Carlo Di Mario1,2, Carlotta Sorini Dini1*, William Wijns3
1 Structural Interventional Cardiology Division, Careggi University Hospital, Largo Brambilla 3, 50134 Florence, Italy
2 National Heart and Lung Institute, Imperial College, Sydney Street, Chelsea, London SW3 6NP, London
3 Lambe Institute for Translational Medicine and Curam, National University of Ireland, Galway and Saolta University Healthcare Group, University Road, Galway, Ireland
PREAMBLE
2016 can be hardly described as a year of revolutions in interventional cardiology. Still multiple randomized studies supported the 3 to 5 year efficacy of metallic drug eluting stents in left main disease, cast doubts on long-term outcome after fully biodegradable stents, discouraged routine thrombectomy during primary PCI, gave a mixed message on the importance of physiological guidance of coronary revascularization, helpful for non-culprit lesions in STEMI, questionable for multivessel disease in allcomers, and defeated the paradigm that a fully arterial surgical revascularization delivers better clinical outcome.
MYOCARDIAL REVASCULARIZATION
Revascularization vs. medical therapy
We start this review of transcatheter interventions with a trial where bypass surgery rather than stent assisted angioplasty was used and compared with medical therapy. STICH1 (Surgical Treatment for Ischemic Heart Failure) compared optimal medical therapy (OMT) and surgical revascularization with coronary artery by-pass grafting (CABG) in 1215 patients with left ventricular ejection fraction (LVEF) ≤35%. The negative results at 4.8 years of the original presentation in 2010 (death from any cause in 41% of patients in OMT and 36% in the CABG group, P = 0.12) justified the conservative attitude of our heart failure colleagues towards myocardial revascularization. In 2010–2013, in USA only 17.5% of 67 161 patients hospitalized for new onset heart failure underwent testing for ischemic CAD during the index hospitalization.2 The highlight of the European Society of Cardiology Congress 2016 in Rome was the presentation of the results at 9.8 years of STICHES3 (Surgical Treatment for Ischemic Heart Failure Extension Study), showing progressive divergence of the curves for mortality (66.0% in OMT group vs. 58.9% in the CABG group, P = 0.02) and cardiovascular mortality (49.3% vs. 40.5%, respectively, P = 0.006), with a significant difference in favour of the surgical arm. In the CABG group, the NNT to prevent one death was 14, though mortality was high in both groups. This trial has implications for transcatheter myocardial revascularization as well because surgery often has a prohibitive risk in patients with very low LVEF and angioplasty with drug eluting stents (DES) may represent a valid alternative in selected patients.
CABG technique
There is clear evidence of excellent long-term patency of the left internal mammary artery on the left anterior descending artery (LAD) with greater late mortality in patients only receiving vein grafts. Fully arterial revascularization is expected to improve the longterm results of CABG and this may have implications for trials comparing CABG and PCI with DES which typically have a rate of utilization of bilateral mammary of <25%. In the recent ART trial4 (Arterial Revascularization Trial), 3102 patients treated with CABG were randomized to single internal-thoracic-artery grafting or bilateral-internal thoracic-artery grafting. After 5 years of follow-up, no differences in mortality rate (8.5% in bilateral arterial graft group vs. 8.4% in single graft group, P = 0.77) were observed. In addition, death from any cause, myocardial infarction (MI) or stroke were similar in the two groups (12.2% vs. 12.7%, respectively). As already shown in the 1 year follow-up report, wound infections and need for sternal reintervention were higher in the bilateral mammary group (P = 0.005 and P = 0.002).
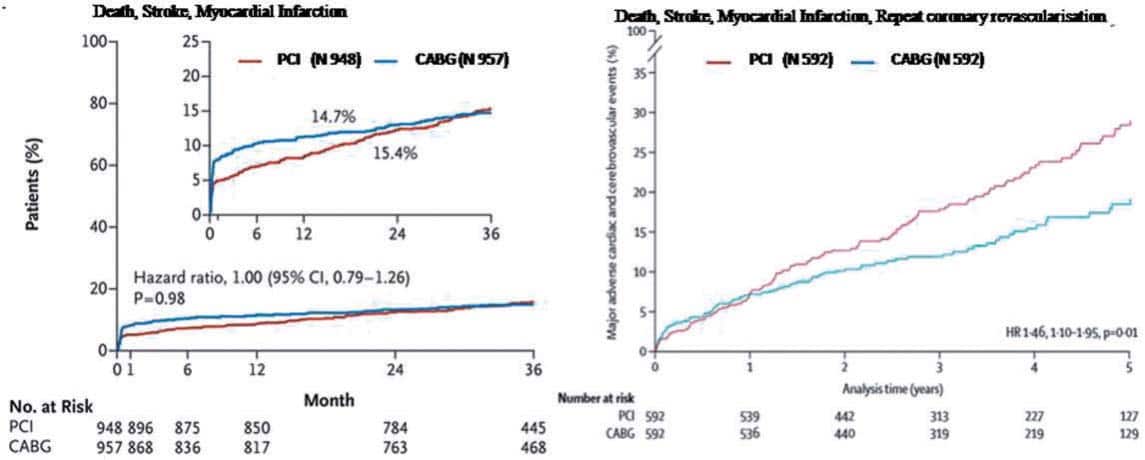
Figure 1. EXCEL trial: primary composite end-point of death, stroke, or myocardial infarction at 3 years (on the left). From NEJM, 2016. NOBLE trial: primary composite end-point of death, stroke, myocardial infarction and repeat coronary revascularization at 5 years (on the right). Reproduced with permission from Mäkikallio et al.7
PCI vs. CABG in diabetes
In the choice between PCI and CABG appropriate patient selection appears the key factor. In 451 patients with diabetes mellitus and renal failure (GFR <60 ml/min) presented in a subanalysis of the FREEDOM trial5 (Future Revascularization Evaluation in Patients with Diabetes Mellitus: Optimal Management of Multivessel Disease), CABG was shown to be superior to percutaneous
coronary intervention (PCI) in major adverse cardiovascular and cerebral events (MACCE), particularly in terms of rates of spontaneous MI (HR 0.27, CI 95%: 0.11–0.65) and repeat revascularization (HR 0.30, IC 95%: 0.18–0.50).
PCI vs. CABG for left main disease
Two trials, EXCEL6 (evaluation of XIENCE vs. Coronary Artery Bypass Surgery for Effectiveness of Left Main Revascularization) and NOBLE7 (NOrdic-Baltic-British LEft main revascularization), randomly compared PCI and CABG in 1905 and 1201 patients, respectively. The different results of the two trials are likely explained by differences in the stents used (the first generation Cypher and Biomatrix in NOBLE and the second generation everolimus eluting XIENCE stent in EXCEL) and in the prespecifi ed components of the primary end-point (new revascularization in NOBLE, periprocedural large MI in EXCEL). At 3 years median follow-up EXCEL confi rmed and improved the results of PCI in the left main (LM) subset of the SYNTAX trial, showing a clear non-inferiority of PCI vs. surgery in patients with low to intermediate disease burden (SYNTAX score ≤33) and critical LM disease (incidence of the combined end-point of death from any cause, MI or stroke was 15.4% in the PCI group vs. 14.7% in the CABG group; P= 0.02 for non-inferiority). PCI enthusiasts will claim that lower initial mortality, stroke and large MI and the absence of periprocedural surgical complications (bleeding, infections, major arrhytmias, present in 3.7%, 2.5% and 2.1% of the CABG patients, respectively) are sufficient reasons to prefer PCI over surgery. A more balanced view will stress the equalization of late results in the Kaplan–Meier curves for the MACCEs included in the primary end-point. The initial advantage of PCI is lost at 3 years in EXCEL, when the two curves cross each other, and there is more frequent revascularization in the PCI group (12.6% vs. 7.5% in the CABG group, P < 0.01). Still revascularization and stent thrombosis (0.7%) were much lower than in SYNTAX, while the incidence of symptomatic graft occlusion was similar in EXCEL and SYNTAX (5.4% and 4.2%, respectively). NOBLE showed an unexpectedly low incidence of stroke in the surgical arm, particularly in the first weeks and an inferiority of PCI vs. CABG when revascularization is included in the primary end-point (29% for PCI vs. 19% for CABG, P =0.006) (Figure 1), with a statistically non-signifi cant mortality difference in favour of surgery at 5 years (12% in PCI group vs. 9% in CABG group, P = 0.77).
