICD) implant were evaluated. The clinical and implant notes of all patients were examined and the relevant data – demographics, comorbidities, implant details and complications – were entered into a structured database and then evaluated. From this large group we selected the patients with pacemaker removal procedures. These patients were accepted for transvenous lead extraction according to the currently used guidelines21. Besides the general data recorded in all patients in this group additional information was collected – device removal indication, precipitating factors for device associated infection, intraprocedural and periprocedural management, removal technique, procedural success and reimplantation data.
Device removal procedure
Preprocedural management
A careful preprocedural evaluation was performed in all patients admitted for transvenous device removal. The pacemaker and leads type and age were determined. Chest X-ray was performed in various incidences as to assert the leads intravascular route and eventual areas of damage. Pacemaker interrogation was performed with evaluation of the leads functioning and of patients underlying rhythm. Inflammation markers and haemocultures were taken in those with device related infections. Transthoracic cardiac echography (TTE) determined lead position, the presence of cardiac vegetations and the association of vegetations with the leads as well as data regarding the patients cardiac function. Transesophageal echo (TOE) was performed when it was deemed necessary A broad spectrum antibiotherapy was initiated before the procedure in all device infection patients as to minimize the extent of the infection. An informed consent was obtained in
all patients after a detailed explanation of risks and outcomes.
The first procedure
In all patients accepted for device removal in our clinic a first procedure using only the tools provided in the implant kit by the manufacturer was performed. These procedures were performed in the cardiac electrophysiology laboratory, in a fasting state, after obtaining informed consent, without cardiothoracic surgery standby. The patient was prepared with ECG monitoring,
pulsoximetry, blood pressure monitoring. Echocardiography and pericardiocentesis kit were available on site. In pacemaker-dependent patients a temporary pacing wire was placed from femoral approach. Local anesthesia was performed with Lidocain 1%. After careful dissection of all the leads up to the venous entry site a firm stylet from the normal implant kit was placed as close to the lead tip as possible. In active fixation leads the distal electrode was unscrewed. Gentle traction was performed on the leads, for a period of maximum 10 minutes or until lead removal. After complete removal, the pacemaker pocket was debrided for fibrous or infected tissue and a drainage was left in place. In case of failure a second lead extraction procedure was proposed to the patient after a detailed explanation of risks and benefits.
The second procedure
After failure of complete device removal by simple traction patients wit ICD leads were referred to the lead management center in Szeged, Hungary for extraction using a laser sheath. Patients with pacemaker leads were proposed either to go for extraction with laser sheath in Szeged or to go for an approach with mechanical dissection and femoral approach in our center. The procedures were performed in the cardiac electrophysiology laboratory, under local anesthesia, in a fasting state, after obtaining informed consent. Before the extraction procedures the patients were prepared with special draping as to provide access for pericardiocentesis, transvenous temporary pacing through the left femoral vein, ECG, arterial blood pressure and pulse oximetry monitoring. The extraction procedures were performed by three trained interventional cardiologists, with anesthesiologist on site and with cardiothoracic surgery standby available. Once the patient was prepared, draped, and sedated, the pulse generator pocket was opened and the leads freed by dissection from their adhesions down to the venous insertion site or as far as possible. The leads were cut 10-15 cm out of the venous entry site. A stiff normal stylet supplied by the lead manufacturer, of appropriate length for the lead, was introduced into the lead body with its tip as close as possible to the lead tip to stiffen it. One or two (in the presence of unipolar or bipolar leads, respectively) ties of silk suture material were secured, respectively, around the outer insulation of the lead. Once the lead was freed and secured, we used a modified percutaneous dilatation technique. Dilatation was performed using standard peel away sheaths size 7-10 Fr (StJude Medical, Medtronic) starting with a sheath with the inner diameter as close as possible to the lead body diameter. Traction was maintained on the silk ties while the sheath was advanced under fluoroscopy following the lead course and avoiding any angle. The advancement of the sheath was made by rotating it alternatively clockwise and counter-clockwise. While dilating, smooth traction was performed to keep the lead in tension, but avoiding myocardial wall invagination or lead damage. When the advancement of the sheath was difficult, it was retrieved for a few millimetres and dilatation restarted. If unsuccessful, the dilator was changed to a new one of larger diameter. Once the sheath advancement was no longer possible we switched to femoral approach. The over the lead suture and the stylet were removed. A 14 Fr 23 cm and a 7 Fr 14 cm sheaths (StJude Medical) ware pleced on the right femoral vein. A standard 4 mm tip D curve ablation catheter (Biosense Webster, St Jude Medical) was advanced on the 7 Fr sheath to the right atrium where the tip was deflected over the lead. A snare (Cook Vascular Inc.) was advanced over the 14 Fr sheath to the right atrium and the ablation catheter’s tip was caught. Traction was performed on both the ablation catheter and the snare as to pull down the lead. Once the lead’s proximal end was pulled down free in the right atrium the ablation the snare was advanced over the lead up to the lead’s tip and traction was performed from this point until the lead was freed. The lead was then removed with the snare through the 14 Fr sheath. The pacemaker pocket was closed with a single layer suture and a drainage was left in place for 48 hours. Procedural outcome was defined according to the radiological outcome: complete success (removal of the whole lead), partial (a fragment of less than 4 cm is left), and failure (a significant fragment is left, or the procedure was stopped because of a major complication).
