Minodora Teodoru1, I. Maniţiu2, A. Teodoru2, Raluca Matei3, Cristina Chircu3
Article received on the 12th December 2012. Article accepted on the 3rd February 2012.
1 “Victor Papilian” Faculty of Medicine
2 Sibiu County Emergency Clinical Hospital, “Victor Papilian” Faculty of Medicine
3 Sibiu County Emergency Clinical Hospital
Minodora Teodoru, Cardiology Clinic, Sibiu County Emergency Clinical Hospital, 2-4 Corneliu Coposu Bvd., Sibiu
E-mail: dbedreaga@yahoo.com
Abstract: Cardiorenal syndrome is often described in heart failure patients and it represents the deterioration of renal function in the context of heart failure. When it occurs, the prognosis of these patients is affected by the combination of these pathologies. The treatment for cardiorenal syndrome should be applied individually in order to achieve the improvement of the patient’s clinical status, the preservation of the heart and kidney function and a better outcome. Along with traditional therapies (diuretics, inotropes), which often develop resistance, the beneficial effect of new therapeutic options (ultrafiltration, vasopressin and adenosine antagonists) is being evaluated in different trials.
Keywords: cardiac insufficiency, renal insufficiency, cardiorenal syndrome
Rezumat: Sindromul cardiorenal se întâlneşte deseori în cursul evoluţiei insuficienţei cardiace şi reprezintă deteriorarea funcţiei renale în contextul insuficienţei cardiace. Atunci când el apare, prognosticul pacienţilor este grevat de combinaţia acestor patologii. Tratamentul sindromului cardiorenal trebuie aplicat individualizat pentru a realiza performanţele sperate, având mereu în vedere ameliorarea statusului pacientului, prezervarea funcţiei cordului şi a rinichiului şi îmbunătăţirea prognosticului. Alături de tratamente clasice (diuretice, medicaţia inotropă), care deseori se însoţesc de rezistenţă, este în curs de evaluare eficienţa unor opţiuni terapeutice noi (ultrafiltrarea, antagonşti ai vasopresinei şi ai adenozinei).
Cuvinte cheie: insuficienţă cardiacă, insuficienţă renală, sindromul cardiorenal
Definition and importance
Cardiorenal syndrome (CRS) is an individual pathology rather than a simple association between heart and renal failure. CRS develops a different, complex physiopathology, requiring a special treatment, which has not been studied sufficiently. CRS represents “the heart and kidneys’ physiopathological modifications, when the acute or chronic dysfunction of one organ determines the failure of the other” as it was defined by Ronco at the World Nephrology Congress in 20081.
CRS was classified in 5 types according to the organ which generated the lesion and to its debut (acute or chronic). This classification is presented in Table 1.
Table 1. The classification of cardiorenal syndromes
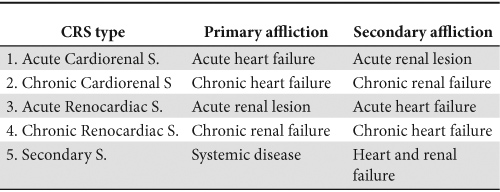
These types of CRS are in fact five different syndromes from an epidemiologic, clinical and therapeutic point of view. Often different components, from the different types of CRS, interact with one another.
In this article we will refer mostly to the first two types (cardiorenal syndromes).
Renal dysfunction associated with heart failure leads to a severe prognostic. According to some studies, this association increases the mortality rate in these patients with up to 20%2-4.
The physiopathological mechanisms which intermediate the disequilibrium present in CRS is presented in Figure 1, according to the Guyton model5.
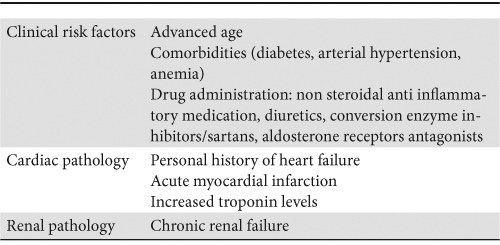
The profile of patients at risk of developing CRS is presented in Table 2.
Table 2. Risk factor for CRS
In daily practice, the diagnosis of CRS is based on the existence of an association between heart failure and the onset, or exacerbation, of a renal dysfunction. Current diagnostic elements include the increase of serum creatinine levels by more than 30% compared to initial levels, reducing the diuresis with adequate doses of diuretics, the aggravation of heart failure signs and symptoms, or the absence of regression of hemodynamic disturbances and biological constants modifications. Because a consensus regarding the definition of acute renal lesions could not be reached in practice, differing from one study to another, the Acute Dialysis Quality Initiative defined the RIFLE criteria based on the increase of serum creatinine values and decreasing urinary debit values6.