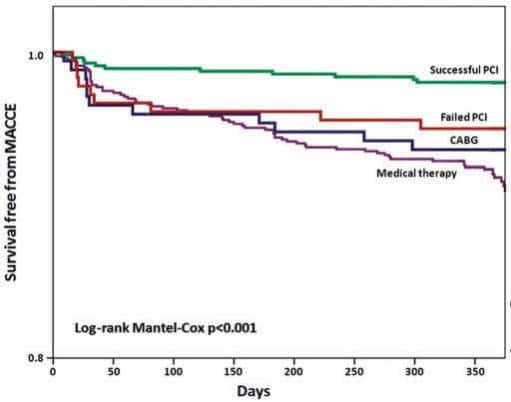
Figure 2. Kaplan–Meier analysis of freedom from major adverse cardiac and cerebrovascular events in patients with successful PCI, failed PCI, patients managed with OMT or CABG, before propensity score adjustment. Reproduced with permission from Tomasello et al.12
CTO treatment
van der Schaaf et al.8 were the first to demonstrate that patients with ST-elevation myocardial infarction (STEMI) and a second occluded artery had a much worse prognosis, results later confirmed by many other registries and subanalysis of randomized trials (HORIZONS).9 The EXPLORE trial10 (Evaluating Xience and Left Ventricular Function in Percutaneous Coronary Intervention on Occlusions After ST-Segment Elevation Myocardial Infarction), enrolled 304 STEMI patients with coronary total occlusion (CTO) in a non-culprit vessel and randomized them to receive recanalization within 7 days from primary PCI or to culprit lesion treatment alone. The primary end-point of LVEF and end-diastolic volume (EDV) at 4 months post-MI was negative (LVEF: 44.1 ± 12.2% in the CTO PCI arm vs. 44.8 ± 11.9% in the conservative arm, P = 0.60) with a favourable difference in favour of PCI only in patients receiving treatment of a CTO of the LAD. This result, the first reported of three randomized trials addressing the prognostic value of CTO treatment (the EuroCTO trial and a similar size Korean study have both completed enrolment last year), contradicts the outcome of many large registries showing a favorable outcome of successful CTO recanalization. This was confi rmed in the 14 441 patients included in the SCAAR registry11 (Swedish Coronary Angiography and Angioplasty Registry) in the period 2005–2012. The presence of a CTO was an independent predictor of long term mortality (HR 1.29, 95% CI: 1.22–1.37, P < 0.01), with an average mortality increase of 6.6% each year. Successful CTO recanalization (54% of 6442 patients underwent PCI for CTO) was associated with a reduced mortality risk (HR 0.85, 95% CI: 0.73–0.98, P < 0.034). In an Italian multicenter registry12 (IRCTO), 776 patients (43.7%) were treated with PCI, showing lower mortality rate at 1 year than the patients on OMT or undergoing surgical revascularization (1.4% PCI vs. 4.7% and 6.3% surgery and medical therapy, respectively; both P < 0.001) and lower MACCE (2.6% vs. 8.2% and 6.9%, P < 0.001) (Figure 2). Registry data have obvious drawbacks and the most important is that they do not consider the entire population of the patients treated but only the 60–70% that typically achieve successful procedures. While waiting for the results of true randomized trials including in the CTO group all the patients with attempted recanalization (intention to treat), it is encouraging to see a progressive improvement of success rate (80–90%) in large consecutive registries. The RECHARGE13 registry (REgistry of Crossboss and Hybrid procedures in FrAnce, NetheRlands, BelGium and UnitEd Kingdom) analysed 1177 patients with CTO (59% lesion length >20 mm, 58% calcific lesions, J-CTO score 2.2 ± 1.3) and showed a high overall procedure success (86%) following a hybrid algorithm (anterograde wire escalation 77%, retrograde dissection re-entry 17% and anterograde dissection re-entry 7%). Major in-hospital complications were low (2.6%) with very low periprocedural mortality (0.2).
Bifurcations
The guidelines14 recommending provisional stenting as default strategy in bifurcational lesions were supported by results of 5 year survival of a patient level pooled analysis of two randomized trials.15 In both studies, 500 patients in the BBC-ONE trial (British Bifurcation Coronary study One) and 413 patients in the NORDIC trial (Nordic Bifurcation study) were randomly assigned to provisional stent or a two-stent approach with the first generation drug eluting stents (DES) Cypher and Taxus. Five year mortality was lower among patients who underwent a provisional strategy, 17 patients (3.8%) vs. 31 patients (7.0%) in the two-stent strategy (P < 0.04). When a second stent is required, the Bifurcation Bad Krozingen16 (BBK) randomized trial showed that the two most widely used techniques applicable in a provisional fashion (T-stenting and culotte) deliver different results, with a significantly lower maximal in-stent % diameter stenosis at angiographic followup (P< 0.038) and lower binary restenosis at 1 year follow-up in the 150 patients treated with culotte than in the equal number of patients receiving T-stenting (6.5% vs. 17.0%, P = 0.006). The clinical relevance of these findings is challenged by the absence of significant differences in TLR between the two groups (6% in culotte group vs. 12% in TAP group, P = 0.069). This trial that utilized modern second generation DES had excellent overall results, and only one stent thrombosis at 1 year.
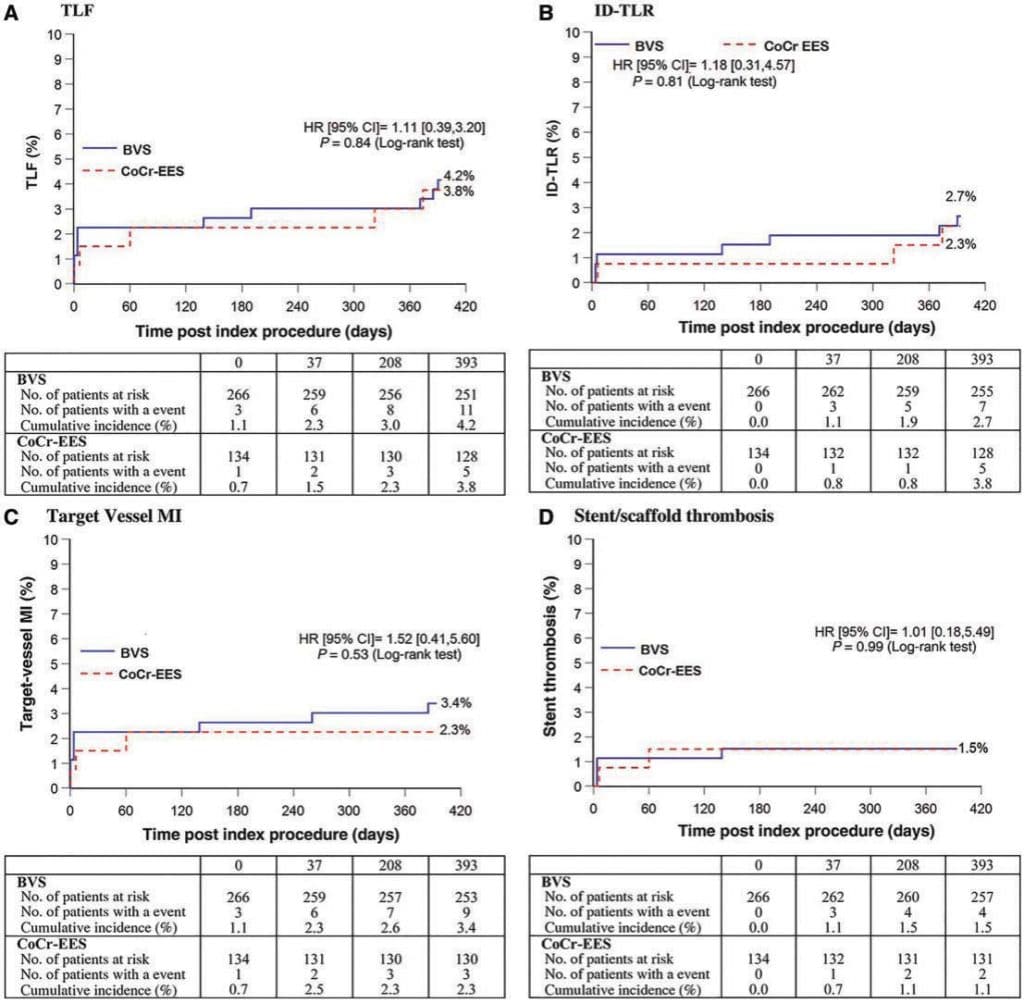
Figure 3. Primary and secondary end-points: (A) target lesion failure, (B) ischemia driven TLR, (C) target vesselMI, (D) stent or scaffold thrombosis in Absorb Japan trial. Reproduced with permission from Kimura et al.40
ACUTE CORONARY SYNDROMES
Thrombectomy for STEMI
The TOTAL trial17 (ThrOmbecTomy with percutaneous coronary intervention vs. PCI ALone in patients with ST-elevation myocardial infarction undergoing primary PCI) is a large multicenter trial where patients with STEMI were randomized to manual thrombectomy and PCI or PCI alone. The absence of any evidence of improved prognosis at 30 days with the routine use of manual thrombectomy was confirmed at 1 year, with a small but worrisome increase in neurological events in patients receiving manual thrombectomy (1.2% vs. 0.7%, P = 0.015). In the angiographic sub-study of the TOTAL trial18, routine thrombectomy did not improve final MBG (myocardial blush grading) or TIMI flow (thrombolysis in myocardial infarction) when compared with PCI alone, but it was associated with a decreased rate of distal embolization (7.1% in thrombectomy group vs. 10.7% in PCI alone group, P < 0.01), a complication confirmed as an independent predictor of mortality (HR 3.00, 95% CI: 1.19–7.58). These two articles confirmed the recent change in the US and European revascularization and STEMI guidelines that discouraged the routine use of manual thrombectomy14,19 in primary PCI.
STEMI, immediate vs. deferred stent implantation
The Danish registry DANAMI (DANish Study of Optimal Acute Treatment of Patients With ST-elevation Myocardial Infarction) analysed 3854 patients in the period 2011–2014. In the DANAMI-3-DEFER,20 1215 patients were randomized to receive standard primary PCI with immediately stent implantation or deferred stent implantation (median of 3 days) to reduce the risk of embolization and no-reflow phenomenon. The primary end-point, a composite of all-cause mortality, hospital admission for heart failure, recurrent infarction, and unplanned TLR, was similar in the two groups (18% in immediate PCI, 17% in deferred PCI, P = 0.92). Distal embolization was observed in <10% and was equal in the two groups (but crossover from deferred to immediate stent implantation was frequent, occurring in 22% of cases).
Non-culprit lesion treatment in STEMI
The multicenter randomized DANAMI-3 PRIMULTI21 trial addressed the recurrent question of the optimal treatment of critical lesions in non-culprit vessels in 627 patients undergoing primary PCI. FFR-guided complete revascularization during the initial hospitalization of patients with STEMI and multivessel disease improved outcome after 2 years of follow-up. Mortality and non-fatal MI were similar in the two groups but ischemia-driven repeat revascularization was 69% lower in the FFR guided group (17.0% vs. 5.0%, P < 0.0001). This result suggests that a delayed in-hospital FFR guided revascularization should be preferred to a conservative symptoms guided strategy.
Timing of PCI in NSTEMI
The European guidelines22 recommend coronary angiography and possible PCI within 24 h from hospital admission in patients with positive troponin or ischemic ECG changes. In the RIDDLE-NSTEMI trial23 (Immediate Versus Delayed Invasive Intervention for Non-STEMI Patients), 323 patients with NSTEMI were randomized to immediate intervention (< 2 h) and delayed intervention (2–72 h). At 30 days, mortality and new MI were lower in the immediate intervention group (4.3% vs. 13%, P = 0.008), a difference that was confirmed at 1 year (6.8% vs. 18.8%, P = 0.002).