Posprocedural management
Cardiac echography was performed in the electrophysiology lab in all patients immediately after the procedure. All patients remained in observation in the intensive care unit in the first 24 hours. Antibiotheraphy was maintained for minimum 7 days in pocket infection patients and minimum 2 weeks in systemic infections. Inflammation markers and clinical evolution were used to determine the evolution of local infection while cardiac echography (TTE or TOE) and blood cultures were used for systemic infections.
Reimplant procedure
Implant indication was reassessed in all patients. A reimplant procedure was performed if the patients maintained an indication according to the current guidelines. The reimplant procedure in pocket infection was peformed contralateral, after a couple of days in pacemaker dependent patients or after minimum two weeks in non-pacemaker dependent ones. In case of endocarditis/systemic infection normalization of cardiac echo, blood cultures, inflammation markers and a period of one month and was required for pacemaker dependent patients and 3 months for non-pacemaker dependent ones. Temporary pacing via the right internal jugular vein was left in place during this period for the pacemaker dependents. Contralateral implant was preferred in all patients
except those with a waiting period of more than 6 months.
Statistical analysis
All numerical values were expressed as means and standard deviations. Student’s T test with two tails, unpaired samples with unequal variances was used as to determine statistically significant differences between the groups regarding numerical data. Chi square test was used to determine differences between groups regarding discrete variables. A p-value of less than 0.05
was considered the cut-off for statistical significance.
RESULTS
Between January 2008 and May 2016 a total of 2028 procedures were performed in our laboratory, 1217 in male patients (60%). From this 1933 were implant procedures and the rest were complications related – lead repositioning, hematoma evacuation, suture granuloma excisions, loose screw or device removal. There were a total of 25 patients with device removal procedures, representing 1.23% of the total number of procedures. 13 patients (52%) were males. The indication was represented by device infection in all patients, with 2 endocarditis and 23 pacemaker pocket infections. Data about the populations and device types are summarized in Table 1. The device removal group were significantly younger, had more diabetes and more CRT devices than the
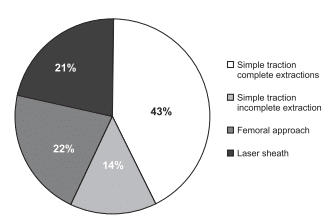
general implants group. In the device removal group there were 11 devices removed during the fist year after implant, without special tools, which were classified as explants and 14devices (56%) older than one year and/or using special extraction tools which were considered extractions. Practically there was no statistical significant difference between these groups. The characteristics of the two groups are listed in Table 2. All devices less than a year old were removed by simple traction. Devices in the extraction group required special extraction tools in 6 patients (43%) – laser sheath in 3 and femoral snare in 3 patients. In the other 8 patients simple traction led to complete device removal in 6 patients and incomplete device removal in two patients, who subsequently refused an extraction procedure using dedicated equipment (Figure 1). The mean number of leads removed was 2.2 per patient. Of the 4 ICD leads older than one year one could be removed by simple traction, one failed to be removed by simple traction but the patient refused further procedures and two were removed by laser sheath. There was one death in the explant group, in a CRT patient who died of sudden cardiac death at 72 hours after an uneventful procedure. The cause of death was probably arrhythmia related with the underlying cardiac disease and the death was not considered as procedure related. No other procedure related complications were noted. Mean lead age was 39.7(±19) months in the extraction group, with 33.5 (±16.7) in the simple traction and 48(±20) in the extraction tools patients, without reaching statistical significance – p 0.17. 7 (50%) of this group have had a box change or upgrade procedure before the infection occurred. 20 of the 25 patients were reimplanted – giving an 80% reimplant rate: 3 patients in the extraction group (21%) – one with cancer, one with patient refusal in a primary prevention ICD and one due to indication revision – and 2 patients in the explant group (19%) – one indication revision and one death.