Defining chronic renal failure requires the estimation of the glomerular filtration rate (the MDRD and Cockroft – Gault formulas)7. But there are limitations in both cases when these criteria apply to patients suffering from heart failure8.
More recently, biomarkers have been promoted as diagnostic instruments for the various types of CRS, risk stratification tools, as well as “targets” for its treatment9.
Diuretics resistance
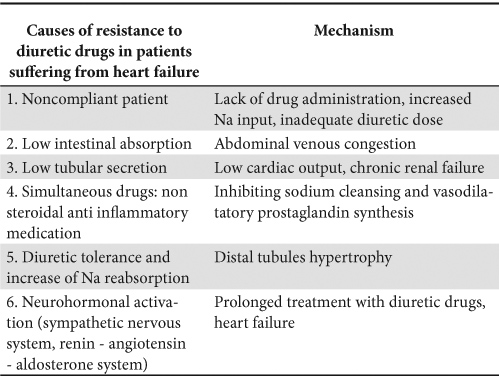
Resistance to diuretic drugs is not entirely defined and presents a great diversity of terms characterizing it. A practical definition is the persistence of pulmonary congestion despite the repeated use of 80 mg of furosemide, or of more than 240 mg of furosemide per day (including continuous perfusion), or in spite of the use of combined diuretics (loop and thiazide diuretics, or aldosterone antagonists).
The development of resistance to diuretics can be considered a bad prognostic indicator in patients suffering from chronic heart failure10.
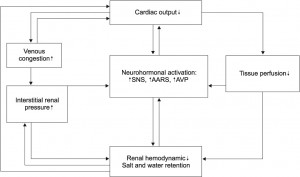
Figure 1. Mechanisms involved in CRS.
There are numerous mechanisms involved in this process. Two types of diuretics resistance were described: short and long term resistance. The first appears after the administration of a single first dose due to neurohormonal activation. Long term resistance develops after long term treatment with loop diuretics, inducing modifications in the renal structure, such as epithelial cell hypertrophy in the distal tubules, increasing Na reabsorption and decreasing diuresis11.
A summary of the mechanisms involved in the development of resistance to diuretics is illustrated in Table 3.
Table 3. Causes and mechanisms of developing resistance to diuretics
CRS management
Considering the complex and heterogeneous physiopathology of CRS, patient management is a real challenge. Up to the present time, there is no treatment ensuring guaranteed success because each patient has his/her own personal history, risk profile and a combination of comorbidities. The medication used for the treatment of CRS is illustrated in Table 4.
Table 4. The medication used for the treatment of CRS
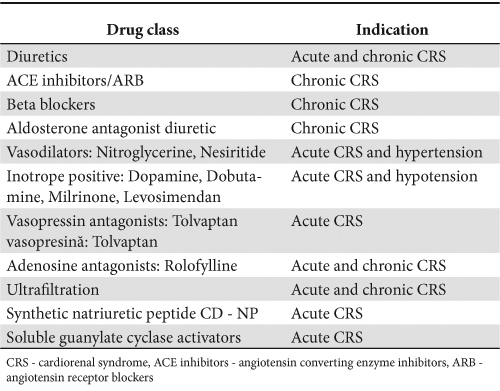
Optimizing the treatment for heart and renal failure
The best means to control CRS is to prevent it from occurring. This implies adapting heart failure therapy to hemodynamic and clinical conditions, to the presence of associated diseases and to the use of therapeutic agents capable of preserving or improving the renal function. The patient requires permanent hemodynamic monitoring, especially in case of arterial hypotension and a limitation of the sodium input to less than 2g, as well as a fluid intake limitation to less than 1000 ml/24h, in case the patient presents hyponatremia. It is necessary to monitor weight, ionogram, serum values for urea and creatinine, GFR, diuresis. A serial echocardiography monitoring is also useful. The use of bioimpedance vector analysis has significant prognostic results, positively correlating itself with the BNP values in heart failure12. This could prove useful in the management of these patients, to maintain hemodynamic stability and an optimal volemic control13.