PCI in out-of-hospital cardiac arrest (OHCA)
The optimal timing of coronary angiography is unclear in patients with OHCA and without ST-elevation. In the PROCAT II24 registry (Parisian Region Out of hospital Cardiac ArresT), 695 patients after OHCA without ST elevation and without extracardiac causes of the event underwent emergency coronary angiography (immediate transfer to cath lab like for primary angioplasty). In 403 patients (58%), at least one signifi cant coronary lesion was observed and in 199 patients (29%), emergency PCI was performed. Successful recovery was observed in 43% of patients after emergency PCI and 33% of patients treated conservatively, with successful PCI emerging as an independent predictor of recovery (HR 1.8, 95% CI: 1.09–2.97, P =0.02).
ACS in elderly patients
Patients older than 80 years with ACS are quickly increasing as a consequence of the aging world population but these patients are underrepresented in clinical trials and undertreated with invasive and pharmacological therapy. In the EIGHTY trial25, 457 octuagenerians were randomized to invasive treatment or medical treatment. After 1.53 years of follow-up, the combined
primary end-point of death, MI, urgent revascularization and stroke occurred in 40.6% of invasive group vs. 61.4% of conservative group (P = 0.001), with significant differences for MI and urgent revascularization. No differences in bleeding complications were observed.
Routine follow-up angiography after PCI
The REACT trial26 (Randomized Evaluation of Routine Follow-up Coronary Angiography after Percutaneous Coronary Intervention) investigated the value of routine coronary angiography 9–12 months after the initial angioplasty, a practice widely used in all patients in Japan and reserved outside Japan to selected subgroups such as LM or diffuse disease in diabetic patients. Unfortunately, the trial enrolled only 700 out of the 3300 originally planned patients which precludes meaningful subgroup subanalysis. Results showed a 15% increase in revascularization as a consequence of the follow-up angiogram, not translating into a reduction of MI or mortality at 5 years follow-up. The result discourages routine use of diagnostic angiography but also shows that over the first 5 years, the conservative group reaches the same revascularization rate, a clinically mandated catch-up phenomenon that partially justifies the clinical value of elective early retreatment.
DEVICES
Bare metal stent (BMS) vs. drug eluting stent (DES)
The universal use of DES was challenged by the results of the NORSTENT trial27 (Norwegian Coronary Stent Trial), a randomized multicenter comparison of BMS vs. DES (82.9% everolimus-eluting stents and 13.1% zotarolimus-eluting stents) in 9013 Norwegian patients. The study excluded, among others, patients with previous coronary stenting or with bifurcation lesions requiring treatment with a two-stent technique. Mortality and spontaneous MI, the primary end-point of the trial, were similar in the two groups at 6 years (median follow-up 59 months). The DES group showed a reduction in target lesion revascularization (TLR) (19.8% in BMS group vs. 16.5% in DES group, P < 0.001). The number needed to treat to prevent one repeat revascularization (NNT = 30) was high in comparison to previous DES studies but low when compared with the typical NNT of pharmacological studies. Concerns about high cost could have deterred doctors from using DES 5 years ago when DES were much more expensive than BMS. This smaller than expected difference is unlikely to have an impact now, especially in the absence of any signal of possible increase of late in-stent thrombotic events (in fact there was a small but significant difference in favour of DES, definite or probable ST was 1.2% in the BMS group and 0.8% in the DES group, P = 0.0498). For many years, BMS were preferred in patients at high risk of bleeding to avoid the need of a prolonged double antiplatelet therapy or, worse, triple antiplatelet/ antithrombotic therapy. Recently, this practice has been challenged by the availability of data showing safety of early withdrawal of dual antiplatelet therapy (DAPT) in patients treated with second generation zotarolimus or everolimus eluting stents. In the randomized multicenter LEADERS-FREE trial28 better results were obtained with a polymer free biolimus A9 (umirolimus) DES used with only 1 month of DAPT in comparison with BMS. The significant reduction of TLR observed at 1 year (5.1% in DES group vs. 9.8% in BMS group, P < 0.01) was confi rmed at 2 years29 (6.8% in DES group vs. 12.0% in BMS group, P < 0.001). In addition, a post hoc analysis of the LEADERS FREE trial confirmed these results in 1575 elderly patients30 (>75 years). The composite safety end-point of cardiac death, MI and ST was reached in 14.3% of the BMS group and 10.7% of the DES group, (P = 0.03). In addition, the primary end-point of clinically driven TLR was worse in the BMS group (10.8%) than in the DES group (5.8%, P < 0.01). The pre-specified subanalysis of the ZEUS trial31 (Zotarolimus Eluting Endeavor Sprint Stent in Uncertain DES Candidates) showed that MACE were lower in high risk bleeding patients treated with zotarolimus eluting stent than in BMS patients (22.6% in DES group vs. 29.0% in BMS group, P > 0.01). A prespecified post hoc analysis of the PRODIGY trial32 (PROlonging Dual antiplatelet treatment after Grading stent-induced Intimal hyperplasia studY), analysed 323 patients with chronic renal failure (eGFR < 60ml/min/1.73 m2) with stable coronary disease or ACS undergoing PCI. It showed a lower risk of stent thrombosis and lower MACE rate at 2 years in patients treated with everolimus or zotarolimus eluting stent than in patients treated with paclitaxel eluting stents or BMS.
Late follow-up after DES
The SIRTAX-very late trial33 (Sirolimus-Eluting vs. Paclitaxel-Eluting Stents for Coronary Revascularization) reported 10 year results of a Swiss randomized comparison study between sirolimus eluting (CYPHER) and paclitaxel eluting stents (TAXUS). The fear was that a continuous progression of late lumen loss and the development of accelerated neoatherosclerosis in DES could induce a poor long-term outcome. Results showed that the annual risk of TLR between 5 and 10 years was >60% lower than in the period between 1 and 5 years (0.7%/year vs. 1.8%/year, P <0.001). Equally, the annual risk of very late stent thrombosis decreased during the extended follow-up period (5–10 years: 0.23%/year vs. 1–5 years: 0.67%/year, P < 0.01). In the ISAR-Test 5 trial34 (Intracoronary Stenting and Angiographic Results: Test Efficacy of Sirolimus and Probucol and Zotarolimus Eluting Stents), 3,002 patients were randomly assigned to treatment with polymer-free sirolimusand probucol-eluting stents (n = 2002) or Resolute stent (biostable polymer eluting zotarolimus, n = 1000). The 5 year follow-up confirmed the non-inferiority of the first group for a composite of cardiac death, target-vessel related myocardial infarction, or TLR (23.8% vs. 24.2%, P = 0.80). Stent thrombosis was low and similar in the two groups (1.3% vs. 1.6%, P = 0.64). In the EXAMINATION trial35 (A Clinical Evaluation of Everolimus Eluting Coronary Stents in the Treatment of Patients With ST-segment Elevation Myocardial Infarction), 1498 patients with STEMI treated with primary PCI were randomly assigned to receive everolimus eluting stent in or BMS. After 5 years, a composite end-point of allcause death, any myocardial infarction, or any revascularization was met in 21% of patients in the EES group vs. 26% in the BMS group (P = 0.033), mainly driven by a lower rate of all-cause mortality (P = 0.047).
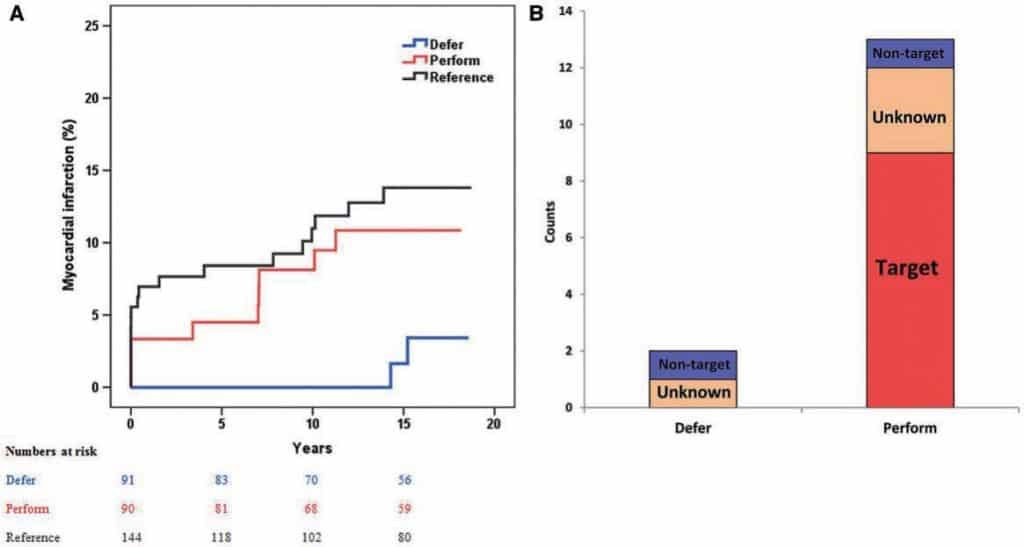
Figure 4. Kaplan–Meier of myocardial infarction (A) and relation of myocardial infarction with study vessel territory. Reproduced with permission from Zimmermann et al.46
Comparison of modern second generation DES
The BIO-RESORT TWENTE III trial36 compared two third generation DES (sirolimus eluting Orsiro stent and everolimus eluting Synergy) with biodegradable polymer coatings with a second generation durable polymer DES (zotarolimus eluting Resolute Integrity). Results in 3514 allcomer patients showed very low incidence of new revascularization and very low stent thrombosis (0.3%), equivalent for the three platforms (P = 0.7). The PRISON IV trial37 is a randomized, multicenter trial designed to evaluate the safety and efficacy of hybrid sirolimus-eluting stents (SES) with bioresorbable polymer (Orsiro) compared with everolimus- eluting stents (EES) with durable polymers (Xience Prime) after CTO recanalization. In 330 patients analysed in this trial, new generation DES did not meet the non-inferiority criteria for in-segment late lumen loss when compared with second generation DES (0.13 ± 0.63 mm in SES group vs. 0.02 ± 0.47 mm in EES, P for superiority = 0.08). In addition, binary restenosis was significantly higher in SES (8.0% in SES vs. 2.1% in EES, P = 0.028), while the clinical end-points were similar in both groups.