Figure 1. Success rates of different extraction techniques.
DISCUSSIONS
Overall the number of device removal procedures is quite low but one must consider the high mortality rates of these infections, which makes them a serious clinical problem. More than half of these infections occurred in older implanted devices. Consistent with the European data22, resynchronization therapy devices had the higher risk, bringing 48% of the cases. Box changes and upgrades are also of considerable risk, being responsible for half of the cases requiring extraction. Considering the continuously rising number of device implants and the even higher increase for ICD and CRT devices in the last years in our country as well as the battery life span of about 5-7 years of such a device we can conclude that the need for lead extractions will increase significantly in the following years. Simple traction could achieve complete device removal in less than half of the devices older than one year. Femoral approach and laser extractions were effective but they bring higher risks and require specialized tools and operating teams. Femoral approach was preferred for pacing leads in our study while laser sheath was the choice for ICD leads – taking into consideration the stronger adherences in the superior vena cava in the later. Mechanical sheaths and radiofrequency sheaths could also be effective24-27, but, like the laser, they are not available in our country. Another issue to be considered is patient management – lead removal being only one part in device infection treatment. Adequate support from the cardiothoracic surgeon, appropriate antibiotic therapy, good surgical treatment of the infected pocket, careful patient monitoring and accurate reimplant timing are required to achieve clinical success.
LIMITATIONS
The data shown in this study represent the clinical practice in one center, so they cannot be generalized. However, compared with the data in the literature the implanted population is quite similar with the general data in Europe regarding the types of devices, patients age and infection rates22. Device removal data are different to the general practice22 since only infection
patients have been addressed. The lack of other indications is related with the centers lack of resources for transvenous lead extraction – as required in the current European recommandations23.
CONCLUSIONS
Complex devices, box changes and upgrades bring the highest risk for infections. Transvenous lead removal is an effective treatment for these device related infections. While simple traction is effective in recently implanted devices, in more than half of the devices older than one year special extraction tools are required. Given the complexity of the procedures, creation of
dedicated, high volume centers, fully equipped with dedicated techniques and trained operators is required.
Conflict of interest: none declared.
References
1. Furman S, Behrens M, Andrews C, Klementowicz P. Retained pacemaker leads. J Thoracic Cardiovasc Surg 1987;94:770–772.
2. Zerbe F, Ponizynski A, Dyszkiewicz W, Ziemiansk A, Dziegielewski T, Krug H. Functionless retained pacing leads in the cardiovascular system. Br Heart J 1985; 54:76–79.
3. Rettig G, Doenecke P, Sen S, Volkmer I, Bette L. Complications with retained transvenous pacemaker electrodes. Am Heart J 1979;98:587–594.
4. Parry G, Goudevenos J, Jameson S, Adams PG, Gold RG. Complications associated with retained pacemakers leads. Pacing Clin Electrophysiol 1991;14: 1251–1257.
5. Byrd CL, Schwartz SJ, Hedin NB, Goode LB, Feranot NE, Smith HJ. Intravascular lead extraction using locking stylets and sheaths. Pacing Clin Electrophysiol 1990;13: 1871–1875.
6. Byrd CL, Schwartz SJ, Hedin NB. Intravascular techniques for extraction of permanent pacemakers leads. J Thoracic Cardiovasc Surg 1991;101:989–997.
7. Bongiorni MG, Petz E, Levorato D, Soldati E, Arena G, Quirino G, Vagheggini G, Malamuth D, Biagini A, Camerini F. Removal of chronic leads for permanent pacing. Clinical experience with transvenous extractors. In: Antonioli GE, ed. Pacemaker Leads 1991. Amsterdam, Elsevier Science Publishers; 1991. p289–294.
8. Byrd CL, Schwartz SJ, Hedin NB. Lead extraction: indications and techniques. Cardiol Clin 1992;10:735–748.
9. Smith HJ, Fearnot NE, Byrd CL, Wilkoff BL, Love CJ, Sellers TD. Fiveyears experience with intravascular lead extraction. U.S. Lead Extraction Database. Pacing Clin Electrophysiol 1994;17:2016–2020.