Neurohormonal medication
There are therapies used in heart failure which have proved their importance in the increase of survival, which target mostly the neurohormonal modifications. Such therapeutic schemes include beta blockers, ACE inhibitors and aldosterone antagonists.
a) Although the use of ACE inhibitors is frequently avoided, or interrupted, in order to prevent the alteration of the renal function, the increase of creatinine levels after initiating a treatment with ACE inhibitors can actually identify a subgroup of patients which will have a maximum benefit from their use. This requires careful monitoring of the renal function and blood pressure. In addition to all of these, interrupting the use of ACE inhibitors for this group of patients leads to the increase of the mortality risk. The effects of ACE inhibitors in the treatment of heart failure with associated renal failure are difficult to evaluate, because the extended studies which assessed their effects did not include patients with altered renal function. Most of these studies had an exclusion threshold of 2 mg/dL for the serum creatinine14. Even when renal failure was present, ACE inhibitors demonstrated the reduction of proteinuria and long term benefits regarding survival15.
b) Beta blockers represent a heterogeneous class of drugs which have beneficial effects on the evolution of heart failure when renal dysfunction is associated. This is due to the higher levels of plasmatic norepinephrine, compared to individuals with a normal renal function16. This prompts the enquiry as to what beta blocker is the most appropriate to be used in CRS; metoprolol and carvedilol are eliminated through the liver, leaving open the opportunity to be administered in unmodified doses, whereas bisoprolol is eliminated through both the liver and the kidneys, necessitating an adjustment of the dose to the renal function.
Diuretic treatment
Diuretics have represented for a long time the initial and essential component in the management of patients suffering from heart failure. They also represent an important element in the elimination of volemic overload in CRS, but they must be used judiciously, under careful monitoring of the renal function and of the volemic status.
Diuretics increase neurohormonal activity, the activity of plasmatic renin and aldosterone and the plasmatic levels of norepinephrine and arginine-vasopressin. They also increment peripheral vascular resistance and indirectly deteriorate left ventricle function. By cumulating these effects, diuretics increase the mortality risk17,18.
The first therapeutic measure is to administer intravenously a loop diuretic for a better effect on a renal level. It is often necessary to double the dose when an appropriate diuretic effect is not obtained. It can be administered in a continuous venous perfusion, with doses adjusted to the severity of the renal dysfunction, for 2-4 hours19. Some authors have observed the increase of urinary output, the decrease of the frequency of ototoxicity and a shorter period of admittance20. This practice was not confirmed by the randomized prospective trial The Diuretic Optimization Strategies Evaluation (DOSE), which included 308 patients with acute decompensated heart failure. DOSE did not reveal significant differences in the evolution of patients after receiving diuretic treatment in bolus, compared to those who received the treatment through continuous endovenous perfusion. As a result of administering high doses of diuretics, as opposed to the administration of low doses, a transitory deterioration of the renal function could be observed. This had no adverse effects regarding the patient’s prognostic21. Another option taken into study when hypoalbuminemia is associated considers the combined use of furosemide with low sodium albumin. A furosemide – albumin complex is formed, increasing the disponibility of the diuretic by maintaing it in the vascular stream22.
Ultrafiltration
Ultrafiltration is a treatment option which is increasingly used in the treatment of CRS. It successfully eliminates the liquid excesses in cases with severe heart failure, associated with refractory hydrosaline retention and resistance to optimal diuretic treatment. Ultrafiltration is a mechanical process which consists of removing isotonic liquid and low molecular weight molecules from the circulatory system, based on a pressure gradient and using a semi permeable membrane. In a classic manner a central venous catheter is required, especially for patients with edema, but modern methods allow the use of the cubital vein and the use of a low flow catheter23. Its effects include the decrease of the right atrial pressure and the downgrade of the pressure blocked in the pulmonary capillaries, without a significant impact on the cardiac output and stroke volume24. Ultrafiltration eliminates a larger quantity of water and sodium compared to diuretics, without neurohormonal activation, allowing the elimination of approximately 3-4 liters of liquid per session. Because of the benefits observed in the treatment of CRS patients a large number of studies were dedicated to the evaluation of the efficiency of ultrafiltration in their treatment. RAPID CHF studied liquid elimination and the evolution of some patients admitted for CRS, finding better results in cases where ultrafiltration was used, rather than a classic treatment25.
Another study conducted on patients with acute heart failure was the UNLOAD trial. Patients who underwent ultrafiltration lost more weight than those who were given diuretics, but without any statistically significant differences concerning the reduction of dyspnea or the improvement of the renal function. However, the lot of patients treated with ultrafiltration had a lower re-admittance rate after 90 days from the initial admission26.
The indications of ultrafiltration in the treatment of heart failure are limited in day to day practice to patients with edema syndromes refractory to optimal doses of diuretics, or in cases with aggravating renal failure. Due to the high costs it implies and the need for a better patient surveillance, new studies are needed to evaluate ultrafiltration from the perspective of cost-efficiency, moment of administration (clinical context, the gravity of the organ dysfunction) and protocol (type of treatment, rhythm and period of administration). The Cardiorenal Rescue Study in Acute Decompensated Heart Failure (CARRESS) study is currently in progress and tries to answer some of these questions. The patients included in this controlled and randomized trial have been hospitalized for acute, destabilized heart failure and developed cardiorenal syndrome. Serum creatinine and weight were monitored for any modifications at 96 h after admission. Considering the already existing proof, ultrafiltration should be considered and used as first line therapy in CRS.