Bioresorbable Scaffolds
Four large multicenter randomized trials from Europe (ABSORB II,38 501 patients), USA (ABSORB III,39 the largest with 2,008 patients), Japan and China (ABSORB Japan40 and ABSORB China41, 400 and 480 patients respectively) compared a bioresorbable or a metallic stent eluting everolimus. ABSORB III showed non-inferiority of Absorb vs. Xience in terms of target lesion failure (TLF) at 1 year (7.8% for Absorb vs. 6.1% for Xience, P = 0.16), cardiac death (0.6% vs. 0.1%, P = 0.29) and stent thrombosis at 1 year (1.5% vs. 0.7%, P = 0.13). ABSORB China41 focused on a primary angiographic end-point of in-segment late loss and showed a low late lumen loss at 1 year (0.19 ±0.38 mm) in the Absorb group, non-inferior to the 0.13 ± 0.38 mm observed in the Xience group. Angiographic restenosis at 13 months was low, 1.9% and 3.9% (P = 0.31) in the Absorb Japan trial.40 In a large patient-level pooled meta-analysis, 3389 patients42 from four trials with stable coronary disease or ACS were randomized to receive BVS (2164 patients) or metallic EES (1225 patients). No significant differences were observed in the patient oriented composite end-point of all cause mortality, all MI or all revascularization at 1 year, and in the device oriented composite end-point of TLF, in cluding cardiac mortality, target vessel related MI or ischemia driven TLR (P = 0.17). The most worrisome data came from the 3 year results of the oldest of these studies, ABSORB II.43 Unexpectedly, the primary end-point of vasomotor reactivity did not differ in the two groups (0.047 mm for BVS vs. 0.056 mm for EES, Psuperiority = 0.49). Six patients experienced definite very late scaffold thrombosis in the BVS group (2.0% in total vs. 0% in the XIENCE arm, P = 0.19), with a higher incidence of clinically indicated TLR (6% vs. 2%, P = 0.04). While presenting these results, Serruys called for a prolongation of double antiplatelet therapy and suggested a drastic modifications of the protocol of stent implantation recommended in the ABSORB II study, with rare use of imaging and post-dilatation. More fundamental criticisms pointed to the limitations of the current ABSORB platform (strut thickness of 150 micron, low radial force, 0.5 mm margin for further diameter expansion, lack of X-ray visibility), enhancing the interest for the favorable results of initial registries of thinner biodegradable stents. These results are certainly upsetting those cardiologists who believed that an initial greater complexity of implant and a possible price to pay in terms of higher early stent thrombosis was due to be compensated by an absolute absence of late events after the completion of the absorption process. The follow-up duration might be insufficient for a complete absorption of the PLLA when the struts are detached from the wall and gross persistent abnormalities of vascular rheology due to malapposed struts can trigger thrombosis even after complete absorption if intraluminal strands remain.
Other devices
Severe coronary calcification remains a predictor of worse clinical outcomes. In the ORBIT II trial44 (Evaluate the Safety and Efficacy of Orbital Atherectomy System in Treating Severely Calcifi ed Coronary Lesions), 443 patients with severe calcifi ed lesions were treated with orbital atherectomy for pre-stent implantation preparation. The 2 year follow-up of the ORBIT II trial extended the favorable results previously shown at 30 day and 1 year follow-up: MACE were 19.4%, all-cause death 7.5%, cardiac death 4.3%, and TLR 6.2%. The stratification for stent type showed a worse result in BMS vs. first and second-generation DES (TLR15.1% in BMS vs. 6.3% first-generation DES vs. 5.0% in second-generation DES, P = 0.047). The DISRUPT CAD trial is the first coronary experience with a lithoplasty balloon (Shockwave Medical),45 a device previously used only in peripheral vessels. Sixty patients with moderate or severe calcific lesions (stenosis >50%, reference vessel diameter 2.5–4.0 mm, lesion length <32 mm) were treated before stenting with excellent stent expansion and OCT documented fractures of superficial calcifications in 58%. This device proved to be safe, with no final angiographic complication and a low 30 day MACE (no death, no Q-wave MI, 5% non-Q MI). Both orbital atherectomy and, especially, the revolutionary approach of lithotripsy of coronary calcium have the potential to greatly improve the effects limited to superficial calcium achieved with Rotablator but, so far, no comparison data are available.
FUNCTIONAL AND IMAGING GUIDANCE
Fractional flow reserve (FFR) for intermediate lesions DEFER46 was the first study confirming the clinical usefulness of FFR showing similar freedom from major adverse cardiac events (death, MI, and repeat revascularization), the primary end-point of the trial, withdrawing PCI in patients initially referred for treatment when FFR was >0.75. The long-term safety of deferring treatment of lesions of intermediate angiographic severity when FFR is above the threshold of 0.75 was confirmed by the 15 years results of the 5 years DEFER trial, conducted in the late-90s in 250 patients using POBA or BMS, and the DEFER DES trial,47 randomizing 229 patients using first or second (70%) generation DES. In DEFER, after a 15 year follow-up, there was still no difference in all-cause mortality among the three groups of randomization (36.1% reference group vs. 33% DEFER vs. 31.1% performance, P = 0.79). MI was significantly lower in the DEFER group compared with the perform PCI group (2.2% vs. 10%, P = 0.03), mainly because of a lower target vessel infarction (Figure 4). In DEFER-DES, there was no difference in MACE after 5 year follow-up (11.6 ± 3.0% in the routine-DES group and 14.2 ± 3.3% in the FFR-guided group, P = 0.55). It is obviously dangerous to overemphasize differences in events that were not from the primary end-point of the trial and at different times than the planned followup. The lack of impact of PCI in DEFER-DES can be considered an unavoidable limitation of recanalization treatment in patients mainly with single vessel disease and preserved left ventricular function, a condition where PCI never showed prognostic benefit. This note of caution is particularly needed after publication of the FUTURE trial,48 a multicenter French registry trying to replicate the FAME trial49 results with FFR guided revascularization in multivessel disease patients with nearly equal numbers of stable coronary disease or ACS (46%). This French trial was stopped prematurely (after 836 patients, instead of the 1728 scheduled patients) because a significantly higher mortality was observed in the FFR group (3.9% vs. 1.9%, P = 0.02).
OCT for stent optimization
The DOCTORS trial50 (Does Optical Coherence Tomography Optimize Results of Stenting) is the first randomized study comparing OCT and angiographic guidance of coronary stent implantation. The trial was conducted in 240 NSTEMI patients using as end-point the final post-procedural FFR. The value of FFR was higher when OCT was used pre and post-PCI than in the angiography only group (0.94 ± 0.04 vs. 0.92 ± 0.05, P = 0.005). Post-dilatation was used more frequently in the OCT group (43.0% vs. 12.5%, P < 0.0001), resulting in a lower residual diameter stenosis (7.0 ± 4.3% vs. 8.7 ± 6.3%, P = 0.01). Adverse events were similar in two groups. ILUMIEN II,51 an observational study of OCT in patients undergoing PCI, is a recent post hoc analysis of two studies: ILUMIEN, where stent expansion was guided by OCT in 354 patients, and ADAPTDES, where IVUS guided stent implantation was used in 586 patients. Stent expansion was similar in the two studies (72.8 vs. 70.6% of the average reference area, respectively, P = 0.29). The randomized trial ILUMIEN III52 compared IVUS, OCT and angiographic guidance in 450 patients, showing non-inferiority of the minimal instent lumen area obtained with OCT (5.79 mm2 IQR 4.54–7.34 in the OCT group, 5.89 mm2 IQR 4.67–7.80 in the IVUS group, and 5.49 mm2 IQR 4.39–6.59 in the angiography group) using an imaging end-point based on the reference external elastic membrane, visible also with OCT in >80% of cases. Major malapposition and dissection were more frequent in the IVUS guided group when compared with the OCT group (21% vs. 11%, P = 0.02 for malapposition; 26% vs. 14%, P = 0.009 for dissection). In the Japanese OPINION trial,53 800 patients were randomized to IVUS or OCT-guided PCI, showing no differences in the primary endpoint of target vessel failure, including cardiac death, MI caused by target vessel, clinically driven target vessel revascularization after angiographic control at 12 months (P = 0.833). In the OPINION subanalysis, 100 patients were analysed with OCT (n = 50) and IVUS (n = 50) after 8 months, showing greater stent expansion in the IVUS group, approaching statistical signifi cance for mean in-stent lumen area (6.56 mm2 in OCT vs. 7.51 mm2 in IVUS, P = 0.054). This difference in favour of IVUS came at the cost of a higher frequency of dissection (P = 0.039 for proximal reference). The numerically lower strut malapposition in the OCT group did not lead to an increased coverage at the follow-up study after 9 months which showed 4.67% uncovered struts in the IVUS group vs. 6.97% in the OCT group (P= 0.039).