10. Byrd CL, Wilkoff BL, Love CJ, Sellers TD, Turk KT, Reeves R, Young R, Crevey B, Kutalek SP, Freedman R, Friedman R, Trantham J, Watts M, Schutzman J, Oren J, Wilson J, Gold F, Fearnot NE, Van Zandt HJ. Intravascular extraction of problematic or infected permanent pacemaker leads: 1994–1996. U.S. Extraction Database, MED Institute. Pacing Clin Electrophysiol 1999;22:1348–1357.
11. Saad EB, Saliba WI, Schweikert RA, Al-Khadra AS, Abdul-Karim A, Niebauer MJ, Wilkoff BL. Nonthoracotomy implantable defi brillator lead extraction: results and comparison with extraction of pacemaker leads. Pacing Clin Electrophysiol 2003;26: 1944–1950.
12. Wilkoff BL, Byrd CL, Love CJ, Hayes DL, Sellers TD, Schaerf R, Parsonnet V, Epstein LM, Sorrentino RA, Reiser C. Pacemaker lead extraction with the laser sheath: results of the pacing lead extraction with the excimer sheath (PLEXES) trial. J Am Coll Cardiol 1999;33:1671–1676.
13. Epstein LM, Byrd CL, Wilkoff BL, Love CJ, Sellers TD, Hayes DL, Reiser C. Initial experience with larger laser sheaths for the removal of transvenous pacemaker and implantable defibrillator leads. Circulation 1999;100:516–525.
14. Love CJ. Lead extraction. Heart Rhythm 2007;4:1238–1243.
15. Parsonnet V, Roelke M, Trivedi A, Rizvi SA, Pervez A. Laser extraction of entrapped leads. Pacing Clin Electrophysiol 2001;24:329–332.
16. Byrd CL, Wilkoff BL, Love CJ, Sellers TD, Reiser C. Clinical study of the laser sheath for lead extraction: the total experience in the United States. Pacing Clin Electrophysiol 2002;25:804–808.
17. Moon MR, Camillo CJ, Gleva MJ. Laser-assist during extraction of chronically implanted pacemaker and defi brillator leads. Ann Thorac Surg 2002;73:1893–1896.
18. Wilkoff BL. Transvenous leads extraction with electrosurgical dissection sheaths. Initial experience. Pacing Clin Electrophysiol 2000;23: 679–684.
19. Love CJ. Current concepts in extraction of transvenous pacing and ICD leads. Cardiol Clin 2000;18:193–217.
20. Verma A, Wilkoff BL. Intravascular pacemaker and defi brillator lead extraction: a state-of-the-art review. Heart Rhythm 2004;1:739–745.
21. Wilkoff BL, Love CJ, Byrd CL, et al. Transvenous lead extraction: Heart Rhythm Society expert consensus on facilities, training, indications, and patient management. Heart Rhythm. 2009;6:1085–104
22. Bongiorni MG, Marinskis G, Lip GY,Svendsen JH, Dobreanu D, Blomström-Lundqvist M, et al. How European centres diagnose, treat, and prevent CIED infections: results of an European Heart Rhythm Association survey.Europace 2012;14:1666–
23. Wilkoff BL, Love CJ, Byrd CL, Bongiorni MG, Carrillo RG, Crossley GH III et al.Transvenous lead extraction: Heart Rhythm Society expert consensus on facilities,training, indications, and patient management: this document was endorsed by theAmerican Heart Association (AHA). Heart Rhythm 2009;6:1085–104
24. Kennergren C, Bucknall CA, Butter C, Charles R, Fuhrer J, Grosfeld M, Tavernier R, Morgado TB, Mortensen P, Paul V, Richter P, Schwartz T,Wellens F, PLESSE investigators group. Laser-assisted lead extraction: the European experience. Europace 2007;9:651–656.
25. Neuzil P, Taborsky M, Rezek K, Vopalka R, Sediva L, Niederle P, Reddy V. Pacemaker and ICD lead extraction with electrosurgical dissection sheaths and standard transvenous extraction systems: results of a randomized trial. Europace 2007;9:98–104.
26. Jarwe M, Klug D, Beregi JP, Le Franc P, Lacroix D, Kouakam C, Guedon-Moreau L, Zghal N, Kacet S. Single center experience with femoral extraction of permanent endocardial pacing. Pacing Clin Electrophysiol 1999;22:1202–1209.
27. Klug D, Jarwe M, Messaoudène SA, Kouakam C, Marquiè C, Gay A, Lacroix D, Kacet S. Pacemaker lead extraction with the needle’s eye snare for countertraction via a femoral approach. Pacing Clin Electrophysiol 2002;25:1023–1028.
 This work is licensed under a
This work is licensed under a