Vasodilatation treatment
Vasodilatation treatment determines the rapid reduction of the ventricular filling pressure, central venous pressure and myocardial oxygen consumption, improving cardiac function.
a) Nitroglycerin is frequently used in heart failure to reduce pulmonary congestion. The reduction of venous pressure could be benefic in CRS by reducing the renal venous pressure. However, the benefits to overall survival and renal function improvement deriving from the use of nitroglycerin in CRS are not yet known.
b) Nesiritide is a recombined BNP (brain natriuretic peptide) which induces vasodilatation with the reduction of cardiac afterload and preload, increasing the cardiac output27. Several clinical studies have confirmed anterior favorable results on hemodynamic parameters and have also shown symptomatic improvement. When nesiritide was compared to an inotrope intravenous medication or to vasodilatation therapy, a symptom improvement and increase of diuresis were observed.
Wang’s study was the first to explore the effects of nesiritide on the renal function, in patients with proven cardio-renal dysfunction. The expectations were not matched by the results. This study concluded that nesiritide had no effect on the glomerular filtration rate, renal plasma flow and urine volume or sodium excretion29. Although the initial hypothesis was not confirmed, the study does not exclude all possibilities for this agent to play a role in the treatment of heart failure symptoms.
Recently, the Acute Study of Clinical Effectiveness of Nesiritide in Decompensated Heart Failure Trial (ASCEND-HF) trial included over 7000 patients with acute, decompensated heart failure. The patients included in this study received standard heart failure therapy associated with nesiritide in a continuous perfusion, or placebo. It concluded that nesiritide can be safely administered without adverse effects regarding the renal function or mortality and that it lead to a minor improvement concerning the dyspnea30. There are other studies which have tested the effects of nesiritide administered in a single dose per week, evaluating this type of treatment for high risk patients, Fusion II. However, these studies did not prove any significant improvements in the quality of life, or survival31. The Renal Optimization Strategies Evaluation in Acute Heart Failure (ROSE) trial is currently in progress. It includes patients with acute heart failure, treated with 3 different types of drugs: diuretic treatment, optimal when associated with nesiritide, low doses of dopamine and placebo. At the same time, the efficiency and the study profile of this therapy was studied on patients with acute heart failure.
Positive inotropic agents
Such agents like dopamine, dobutamine, phosphodiesterase inhibitors and levosimendan are used in the treatment of CRS in order to facilitate diuresis, preserving or improving the renal function due to their capacity to increase the cardiac index and renal perfusion32,33. Low doses of dopamine (lower than 5 µg/kg/min) were often used in order to improve renal function, but it appears that this effect is owed mostly to the increase of the cardiac output, rather than a local effect34. Clinical studies have been conducted on the effects of dopamine and they have not revealed any clinical benefit35. Also, in a study performed on patients with acute renal failure it was noticed that dopamine can actually worsen the renal perfusion36.
The OPTIME study investigated the use of milrinone for patients suffering from chronic heart failure. It showed that patients in advanced stages of the disease do not have an improved prognostic if they follow this treatment37.
Inotrope medication can be introduced in the treatment of patients suffering from CRS only in cases in which renal failure develops secondary to low cardiac output. The medication will be administered on short term and under careful monitoring due to the risk of developing arrhythmia. It does not represent a routine treatment for patients suffering from chronic or acute heart failure because it increases the mortality risk and has multiple adverse effects on the heart.
The ROSE study, previously mentioned, proposes the optimization of heart failure therapy, wishing to administer low doses of dopamine in acute heart failure.
The treatment of anemia
The cardiorenal-anemia syndrome was extensively described in the literature. It develops as a result of the combination between heart failure and renal failure, through plurifactorial mechanisms38. In its turn, anemia can exacerbate heart failure and CRS. Thus, a new concept appeared considering a new syndrome, with a different pathology than the one associated with classic CRS and with a diverse impact on patients’ morbidity and mortality.
The treatment of anemia with erythropoietin demonstrated a favorable impact on the cardiac function, increasing left ventricular ejection fraction and physical performance39. However, the correction of the anemia must be only partial, because an aggressive correction is accompanied by a high rate of adverse effects.
Alpha darbepoetin also demonstrated a favorable effect in the treatment of patients suffering from heart failure41. The RED-HF trial studies the effects of long term administration of alpha darbepoetin to patients with left ventricular dysfunction42.