OCT to prevent stent thrombosis
OCT, providing high resolution images, can improve the knowledge of the pathophysiology of stent thrombosis. In the French PESTO registry54 (Morphological Parameters Explaining Stent Thrombosis assessed by OCT), 120 patients with stent thrombosis (75% very late, 6% late, 15% sub-acute, 4% acute) within BMS, DES or bioabsorbable scaffolds were analysed. In 97% of cases an underlying abnormality was found by OCT analysis: malapposition (34%), neoatherosclerotic lesions (22%), (both mainly in late and very late thrombosis), major stent underexpansion (11%), coronary evagination (8%), isolated uncovered struts (8%), edge relateddisease progression (8%) and neointimal hyperplasia (4%). In another multicenter European registry,55 58 patients with very late stent thrombosis with DES were analysed by OCT, conforming that multiple mechanisms cause very late thrombosis. Malapposition (34.5%) and neoatherosclerosis (27.6%), were more frequent than uncovered struts (12.1%) and stent underexpansion (6.9%)
ADJUNCTIVE PHARMACOLOGIC THERAPY
Prasugrel vs. ticagrelor
The PRAGUE-18 study56 is the first multicenter randomized study comparing prasugrel and ticagrelor in acute MI undergoing primary PCI. Approximately 1230 out of the expected 2600 patients were randomized at the time the study was prematurely interrupted for futility because the primary end-point (composite of death, re-infarction, urgent TLR, stroke, serious bleeding requiring transfusion or prolonging hospitalization beyond 7 days) was equal in the two groups (4.0% prasugrel vs. 4.1% ticagrelor, P = 0.939). The premature interruption of the trial and the high frequency of treatment switching, moving to clopidogrel after 7 days, makes this trial unable to provide a credible definitive comparison between ticagrelor and prasugrel.
Onset of action of P2Y12 inhibition
The use of morphine in ACS patients can reduce chest pain but can also delay the onset of action of P2Y12 receptor inhibitors. In the IMPRESSION trial,57 74 MI patients were randomized to receive 5 mg of intravenous morphine or placebo followed by ticagrelor 180 mg. Pharmacokinetic and pharmacodynamic parameters confirmed that morphine reduced total exposure to ticagrelor, showing a concomitant delay in maximal plasma concentration of ticagrelor (4 h in morphine group vs. 2 h in the control group, P < 0.004). In ACS, a rapid effect of P2Y12 inhibitors is very important but also in elective PCI a sufficient platelet inhibition at the start of PCI has to be obtained to decrease MI and stent thrombosis. The EXCELSIOR-LOAD trial58 (Impact of Extent of Clopidogrel-Induced Platelet Inhibition during Elective Stent Implantation on Clinical Event Rate-Advanced Loading Strategies) showed suboptimal still elevated platelet reactivity (defined as >468 AU/ min in aggregometry tests) in 55% of 100 patients loaded with clopidogrel 600 mg, in 37% of 100 patients receiving prasugrel 30 mg, and 33% of 100 patients receiving prasugrel 60 mg (P < 0.01). After two hours the platelet reactivity was not significantly different in the three groups with similar 30 day incidence of bleeding events. In patients not pretreated or not optimally reacting to oral P2Y12 inhibitors, cangrelor plays an important role. A recent subanalysis of the CHAMPION PHOENIX trial59 confirmed that intravenous cangrelor was more effective than clopidogrel to reduce periprocedural MI at 48 h (3.8% in cangrelor patients vs. 4.7% clopidogrel patients), regardless of MI definition. Another subanalysis showed a reduction of periprocedural complications in patients undergoing PCI for stable angina or ACS treated with cangrelor in comparison to clopidogrel.
Duration of antiplatelet therapy
In spite of innovations in stent materials and the consequent shortening of double antiplatelet therapy (DAPT), some patients still remain at high risk for ischemic events. Overall a longer than 1 year DAPT offers advantages, as shown in the PEGASUS-TIMI 54 trial.60 The DAPT score,61 including clinical history and angiographic features, is a useful instrument to identify patients with expected benefit of a longer DAPT. Balancing ischemic and bleeding risk it permits personalized tailored DAPT duration. In the OPTIDUAL trial62 (OPTImal DUAL antiplatelet therapy), 1385 patients were randomly assigned to continuing clopidogrel 75 mg daily after 1 year DAPT (extended-DAPT group) or discontinuing clopidogrel (aspirin group). Due to premature termination of enrolment, no differences were shown in the composite primary end-point of death, MI, stroke, or major bleeding (5.8% in extended-DAPT group and 7.5% in aspirin group, P = 0.17), mortality rate (2.3% vs. 3.5%, respectively, P = 0.18) and major bleedings (2.0% in both groups).
Triple antithrombotic therapy
Approximately 5–21% of patients undergoing PCI for ACS have concomitant atrial fibrillation and a growing number of atrial fibrillation patients are treated with new oral anticoagulants. The PIONEER-AF PCI63 trial is an open-label, randomized, controlled, multicenter study exploring two treatment strategies of rivaroxaban and oral vitamin K antagonist in subjects undergo PCI. The study randomized 2100 patients to receive rivaroxaban 15 mg once daily plus clopidogrel 75 mg for 12 months or rivaroxaban 2.5 mg twice daily plus DAPT or dose-adjusted VKA once daily plus DAPT. The primary end-point (a composite of TIMI major and minor bleeding requiring medical attention) showed a significant advantage of both rivaroxaban groups against the VKA group (16.8% first group, 18% second group, 26.7% in VKA group, P< 0.01). All cause death or hospitalization were reduced in the rivaroxaban arms (first group, NNT 15, second group NNT = 10).
Other drugs
In the GLAGOV trial64 (Global Assessment of Plaque Regression With a PCSK9 Antibody as Measured by Intravascular Ultrasound), Evolocumab was tested against statin therapy in patients with coronary artery disease for 76 weeks. In this multicenter placebo-controlled trial, 484 patients treated with evolocumab showed not only a lowering of LDL-C mean values compared with placebo group (36.6 vs. 93.0 mg/dL, respectively, P < 0.01) but also a signifi cant reduction of percent atheroma volume in IVUS analysis (decrease of 0.95% vs. increase of 0.05%, respectively), and a reduction of total atheroma volume (from 5.8 mm3 with evolocumab to 0.9 mm3 with placebo, P < 0.01). This raised expectations of clinically significant results in the FOURIER trial, with publication expected at ACC in March 2017.
CONCLUSIONS
The main clinically relevant messages from the year 2016 in coronary interventions can be summarized as follows. Follow-up up to 10 years shows that surgical revascularization is advantageous in coronary patients with impaired left ventricular function when compared with optimal medical therapy alone, prompting repeated late analyses of all other trials showing equivalence of optimal medical therapy and revascularization. New randomized data support the bold decision of the 2014 European Society of Cardiology Myocardial Revascularization Guidelines to give an equal recommendation class to PCI and CABG for left main disease. This year was definitely not the year of the universal switch from metallic to bioabsorbable drug eluting stents, in spite of reassuring data from large randomized trials with concerns raised by conflicting data from large randomized trials. Current generation scaffolds are early devices and main need further iterative improvements before being able to compete with currently available high performance DES. The unpredictable absorption time and onset of action also with the newer antiplatelet oral agents offers a window of opportunity for novel intravenous antiplatelet agents, while a longer than 1 year DAPT should be considered with a tailored approach. A triple antiplatelet/antithrombotic therapy remains an unresolved question, with low dose new oral anticoagulants proposed as alternative components of treatment in this setting. New injectable cholesterol lowering drugs for the first time showed a significant reduction of plaque volume that mirrors the greater efficacy in LDL cholesterol reduction.
Conflict of interest: C.D.M reports institutional research grants from Abbott, Medtronic, Shockwave Medical, MiCell, St. Jude. W.W. reports institutional research grants from Abbott Vascular, Biotronik, Boston Scientific, Medtronic, MiCell, Stentys, St Jude, Terumo. W.W. is a non-executive Board member of Argonauts and Genae Inc.
References
1. Velazquez EJ, Lee KL, Deja MA, Jain A, Sopko G, Marchenko A, Ali IS, Pohost G, Gradinac S, Abraham WT, Yii M, Prabhakaran D, Szwed H, Ferrazzi P, Petrie MC, O’Connor CM, Panchavinnin P, She L, Bonow RO, Rankin GR, Jones RH, Rouleau JL; STICH Investigators. Coronary- artery bypass surgery in patients with left ventricular dysfunction. N Engl J Med 2011;364:1607–1616.
2. Doshi D, Ben-Yehuda O, Bonafede M, Josephy N, Karmpaliotis D, Parikh MA, Moses JW, Stone GW, Leon MB, Schwartz A, Kirtane AJ. Underutilization of coronary artery disease testing among patients hospitalized with new-onset heart failure. J Am Coll Cardiol 2016;68:450–458.
3. Velazquez EJ, Lee KL, Jones RH, Al-Khalidi HR, Hill JA, Panza JA, Michler RE, Bonow RO, Doenst T, Petrie MC, Oh JK, She L, Moore VL, Desvigne-Nickens P, Sopko G, Rouleau JL; STICHES Investigators. Coronary-artery bypass surgery in patients with ischemic cardiomyopathy. N Engl J Med 2016;374:1511–20.
4. Taggart DP, Altma DG, Gray AM, Lees B, Gerr S, Benedetto U, Flather M, for the ART Investigator. Randomized trial of bilateral versus single internalthoracic-artery graft. N Engl J Med 2016; doi: 10.1056/ NEJMoa1610021.
5. Baber U, Farkouh ME, Arbel Y, Muntner P, Dangas G, Mack MJ, Hamza TH, Mehran R, Fuster V. Comparative efficacy of coronary artery bypass surgery vs. percutaneous coronary intervention in patients with diabetes and multivessel coronary artery disease with or without chronic kidney disease. Eur Heart J 2016;doi: 10.1093/eurheartj/ehw378.
6. Stone GW, Sabik JF, Serruys PW, Simonton CA, Genereux P, Puskas J, Kandzari DE, Morice MC, Lembo N, Brown WM III, Taggart DP, Banning A, Merkely B, Horkay F, Boonstra PW, van Boven AJ, Ungi
I, Bogats G, Mansour S, Noiseux N, Sabate M, Pomar J, Hickey M, Gershlick A, Buszman P, Bochenek A, Schampaert E, Page P, Dressler O, Kosmidou I, Mehran R, Pocock SJ, Kappetein AP; EXCEL Trial Investigators. Everolimus-eluting stents or bypass surgery for left main coronary artery disease. N Engl J Med 2016;375:2223–2235.