Future directions in the study and treatment of cardiorenal dysfunction
In order to develop new approaches to this syndrome there are several ongoing research projects, testing new therapies. Other options include the early introduction of dialysis and ultrafiltration; in severe cases, left ventricular devices and intra aortic counter pulsation balloons can be used for short term management of these patients.
a) Vasopressin antagonists: anti diuretic hormone, also known as arginin vasopressin (AVP), is secreted when the circulatory volume decreases, or when hyperosmolarity occurs. It exerts its effect through three kinds of receptors, of which the V2 receptors are located in the kidney, more precisely in the distal and collector tubes. AVP acts at this level causing vasoconstriction and water reabsorption. AVP serum levels can be low in heart failure with hypotension and low sanguine circulatory volume. A class of drugs called vaptans was developed in order to antagonize the effects of AVP, increasing water excretion by enhancing the clearance of water and increasing serum sodium levels, useful especially in hyponatremic states43.
The ACTIV study focused on the effects of administering tolvaptan in acute heart failure. It was observed that patients treated with tolvaptan presented a more rapid weight loss and an increased urinary debit compared to those who have received the standard treatment, without modifications to serum creatinine values at discharge44.
A more ample study, EVEREST, confirmed the data already known from ACTIV, but did not demonstrate any prognostic benefits from long term administration of tolvaptan in acute heart failure45.
b) Adenosine receptors antagonists: the energy consumed during sodium excretion in the kidney is generated by adenosine, resulted from the transformation of ATP in ADP in the renal tubules, determining the constriction of the afferent artery through the binding of the A1 receptor, reducing the renal flux and determining sodium reabsorption. Adenosine receptors antagonists represent a class of drugs that determine the increase of renal perfusion, diuresis and sodium excretion. These drugs are prescribed in heart failure with hypervolemia and hyponatremia46. Previous studies indicated a possible role played by these agents in avoiding the decline of the renal function due to the use of loop diuretics47.
Rolofylline stands out from this class of drugs as the one able to increase diuresis and natriuresis in association with lower doses of diuretics48. Rolofylline was studied in the PROTECT multicenter trial, including 300 patients with acute heart failure and altered renal function at the time of admission. It was administered in different doses in continuous perfusion, together with the standard therapy. The final data did not show any benefits regarding the renal function or the prognostic. New studies are necessary in order to test these hypotheses49. The REACH-UP study did not demonstrate a clear benefit of rolofylline in the treatment of patients with acute heart failure and renal dysfunction50.
The potential role can exist, in particular cases, such as the prevention of contrast nephropathy or in case of hypotension and in patients at risk of developing resistance to diuretics51.
c) The synthetic natriuretic peptide
Synthetic natriuretic peptides are molecules synthesized from natural natriuretic peptides, created with the purpose of optimizing the pharmacological actions and minimizing the side effects52. Of these, the synthetic peptide CD-NP was created starting from the type C and type D natriuretic peptide and was used in the treatment of acute heart failure, without inducing arterial hypotension53.
In the first clinical trial favorable effects were proven regarding natriuresis and the preservation of the renal function. Unlike native natriuretic peptides, CD-NP suppresses aldosterone synthesis54. The success of synthetic peptides paves the way to their study and use in the treatment of heart failure and CRS.
d) Soluble guanylate cyclase activators (cinaciguat, atisciguat) can be used in the treatment of heart failure to reduce systemic vasoconstriction by increasing the vasodilatation effect of nitric oxide, thus preventing the development of resistance to nitrates, which usually appears in heart failure55. An experimental study showed the reduction of the preload and afterload, with the increase of the cardiac output in a heart failure model56. The observed renal effect was the preservation of glomerular filtration, possible because of the effects on renal resistance.
e) The intrarenal administration of medication can be an alternative to the classic administration of drugs in CRS, in order to increase the local concentration and inducing local renal effects, with less systemic exposure and side effects. By administering fenoldopam (a dopaminergic agonist) or nesiritide locally, in the renal arteries the renal metabolization occurring when the drug is administered intravenously is by-passed, inducing less systemic side-effects48,57 refI. Phosphodiesteraze V inhibitors are also in study as a possible therapy, administered in association with BNP in order to offer a new vision of CRS physiopathology and to establish a new therapeutic combination, with influence in the treatment and prevention of heart failure58.
Sympathetic renal denervation can have a potential role in the prevention and management of cardiorenal syndrome, without having any clinical or experimental trials to confirm the therapeutic effects of this procedure.