7. Mäkikallio T, Holm NR, Lindsay M, Spence MS, Erglis A, MenownI BA,Trovik T, Eskola M, Romppanen H, Kellerth T, Ravkilde J, Jensen LO, Kalinauskas G, Linder RBA, Pentikainen M, Hervold A, Banning A, Zaman A, Cotton J, Eriksen E, Margus S, Sørensen HT, Nielsen PH, Niemelä M, Kervinen K, Lassen JF, Maeng M, Oldroyd K, Berg G,Walsh SJ, Hanratty CG, Kumsars I, Stradins P, Steigen TK, Fröbert O, Graham ANJ, Endresen PC, Corbascio M, Kajander O, Trivedi U, Hartikainen J, Anttila V, Hildick-Smith D, Thuesen L, Christiansen EH; NOBLE Study Investigators. Percutaneous coronary angioplasty versus coronary artery bypass grafting in treatment of unprotected left main stenosis (NOBLE): a prospective, randomized, open-label, non-inferiority trial. Lancet 2016;388:2743–2752.
8. Van der Schaaf RJ, Vis MM, Sjauw KD, Koch KT, Baan J Jr, Tijssen JG, de Winter RJ, Piek JJ, Henriques JP. Impact of multivessel coronary disease on long-term mortality in patients with ST-elevation myocardial infarction is due to the presence of a chronic total occlusion. Am J Cardiol 2006;98:1165–1169.
9. Claessen BE, Dangas GD, Weisz G, Witzenbichler B, Guagliumi G, Möckel M, Brener SJ, Xu K, Henriques JP, Mehran R, Stone GW. Prognostic impact of a chronic total occlusion in a non-infarct-related artery in patients with STsegment elevation myocardial infarction: 3-year results from the HORIZONSAMI trial. Eur Heart J 2012;33:768–775.
10. Henriques JP, Hoebers LP, Råmunddal T, Laanmets P, Eriksen E, Bax M, Ioanes D, Suttorp MJ, Strauss BH, Barbato E, Nijveldt R, van Rossum AC, Marques KM, Elias J, van Dongen IM, Claessen BE, Tijssen JG, van der Schaaf RJ; EXPLORE Trial Investigators. Percutaneous intervention for concurrent chronic total occlusions in patients with STEMI. The EXPLORE Trial. J Am Coll Cardiol 2016;68:1622–1632.
11. Råmunddal T, Hoebers LP, Henriques JP, Dworeck C, Angerås O, Odenstedt J, Ioanes D, Olivecrona G, Harnek J, Jensen U, Aasa M, Albertsson P, Wedel H, Omerovic E. Prognostic impact of chronic total occlusions: a report from SCAAR (Swedish Coronary Angiography and Angioplasty Registry). J Am Coll Cardiol Cardiovasc Interv 2016;9:1535–1544.
12. Tomasello SD, Boukhris M, Giubilato S, Marz a F, Garbo R, Contegiacomo G, Marzocchi A, Niccoli G, Gagnor A, Varbella F, Desideri A, Rubartelli P, Cioppa A, Baralis G, Galassi AR. Management strategies in patients affected by chronic total occlusions: results from the Italian Registry of Chronic Total Occlusions. Eur Heart J 2015;36:3189–3198.
13. Maeremans J, Walsh S, Knaapen P, Spratt JC, Avran A, Hanratty CG, Faurie B, Agostoni P, Bressollette E, Kayaert P, Bagnall AJ, Egred M, Smith D, Chase A, McEntegart MB, Smith WH, Harcombe A, Kelly P, Irving J, Smith EJ, Strange JW, Dens J. The hybrid algorithm for treating chronic total occlusions in Europe: the RECHARGE Registry. J Am Coll Cardiol 2016;68:1958–1970.
14. Windecker S, Kolh P, Alfonso F, Collet JP, Cremer J, Falk V, Filippatos G, Hamm C, Head SJ, Jüni P, Kappetein AP, Kastrati A, Knuuti J, Landmesser U, Laufer G, Neumann FJ, Richter DJ, Schauerte P, Sousa Uva M, Stefanini GG, Taggart DP, Torracca L, Valgimigli M, Wijns W, Witkowski A, 2014 ESC/EACTS Guidelines on myocardial revascularization: The Task Force on Myocardial Revascularization of the European Society of Cardiology (ESC) and the European Association for Cardio-Thoracic Surgery (EACTS) Developed with the special contribution of the European Association of Percutaneous Cardiovascular Interventions (EAPCI). Eur Heart J 2014;35:2541–619.
15. Behan MW, Holm NR, de Belder AJ, Cockburn J, Erglis A, Curzen NP, Niemelä M, Oldroyd KG, Kervinen K, Kumsars I, Gunnes P, Stables RH, Maeng M, Ravkilde J, Jensen JS, Christiansen EH, Cooter N, Steigen TK, Vikman S, Thuesen L, Lassen JF, Hildick-Smith D. Coronary bifurcation lesions treated with simple or complex stenting: 5-year survival from patient-level pooled analysis of the Nordic Bifurcation Study and the British Bifurcation Coronary Study. Eur Heart J 2016;37:1923–1928.
16. Ferenc M, Gick M, Comberg T, Rothe J, Valina C, Toma A, Löffelhardt N, Hochholzer W, Riede F, Kienzle RP, Achtari A, Neumann FJ. Culotte stenting vs. TAP stenting for treatment of de-novo coronary bifurcation lesions with the need for side-branch stenting: the Bifurcations Bad Krozingen (BBK) II angiographic trial. Eur Heart J 2016;37:3399–3405.
17. Jolly SS, Cairns JA, Yusuf S, Rokoss MJ, Gao P, Meeks B, Kedev S, Stankovic G, Moreno R, Gershlick A, Chowdhary S, Lavi S, Niemela K, Bernat I, Cantor WJ, Cheema AN, Steg PG, Welsh RC, Sheth T, Bertrand OF, Avezum A, Bhindi R, Natarajan MK, Horak D, Leung RC, Kassam S, Rao SV, El-Omar M, Mehta SR, Velianou JL, Pancholy S, Džavık V; TOTAL Investigators. Outcomes after thrombus aspiration for ST elevation myocardial infarction: 1-year follow-up of the prospective randomized TOTAL trial. Lancet 2016;387:127–135.
18. Sharma V, Jolly SS, Hamid T, Sharma D, Chiha J, Chan W, Fuchs F, Bui S, Gao P, Kassam S, Leung RC, Horak D, Romppanen HO, El-Omar M, Chowdhary S, Stankovic G, Kedev S, Rokoss MJ, Sheth T, Džavık V, Overgaard CB Myocardial blush and microvascular reperfusion following manual thrombectomy during percutaneous coronary intervention for ST elevation myocardial infarction: insights from the TOTAL trial. Eur Heart J 2016;37:1891–1898.
19. Levine GN, Bates ER, Blankenship JC, Bailey SR, Bittl JA, Cercek B, Chambers CE, Ellis SG, Guyton RA, Hollenberg SM, Khot UN, Lange RA, Mauri L, Mehran R, Moussa ID, Mukherjee D, Ting HH, O’Gara PT, Kushner FG, Ascheim DD, Brindis RG, Casey DE Jr, Chung MK, de Lemos JA, Diercks DB, Fang JC, Franklin BA, Granger CB, Krumholz HM, Linderbaum JA, Morrow DA, Newby LK, Ornato JP, Ou N, Radford MJ, Tamis-Holland JE, Tommaso CL, Tracy CM, Woo YJ, Zhao DX. 2015 ACC/AHA/SCAI Focused Update on Primary Percutaneous Coronary Intervention for Patients With ST-Elevation Myocardial Infarction: An Update of the 2011 ACCF/AHA/SCAI Guideline for Percutaneous Coronary Intervention and the 2013 ACCF/AHA Guideline for the Management of STElevation Myocardial Infarction: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Society for Cardiovascular Angiography and Interventions. Circulation 2016;133:1135–47.
20. Kelbæk H, Høfsten DE, Køber L, Helqvist S, Kløvgaard L, Holmvang L, Jørgensen E, Pedersen F, Saunamäki K, De Backer O Bang, LE Kofoed, KF Lønborg, J Ahtarovski, K Vejlstrup, N Bøtker, HE Terkelsen, CJ Christiansen, EH Ravkilde, J Tilsted, HH Villadsen, AB Aarøe, J Jensen, SE Raungaard, B Jensen, LO Clemmensen, P Grande, P Madsen, JK Torp-Pedersen, C Engstrøm, T. Deferred versus conventional stent implantation in patients with ST-segment elevation myocardial infarction (DANAMI3-DEFER): an open-label, randomized controlled trial. Lancet 2016;387:2199–2206.
21. Engstrøm T, Kelbæk H, Helqvist S, Høfsten DE, Kløvgaard L, Holmvang L, Jørgensen E, Pedersen F, Saunam€aki K, Clemmensen P, De Backer O, Ravkilde J, Tilsted HH, Villadsen AB, Aarøe J, Jensen SE, Raungaard B, Køber L; Danami-3—PRIMULTI Investigators. Complete revascularisation versus treatment of the culprit lesion only in patients with ST-segment elevation myocardial infarction and multivessel disease (DANAMI-3—PRIMULTI): an open-label, randomized controlled trial. Lancet 2015;386:665–671.
22. Roffi M, Patrono C. 2015 ESC Guidelines for the management of acute coronary syndromes in patients presenting without persistent ST-segment elevation Task Force for the Management of Acute Coronary Syndromes in Patients Presenting without Persistent ST-Segment Elevation of the European Society of Cardiology (ESC). Eur Heart J 2016;37:267–315.
23. Milosevic A, Vasiljevic-Pokrajcic Z, Milasinovic D, Marinkovic J, Vukcevic V, Stefanovic B, Asanin M, Dikic M, Stankovic S, Stankovic G. Immediate versus delayed invasive intervention for non-STEMI patients: the RIDDLE-NSTEMI study. J Am Coll Cardiol Cardiovasc Interv 2016;9:541–549.