Conclusions
According to the presented facts we can safely affirm that the onset of cardiorenal syndrome is a bad, but frequent development in the evolution of chronic heart failure. Understanding the mechanisms involved in the development of CRS is rudimentary at best, lacking efficient therapies. The treatment of heart failure is nowadays mostly the same as it was a few decades ago, especially from the point of view of cardiorenal interactions. Our hope is that new and efficient therapies will be developed in order to prevent this challenging syndrome.
Funding: Research conducted through the POSDRU/ 88/1.5/S/60370 – The integration of Romanian research in the context of European research projects – doctoral fellowships, co-financed from the European Social Fund, via the Sectoral Operational Programme Human Resources Development 2007-2013.
Bibliography
1. Ronco C, Haapio M, House AA, Anavekar N, Bellomo R., Cardiorenal syndrome, J Am Coll Cardiol. 2008;52(19):1527-39.
2. Giamouzis G, Kalogeropoulos A, Georgiopoulou V, et al. Hospitalization epidemic in patients with heart failure: risk factors, risk prediction, knowledge gaps, and future directions. J Card Fail. 2011;17(1):54–75
3. Bhatia RS, Tu JV, Lee DS, et al. Outcome of heart failure with preserved ejection fraction in a population-based study. N Engl J Med 2006; 355:260-9.
4. Owan TE, Hodge DO, Herges RM, et al. Trends in prevalence and outcome of heart failure with preserved ejection fraction. N Engl J Med 2006; 355:251-9.
5. Bongartz LG, Cramer MJ, Doevendans PA, Joles JA, Braam B. The severe cardiorenal syndrome: ‘Guyton revisited’. Eur Heart J. 2005; 26:11-7.
6. Ronco C, Kellum JA, Mehta R. Acute dialysis quality initiative (ADQI). Nephrol Dial Transplant 2001; 16:1555.
7. Winearls CG, Glassock RJ (2009) Dissecting and refining the staging of chronic kidney disease. Kidney Int 75:1009–1014
8. Bellomo R, Ronco C, Kellum JA, Mehta RL, Palevsky P ,Acute renal failure—definition, outcome measures, animal models, fluid therapy and information technology needs: the second international consensus conference of the acute dialysis quality initiative (adqi) group. Crit Care 2004;8:R204–R212
9. Francis G. Acute decompensated heart failure: The cardiorenal syndrome. Clev Clin J Med .2006; 73(Suppl 2):S8–13
10. Schrier RW, Abraham WT. Hormones and hemodynamics in heart failure. N Engl J Med 1999; 341:577-85.
11. Chiong JR, Cheung RJ. Loop diuretic therapy in heart failure: the need for solid evidence on a fluid issue. Clin Cardiol. 2010;33(6):345– 352.
12. Di Somma S, De Berardinis B, Bongiovani C, Marino R. Use BNP and Bioimpedance to drive therapy in heart failure patients. Congest Heart Fail 2010; 16(Suppl 1):S56-S61.
13. Montejo JD, Bajo MA, Del Peso G, Selgas R. Papel de la diálisis peritoneal en el tratamiento de la insuficiencia cardíaca refractaria. Nefrologia 2010; 30:21-7.
14. Shlipak MG. Pharmacotherapy for heart failure in patients with renal insufficiency. Ann Intern Med. 2003;138:917-24.
15. Hou FF, Zhang X, Zhang GH, et al. Efficacy and safety of benazepril for advanced chronic renal insufficiency. N Engl J Med. 2006;354:131-40.
16. Ghali JK, Wikstrand J, Van Veldhuisen DJ,et al. The influence of renal function on clinical outcome and response to beta-blockade in systolic heart failure: insights from Metoprolol CR ⁄ XL Randomized Intervention Trial in Chronic HF (MERIT-HF). J Card Fail. 2009; 15(4):310–318.).
17. M. R. Costanzo, J. T. Heywood, T. DeMarco, et al., “Impact of renal insufficiency and chronic diuretic therapy on outcome and resource utilization in patients with acute decompensated heart failure,” Journal of the American College of Cardiology, vol. 43, supplement 1, p. A180, 2004.
18. J. Bayliss, M. Norell, R. Canepa-Anson, G. Sutton, and P. Pole-Wilson, “Untreated heart failure: clinical and neuroendocrine effects of introducing diuretics,” British Heart Journal, vol. 57, no. 1, pp. 17–22, 1987.
19. D. C. Brater, “Diuretic therapy,” The New England Journal of Medicine, 1998, vol. 339, no. 6, pp. 387–395.
20. D. R. Salvador, N. R. Rey, G. C. Ramos, and F. E. Punzalan, “Continuous infusion versus bolus injection of loop diuretics in congestive heart failure,” Cochrane Database of Systematic Reviews, 2004;(1): CD003178.
21. Felker GM, Lee KL, Bull DA, et al. Diuretic strategies in patients with acute decompensated heart failure. N Engl J Med 2011; 364:797–805
22. D. Fliser, I. Zurbrüggen, E. Mutschler et al., “Coadministration of albumin and furosemide in patients with the nephrotic syndrome,” Kidney International, 1999, vol. 55, no. 2, pp. 629–634.
23. B. E. Jaski and D. Miller, “Ultrafiltration in decompensated heart failure,” Current Heart Failure Reports, 2005, vol. 2, no. 3, pp. 148–154.
24. G. Marenzi, G. Lauri, M. Grazi, et al, “Circulatory response to fluid overload removal by extracorporeal ultrafiltration in refractory congestive heart failure,” Journal of the American College of Cardiology, 2001,vol. 38, no. 4, pp. 963–968.
25. M. R. Costanzo, M. E. Guglin, M. T. Saltzberg et al., “Ultrafiltration versus intravenous diuretics for patients hospitalized for acute decompensated heart failure,” Journal of the American College of Cardiology, 2007, vol. 49, no. 6, pp. 675–683.
26. Costanzo MR, Guglin ME, Saltzberg MT, et al. Ultrafiltration versus intravenous diuretics for patients hospitalized for acute decompensated heart failure. J Am Coll Cardiol. 2007;49(6):675–683.
27. Abraham WT, Lowes BD, Ferguson DA, et al. Systemic hemodynamic, neurohormonal, and renal effects of a steady-state infusion of human brain natriuretic peptide in patients with hemodynamically decompensate heart failure. J Card Fail. 1998; no.4, page: 37
28. Colucci WS, Elkayam U, Horton DP, et al. Intravenous nesiritide, a natriuretic peptide, in the treatment of decompensated congestive heart failure. Nesiritide Study Group. N Engl J Med. 2000; no.343, page: 246
29. Wang DJ, Dowling TC, Meadows D, et al. Nesiritide does not improve renal function in patients with chronic heart failure and worsening serum creatinine. Circulation. 2004;no. 110, page: 1620
30. Hernandez AF et al. Rationale and design of the Acute Study of Clinical Effectiveness of Nesiritide in Decompensated Heart Failure Trial (ASCEND-HF). Am Heart J. 2009;157:271–7.
31. Yancy CW, Krum H, Massie BM, et al. Safety and efficacy of out patient nesiritide in patients with advanced heart failure. Results of the serial infusion of nesiritide (FUSIONII) trial. Circ Heart Fail. 2008; 1:9-16.
32. Drexler H, Höing S, Faude F, Wollschläger H, Just H. Central and regional vascular hemodynamics following intravenous milrinone in the conscious rat: comparison with dobutamine. J Cardiovasc Pharmacol. 1987; 9: 563–569.
33. Sato Y, Matsuzawa H, Eguchi S. Comparative study of effects of adrenaline, dobutamine and dopamine on systemic hemodynamics and renal blood flow in patients following open heart surgery. Jpn Circ J. 1982; 46: 1059–1072.
34. Ungar A, Fumagalli S, Marini M, et al. Renal, but not systemic, hemodynamic effects of dopamine are influenced by the severity of congestive heart failure. Crit Care Med. 2004; 32: 1125–1129)
35. Kellum JA, Decker J. Use of dopamine in acute renal failure: a meta-analysis. Crit Care Med. 2001; 29: 1526–1531.
36. A. Lauschke, U. Teichgräber, U. Frei, and K.-U. Eckardt, “‘ Low-dose’ dopamine worsens renal perfusion in patients with acute renal failure,” Kidney International, 2006, vol. 69, no. 9, pp. 1669–1674.
37. Klein L, Massie BM, Leimberger JD, et al. Admission or changes in renal function during hospitalization for worsening heart failure predict postdischarge survival: results from the Outcomes of a Prospective Trial of Intravenous Milrinone for Exacerbations of Chronic Heart Failure (OPTIME-CHF). Circ Heart Fail. 2008; 1: 25–33.
38. A. Kazory, E. A. Ross, “Anemia: the point of convergence or divergence for kidney disease and heart failure?” Journal of the American College of Cardiology, 2009, vol. 53, no. 8, pp. 639–647.
39. D. Silverberg, D. Wexler, M. Blum, Y. Wollman, and A. Iaina, “The cardio-renal anaemia syndrome: does it exist?” Nephrology Dialysis Transplantation, 2003, vol. 18, no. 8, supplement, pp. viii7–viii12.