24. Dumas F, Bougouin W, Geri G, Lamhaut L, Rosencher J, Pène F, Chiche JD, Varenne O, Carli P, Jouven X, Mira JP, Spaulding C, Cariou A. Emergency percutaneous coronary intervention in post- cardiac arrest patients without STsegment elevation pattern: insights from the PROCAT II Registry. J Am Coll Cardiol Cardiovasc Interv 2016;9:1011–1018.
25. Tegn N, Abdelnoor M, Aaberge L, Endresen K, Smith P, Aakhus S, Gjertsen E, Dahl-Hofseth O, Ranhoff AH, Gullestad L, Bendz B; After Eighty Study Investigators. Invasive versus conservative strategy in patients aged 80 years or older with non-ST-elevation myocardial infarction or unstable angina pectoris (After Eighty study): an openlabel randomized controlled trial. Lancet 2016;387:1057–1065.
26. Shiomi H, Morimoto T, Kitaguchi S, Nakagawa Y, Ishii K, Haruna Y, Takamisawa I, Motooka M, Nakao K, Matsuda S, Satoru, Mimoto, Aoyama Y, Taked T, Murata K, Akao M, Inada T, Eizawa H, Momona E, Awano K, Shirotani M, Furukawa Y, Kadota K, Miyauchi K, Tanaka M, Noguchi Y, Nakamura S, Yasuda S, Miyazaki S, Daida H, Kimura K, Ikari Y, Hirayama H, Sumiyoshi T, Kimura T on behalf of the ReACT Investigators. Randomized evaluation of Routine Follow-up Coronary Angiography after Percutaneous Coronary Intervention Trial (ReACT). J Am Coll Cardiol Cardiovasc Interv 2016; doi: 10.1161/ CIRCINTERVENTIONS.115.003365.
27. Bønaa KH, Mannsverk J, Wiseth R, Aaberge L, Myreng Y, Nygård O, Nilsen DW, Kløw NE, Uchto M, Trovik T, Bendz B, Stavnes S, Bjørnerheim R, Larsen AI, Slette M, Steigen T, Jakobsen OJ, Bleie Ø, Fossum E, Hanssen TA, Dahl-Eriksen Ø, Njølstad I, Rasmussen K, Wilsgaard T, Nordrehaug JE; for the NORSTENT Investigator. Drugeluting or bare-metal stents for coronary artery disease. N Engl J Med 2016;375:1242–1252.
28. Urban P, Meredith IT, Abizaid A, Pocock SJ, Carrie D, Naber C, Lipiecki J, Richardt G, Iniguez A, Brunel P, Valdes-Chavarri M, Garot P, Talwar S, Berland J, Abdellaoui M, Eberli F, Oldroyd K, Zambahari R, Gregson J, Greene S, Stoll HP, Morice MC; LEADERS FREE Investigators. Polymer free drug-coated coronary stents in patients at high bleeding risk. N Engl J Med 2015;373:2038–2047.
29. Garot P, Morice MC, Tresukosol D, Pocock SJ, Meredith IT, Abizaid A, Carrie D, Naber C, Iniguez A, Talwar S, Menown IB, Christiansen EH, Gregson J, Copt S, Hovasse T, Lurz P, Maillard L, Krackhardt F, Ong P, Byrne J, Redwood S, Windhövel U, Greene S, Stoll HP, Urban P; LEADERSFREE Investigators. Twoyear outcomes of high bleeding risk patients after polymer-free drug-coated stents. J Am Coll Cardiol 2016; doi: 10.1016/j.jacc.2016.10.009.
30. PCI for Elderly patients at High Bleeding Risk. A substudy of the LEADERS FREE trial, presented at ESC congress 2016 by Morice oral presentation, ESC 2016. 11688-000-EN – Rev.01.
31. Ariotti S, Adamo M, Costa F, Patialiakas A, Briguori C, Thury A, Colangelo S, Campo G, Tebaldi M, Ungi I, Tondi S, Roffi M, Menozzi A, de Cesare N, Garbo R, Meliga E, Testa L, Gabriel HM, Ferlini M, Vranckx P, Valgimigli M; ZEUS Investigators. Is bare-metal stent implantation still justifi able in high bleeding risk patients undergoing percutaneous coronary intervention?: A pre-specifi ed analysis from the ZEUS Trial. J Am Coll Cardiol Cardiovasc Interv 2016;9:426–436.
32. Crimi G, Leonardi S, Costa F, Adamo M, Ariotti S, Valgimigli M. Role of stent type and of duration of dual antiplatelet therapy in patients with chronic kidney disease undergoing percutaneous coronary interventions. Is bare metal stent implantation still a justifi able choice? A post-hoc analysis of the all comer PRODIGY trial. Int J Cardiol 2016;212:110–117.
33. Yamaji K, Räber L, Zanchin T, Spitzer E, Zanchin C, Pilgrim T, Stortecky S, Moschovitis A, Billinger M, Schönenberger C, Eberli F, Jüni P, Lüscher TF, Heg D, Windecker S. Ten year clinical outcomes of fi rst-generation drug-eluting stents: the sirolimus-eluting vs. paclitaxel-eluting stents for coronary revascularization (SIRTAX) VERY LATE trial. Eur Heart J 2016; doi: 10.1093/eurheartj/ehw343.
34. Kufner S, Sorges J, Mehilli J, Cassese S, Repp J, Wiebe J, Lohaus R, Lahmann A, Rheude T, Ibrahim T, Massberg S, Laugwitz KL, Kastrati A, Byrne RA; ISAR-TEST 5 Investigators. Randomized trial of polymerfree sirolimus- and probucol-eluting stents versus durable polymer zotarolimus-eluting stents: 5-year results of the ISAR-TEST-5 trial. J Am Coll Cardiol Cardiovasc Interv 2016;9:784–792.
35. Sabate M, Brugaletta S, Cequier A, I~niguez A, Serra A, Jimenez-Quevedo P, Mainar V, Campo G, Tespili M, den Heijer P, Bethencourt A, Vazquez N, van Es GA, Backx B, Valgimigli M, Serruys PW. Clinical outcomes in patients with STsegment elevation myocardial infarction treated with everolimus-eluting stents versus bare-metal stents (EXAMINATION): 5-year results of a randomized trial. Lancet 2016;387:357–366.
36. von Birgelen C, Kok MM, van der Heijden LC, Danse PW, Schotborgh CE, Gin MTJ, Somi S, van Houwelingen KG, Stoel MG, de Man HAF, Louwerenburg JW, Hartmann M, Zocca P, Linssen GCM, van
der Palen J, Doggen CJM, Löwik MM. Very thin strut biodegradable polymer everolimus-eluting and sirolimus-eluting stents versus durable polymer zotarolimus-eluting stents in allcomers with coronary artery disease (BIO-RESORT): a three-arm, randomized, non-inferiority trial. Lancet 2016;388:2607–2617.
37. Teeuwen K, van der Schaaf RJ, Adriaenssens T, Koolen JJ, Smits PC, Henriques JPS, Vermeersch PHMJ, Tjon Joe Gin RM, Sch—lzel BE, Kelder JC, Tijssen JGP, Agostoni P, Suttorp MJ. Randomized Multi-center Trial Investigating the Angiographic Outcome of Hybrid Sirolimuseluting Stents with Biodegradable Polymer Against Everolimus-eluting Stents with Durable Polymer in Chronic Total Occlusions (PRISON IV). JACC: Cardiovas Interv 2016; doi: 10.1016/j.jcin.2016.10.017.
38. Chevalier B, Onuma Y, van Boven AJ, Piek JJ, Sabate M, Helqvist S, Baumbach A, Smits PC, Kumar R, Wasungu L, Serruys PW. Randomised comparison of a bioresorbable everolimus-eluting scaffold with a metallic everolimus-eluting stent for ischaemic heart disease caused by de novo native coronary artery lesions: the 2-year clinical outcomes of the ABSORBII trial. Euro Interv 2016;12:1102–1107.
39. Ellis SG, Kereiakes DJ, Metzger DC, Caputo RP, Rizik DG, Teirstein PS, Litt MR, Kini A, Kabour A, Marx SO, Popma JJ, McGreevy R, Zhang Z, Simonton C, Stone GW; ABSORBIII Investigators. Everolimus-eluting bioresorbable scaffolds for coronary artery disease. N Engl J Med 2015;373:1905–1915.
40. Kimura T, Kozuma K, Tanabe K, Nakamura S, Yamane M, Muramatsu T, Saito S, Yajima J, Hagiwara N, Mitsudo K, Popma JJ, Serruys PW, Onuma Y, Ying S, Cao S, Staehr P, Cheong WF, Kusano H, Stone GW; ABSORB Japan Investigators. A randomized trial evaluating everolimuseluting absorb bioresorbable scaffolds vs. everolimus-eluting metallic stents in patients with coronary artery disease: ABSORB Japan. Eur Heart J 2015;36:3332–3342.
41. Gao R, Yang Y, Han Y, Huo Y, Chen J, Yu B, Su X, Li L, Kuo HC, Ying SW, Cheong WF, Zhang Y, Su X, Xu B, Popma JJ, Stone GW; ABSORB China Investigators. Bioresorbable vascular scaffolds versus metallic stents in patients with coronary artery disease: ABSORB China Trial. J Am Coll Cardiol 2015;66:2298–2309.
42. Stone GW, Gao R, Kimura T, Kereiakes DJ, Ellis SG, Onuma Y, Cheong WF, Jones-McMeans J, Su X, Zhang Z, Serruys PW. 1-year outcomes with the Absorb bioresorbable scaffold in patients with coronary artery disease: a patient-level, pooled meta-analysis. Lancet 2016;387:1277–1289.
43. Serruys PW, Chevalier B, Sotomi Y, Cequier A, Carrie D, Piek JJ, Van Boven AJ, Dominici M, Dudek D, McClean D, Helqvist S, Haude M, Reith S, de Sousa Almeida S, Campo G, Iniguez A, Sabate M, Windecker S, Onuma Y. Comparison of an everolimus-eluting bioresorbable scaffold with an everolimus-eluting metallic stent for the treatment of coronary artery stenosis (ABSORB II): a 3 year, randomized, controlled, single-blind, multicentre clinical trial. Lancet 2016;388:2479– 2491.
44. Genereux P, Bettinger N, Redfors B, Lee AC, Kim CY, Lee MS, Shlofmitz RA, Moses JW, Stone GW, Chambers JW. Two-year outcomes after treatment of severely calcifi ed coronary lesions with the orbital atherectomy system and the impact of stent types: Insight from the ORBITII trial. Catheter Cardiovasc Interv 2016;88:369–377.
45. Brinton TJ, Hill J, Meredith I, Ali Z, Maehara A, Illindala U, Gotberg M, Van Miegham N, Whitbourn R, Fajadet J, Di Mario C. Coronary Lithoplasty in Calcifi ed Coronary Stenoses: the DISRUPT CAD study. Presented at TCT 2016.
46. Zimmermann FM, Ferrara A, Johnson NP, van Nunen LX, Escaned J, Albertsson P, Erbel R, Legrand V, Gwon HC, Remkes WS, Stella PR, van Schaardenburgh P, Bech GJ, De Bruyne B. Pijls NH. Deferral vs. performance of percutaneous coronary intervention of functionally non-signifi cant coronary stenosis: 15-year follow-up of the DEFER trial. Eur Heart J 2015;35:3182–3188.
47. Park SH, Jeon KH, Lee JM, Nam CW, Doh JH, Lee BK, Rha SW, Yoo KD, Jung KT, Cho YS, Lee HY, Youn TJ, Chung WY, Koo BK. Long-term clinical outcomes of fractional fl ow reserve-guided versus routine drug-eluting stent implantation in patients with intermediate coronary stenosis: fi ve-year clinical outcomes of DEFER-DES Trial. Circ Cardiovasc Interv 2015;8:e002442.
48. Rioufol G, Mewton N, Rabilloud M, et al. The functional testing underlying coronary revascularization (FUTURE) study: a “real world” comparison of fractional fl ow reserve-guided management vs conventional management in multi vessel coronary artery disease patients. Presented at: the 2016 American Heart Association Scientific Sessions (November 12–16, 2016). New Orleans, LA.
49. Meneveau N, Souteyrand G, Motreff P, Caussin C, Amabile N, Ohlmann P, Morel O, Lefranc¸ois Y, Descotes-Genon V, Silvain J, Braik N, Chopard R, Chatot M, Ecarnot F, Tauzin H, Van Belle E, Belle L, Schiele F. Optical coherence tomography to optimize results of percutaneous coronary intervention in patients with non-st-elevation acute coronary syndrome: results of the multicenter, randomized doctors study (Does optical coherence tomography optimize results of stenting). Circulation 2016;134:906–917.
50. Tonino PA, De Bruyne B, Pijls NH, Siebert U, Ikeno F, Van’ T, Veer M, Klauss V, Manoharan G, Engstrøm T, Oldroyd KG, Ver Lee PN, MacCarthy PA, Fearon WF; FAME Study Investigators. Fractional flow reserve versus angiography for guiding percutaneous coronary intervention. N Engl J Med 2009;360:213–224.
51. Maehara A, Ben-Yehuda O, Ali Z, Wijns W, Bezerra HG, Shite J, Genereux P, Nichols M, Jenkins P, Witzenbichler B, Mintz GS, Stone GW Comparison of stent expansion guided by optical coherence tomography versus intravascular ultrasound: the ILUMIEN II study (Observational study of optical coherence tomography [OCT] in patients undergoing fractional flow reserve [FFR] and percutaneous coronary intervention). J Am Coll Cardiol Cardiovasc Interv 2015;8:1704– 1714.
52. Ali ZA, Maehara A, Genereux P, Shlofmitz RA, Fabbiocchi F, Nazif TM,Guagliumi G, Meraj PM, Alfonso F, Samady H, Akasaka T, Carlson EB, Leesar MA, Matsumura M, Ozan MO, Mintz GS, Ben-Yehuda O, Stone GW, ILUMIEN III: OPTIMIZE PCI Investigators. Optical coherence tomography compared with intravascular ultrasound and with angiography to guide coronary stent implantation (ILUMIEN III: OPTIMIZE PCI): a randomized controlled trial. Lancet 2016;388:2618– 2628.
53. Comparison of Optical Frequency Domain Imaging vs Intravascular Ultrasound in Percutaneous Coronary Intervention: An imaging study from a multicenter randomized, open-label, non inferiority OPINION trial. Presented at TCT 2016 by Otake.
54. Souteyrand G, Amabile N, Mangin L, Chabin X, Meneveau N, Cayla G, Vanzetto G, Barnay P, Trouillet C, Rioufol G, Range G, Teiger E, Delaunay R, Dubreuil O, Lhermusier T, Mulliez A, Levesque S, Belle L, Caussin C, Motreff P. Mechanism of stent thrombosis analysed by optcal coherence tomography: insights from the national PESTO French registry. Eur Heart J 2016;37:1208–1216.
55. Taniwaki M, Radu MD, Zaugg S, Amabile N, Garcia-Garcia HM, Yamaji K, Jorgensen E, Kelbaek H, Pilgrim T, Caussin C, Zachin T, Veugeois A, Abildgaard U, Juni P, Cook S, Koskinas KC, Windecker S, Raber L. Mechanism of very late drug-eluting stent thrombosis assessed by optical coherence tomography. Circulation 2016;133:650–660.
56. Motovska Z, Hlinomaz O, Miklik R, Hromadka M, Varvarovsky I, Dusek J, Knot J, Jarkovsky J, Kala P, Rokyta R, Tousek F, Kramarikova P, Majtan B, Simek S, Branny M, Mrozek J, Cervinka P, Ostransky J, Widimsky P. Prasugrel versus ticagrelor in patients with acute myocardial infarction treated with primary percutaneous coronary intervention: multicenter randomized PRAGUE-18 Study. Circulation 2016;134:1603–1612.
57. Kubica J, Adamski P, Ostrowska M, Sikora J, Kubica JM, Sroka WD, Stankowska K, Buszko K, Navarese EP, Jilma B, Siller-Matula JM, Marszałł MP, Rosc D, Kozinski M. Morphine delays and attenuates ticagrelor exposure and action in patients with myocardial infarction: the randomized, double-blind, placebo-controlled IMPRESSION trial. Eur Heart J 2016;37:245–252.
58. Hochholzer W, Amann M, Titov A, Younas I, Löffelhardt N, Riede F, Potocnik C, Stratz C, Hauschke D, Trenk D, Neumann FJ, Valina CM. Randomized comparison of different thienopyridine loading strategies in patients undergoing elective coronary intervention: the Excelsior LOAD trial. J Am Coll Cardiol Cardiovasc Interv 2016;9:219–227.
59. Abtan J, Steg PG, Stone GW, Mahaffey KW, Gibson CM, Hamm CW, Price MJ, Abnousi F, Prats J, Deliargyris EN, White HD, Harrington RA, Bhatt DL, on behalf of the CHAMPION PHOENIX Investigator. Effi cacy and safety of cangrelor in preventing periprocedural complications in patients with stable angina and acute coronary syndromes undergoing percutaneous coronary intervention. J Am Coll Cardiol Cardiov Interv 2016;9:1905–1913.
60. Bonaca MP, Bhatt DL, Cohen M, Steg PG, Storey RF, Jensen EC, Magnani G, Bansilal S, Fish MP, Im K, Bengtsson O, Oude Ophuis T, Budaj A, Theroux P, Ruda M, Hamm C, Goto S, Spinar J, Nicolau JC, Kiss RG, Murphy SA, Wiviott SD, Held P, Braunwald E, Sabatine MS; PEGASUSTIMI54 Steering Committee and Investigators. Long-term use of ticagrelor in patients with prior myocardial infarction. N Engl J Med 2015;372:1791–1800.
61. Yeh RW, Secemsky EA, Kereiakes DJ, Normand SL, Gershlick AH, Cohen DJ, Spertus JA, Steg PG, Cutlip DE, Rinaldi MJ, Camenzind E, Wijns W, Apruzzese PK, Song Y, Massaro JM, Mauri L; DAPT Study Investigators. Development and validation of a prediction rule for benefi t and harm of dual antiplatelet therapy beyond 1 year after percutaneous coronary intervention. JAMA 2016;315:1735–1749.
62. Helft G, Steg PG, Le Feuvre C, Georges JL, Carrie D, Dreyfus X, Furber A, Leclercq F, Eltchaninoff H, Falquier JF, Henry P, Cattan S, Sebagh L, Michel PL, Tuambilangana A, Hammoudi N, Boccara F, Cayla G, Douard H, Diallo A, Berman E, Komajda M, Metzger JP, Vicaut E; OPTImal DUAL Antiplatelet Therapy Trial Investigators. Stopping or continuing clopidogrel 12 months after drug-eluting stent placement: the OPTIDUAL randomized trial. Eur Heart J 2016;37:365–374.
63. Gibson CM, Mehran R, Bode C, Halperin J, Verheugt FW, Wildgoose P, Birmingham M, Ianus J, Burton P, van Eickels M, Korjian S, Daaboul Y, Lip GY, Cohen M, Husted S, Peterson ED, Fox KA. Prevention of bleeding in patients with atrial fi brillation undergoing PCI. N Engl J Med 2016;375:2423–2434.
64. Nicholls SJ, Puri R, Anderson T, Ballantyne CM, Cho L, Kastelein JJ, Koenig W, Somaratne R, Kassahun H, Yang J, Wasserman SM, Scott R, Ungi I, Podolec J, Ophuis AO, Cornel JH, Borgman M, Brennan DM, Nissen SE. Effect of evolocumab on progression of coronary disease in statin-treated patients: the GLAGOV randomized clinical trial. JAMA 2016;316:2373–2384.
 This work is licensed under a
This work is licensed under a