40. Singh AK, Szczech L, Tang KL, Barnhart H, Sapp S, Wolfson M, Reddan D, for the CHOIR Investigators. Correction of anemia with epoetin alfa in chronic kidney disease. N Engl J Med. 2006; 355: 2085–2098.
41. J. T. Parissis, K. Kourea, F. Panou et al., “Effects of darbepoetin α on right and left ventricular systolic and diastolic function in anemic patients with chronic heart failure secondary to ischemic or idiopathic dilated cardiomyopathy,” American Heart Journal, 2008, vol. 155, no. 4, pp. 751.e1–751.e7.
42. J. J. McMurray, I. S. Anand, R. Diaz, et al., “RED-HF Committees and Investigators. Design of the Reduction of Events with Darbepoetin alpha in Heart Failure (RED-HF): a phase III, anaemia correction, morbidity-mortality trial,” European Journal of Heart Failure, 2009, vol. 11, no. 8, pp. 795–801.
43. S. Nielsen, C.-L. Chou, D. Marples, et al. “Vasopressin increases water permeability of kidney collecting duct by inducing translocation of aquaporin-CD water channels to plasma membrane,” Proceedings of the National Academy of Sciences of the United States of America, 1995, vol. 92, no. 4, pp. 1013–1017.
44. M. Gheorghiade, W. A. Gattis, C. M. O’Connor et al., “Effects of tolvaptan, a vasopressin antagonist, in patients hospitalized with worsening heart failure: a randomized controlled trial,” Journal of the American Medical Association, 2004, vol. 291, no. 16, pp. 1963–1971.
45. M. Gheorghiade, M. A. Konstam, J. C. Burnett Jr. et al., “Short-term clinical effects of tolvaptan, an oral vasopressin antagonist, in patients hospitalized for heart failure: the EVEREST clinical status trials,” Journal of the American Medical Association, 2007, vol. 297, no. 12, pp. 1332–1343.
46. Goldsmith SR, Brandimarte F, Gheorghiade M. Congestion as a therapeutic target in acute heart failure syndromes. Prog Cardiovasc Dis. 2010;52(5):383–392.
47. Dittrich HC, Gupta DK, Hack TC, et al. : The effect of KW-3902, an adenosine A1 receptor antagonist, on renal function and renal plasma fl ow in ambulatory patients with heart failure and renal impairment. J Card Fail 2007, 13: 609– 617.
48. S. S. Gottlieb, D. C. Brater, I. Thomas et al., “BG9719 (CVT-124), an A1 adenosine receptor antagonist, protects against the decline in renal function observed with diuretic therapy,” Circulation, 2002, vol. 105, no. 11, pp. 1348–1353.
49. B. D. Weatherley, G. Cotter, H. C. Dittrich et al., “Design and rationale of the PROTECT study: a placebo-controlled randomized study of the selective A1 adenosine receptor antagonist rolofylline for patients hospitalized with acute decompensated heart failure and volume overload to assess treatment effect on congestion and renal function,” Journal of Cardiac Failure, 2010, vol. 16, no. 1, pp. 25–35.
50. S. S. Gottlieb, M. M. Givertz, M. Metra et al., “The effects of adenosine A1 receptor antagonism in patients with acute decompensated heart failure and worsening renal function: the REACH UP study,” Journal of Cardiac Failure, 2010, vol. 16, no. 9, pp. 714–719.
51. Massie BM, et al. Rolofylline, an adenosine A1-receptor antagonist, in acute heart failure. N Engl J Med. 2010;363:1419–28.
52. Lee CY, Lieu H, Burnett Jr JC. Designer natriuretic peptides. J Investig Med. 2009;57:18–21.
53. Lisy O et al. Design, synthesis, and actions of a novel chimeric natriuretic peptide:CD-NP. JAmCollCardiol. 2008;52:60–8.
54. Lee CY, et al. Pharmacodynamics of a novel designer natriuretic peptide, CD-NP, in a first-in-human clinical trial in healthy subjects. J Clin Pharmacol. 2009;49:668–73.
55. Schmidt HH, Schmidt PM, Stasch JP: NO- and haem-independent soluble guanylate cyclase activators. Handb Exp Pharmacol 2009, 191:309–339.
56. Boerrigter G, Costello-Boerrigter LC, Cataliotti A, et al.: Targeting heme-oxidized soluble guanylate cyclase in experimental heart failure. Hypertension 2007, 49:1128–1133.
58. Chen HH et al. Maximizing the renal cyclic 3′-5′- guanosine monophosphate system with type V phosphodiesterase inhibition and exoge-
nous natriuretic peptide: a novel strategy to improve renal function in experimental overt heart failure. J Am Soc Nephrol. 2006;17:2742–7.
 This work is licensed under a
This work is licensed under a