Riccardo Cappato1,2*, Gerhard Hindricks3, Jan Steffel4
1 Arrhythmia and Electrophysiology Research Center, IRCCS Humanitas Research Center, Milan, Italy
2 Arrhythmia and Electrophysiology II Center, Humanitas Gavazzeni Clinics, Bergamo, Italy
3 University Heart Center Leipzig, Leipzig, Germany
4 University Hospital Zurich, Zurich, Switzerland
PREAMBLE
The year 2016 was characterized by numerous relevant contributions in cardiac arrhythmias. A selected group of articles providing information with potential impact in daily practice has been identified by the authors and is reported in the present article.
CARDIAC ARRHYTHMIAS AND CATHETER ABLATION
Supraventricular tachycardia: diagnosis and treatment
Supraventricular tachycardia (SVT) continues to be a frequent cause of emergency hospital admission. The REVERT study evaluated the best and most efficient acute treatment strategy for SVT and compared postural modification (leg elevation and supine positioning applied for 15 sec at the end of 15 sec) with standardized strain Valsalva manoeuvre (i.e. pressure of 40 mm Hg sustained for 15 s by forced expiration measured by aneroid manometer with the target pressure visible to the treating team).1 T he modified treatment was found to terminate SVTs in a significantly larger proportion of patients (43% of 214) than using conventional manoeuvres (17% of 214; P < 0.0001). As a consequence, significantly less patients in the study arm required adenosine (50% vs. 69%) or emergency anti-arrhythmic treatment (57% vs. 80%) to terminate the incident arrhythmia1 and no differences in time to discharge from hospital. This finding may considerably affect daily practice and reduce drug-related patient discomfort at time of emergency treatment in patients with SVT. The full scope of up-to-date diagnosis and treatment of SVTs can be reviewed best in 2016 EHRA/ESC consensus document on SVT management (Katritsis et al., EHJ 2016 in press)
Atrial fibrillation: pathophysiology, risks, treatment opportunities, and the new ESC AF guidelines
The intense scientific discussion about the pathophysiology of atrial fibrillation and particularly the drivers for AF progression was enriched and stimulated by a very interesting experimental and clinical study on atrial remodeling.2 It was shown that atrial adipose tissue, which has been previously identifi ed as a strong risk factor for AF development, is progressively replaced by fibrotic tissue that serves as the substrate for AF progression.2 These data may further explain the link between obesity and AF recently described in clinical studies.3 However, those studies also showed a significant reduction of AF burden with weight loss and it is now of particular interest whether or not the reduction in AF burden may coincide with reversed structural re-modelling—and vice versa (Figure 1). MRI-based fibrosis detection and quantification holds some promise to document the substrate changes over time and may give further insights into this important aspect of AF pathology in the future (Figure 2).4 However, various methodological hurdles need to yet be overcome, mainly due to the thin wall of the atria, and appropriate protocols are indispensable. Rate control is the most frequent treatment options chosen for and by AF patients world-wide. Data about the best medication to support rate control therapy by symptom relief and reducing AF-related risks are limited and somewhat controversial.5 A recent nationwide study from Taiwan investigated the long-term effects of beta-blockers, calcium-channel blockers or digitalis given for the rate control in ongoing atrial fibrillation on mortality.6 After adjustment for baseline differences, the risk of mortality was found to be significantly lower in the 43 879 patients receiving betablockers and in the 18 466 patients receiving calciumchannel blockers than in a control population of 168 678 patients not receiving any rate-control drug. On the contrary, patients receiving digoxin had a higher risk of mortality. Especially the effects observed for beta-blockers are interesting as a recent metaanalysis on rate control medications did not show such beneficial effects for beta-blockers.7 These findings contribute to the ongoing controversy about the impact of rate-control drugs on the risk of all-cause death in patients with ongoing AF and prompt for the need of future randomized trials to address this relevant question. Patients with aortic stenosis often also have preexisting AF which may be ‘silent’ or develop AF (socalled ‘new-onset AF’) early after surgical or transfemoral aortic valve replacement (TAVI). Indeed, when compared with patients in sinus rhythm, patients with AF undergoing surgical or TAVI interventions have been shown to be at higher risk for stroke and bleeding but also for having a higher total mortality.8 A recent clinical update on this topic pointed out that the incidence of new-onset AF may be lower with TAVI as compared with surgical valve replacement.8 However, the optimal treatment strategy of such patients with respect to rhythm or rate control is still unclear. Particularly the role of amiodarone both for the periprocedural prevention of AF and for classical rhythm control as well as the role of catheter ablation as a rhythm control strategy needs further evaluation in clinical studies and trials. Another field of controversy relates to the optimal anticoagulation regimen especially for TAVI patients: are AF patients after TAVI eligible for NOAC therapy or are vitamin K antagonists the better choice? While there are good arguments in favour of NOACs after TAVI convincing data from specific and large clinical trials are still lacking to answer this important question.9 Catheter ablation of paroxysmal AF: burn it down or freeze it? The comparative effect of catheter-based point-by-point radiofrequency ablation and balloonbased cryo-ablation for the treatment of paroxysmal AF was unknown and had been intensely debate over years. We now know that both ablation techniques result in the same rhythm outcome and have similar complication rates.10 In the FIRE AND ICE international, multicentre, clinical trial 762 patients with paroxysmal AF were randomly assigned to undergo pulmonary vein isolation with RF-ablation or cryo energy. During 1.5 years of follow-up, no differences were found between the two groups in the incidence of post-ablation clinical failure (i.e. recurrence of AF, occurrence of atrial fl utter or atrial tachycardia, use of anti-arrhythmic drugs, or repeat ablation): 34.6% in the cryoballoon arm and 35.9% in the RF arm. The two techniques also proved similarly safe, with an aggregate incidence of death, cerebrovascular events, or serious treatment-related adverse events of 10.2% and 12.8%, respectively (P = ns). This relatively high incidence of side effects is in line with previous data of prospectively investigated populations. Quality-oflife assessment post-ablation did not differ between the two study arms. In a subsequent study, the same authors reported a lower incidence of repeat ablations, direct-current cardioversions, and all-cause rehospitalization during follow-up in the cryo-balloon study arm.11 Similarly, a non-inferiority of cryoballoon-assisted vs. RF-assisted ablation was also documented in the Freeze AF study which randomized 315 patients with paroxysmal AF.12 The results of these two studies, which are characterized by a limited adoption in the RF arm of the most recently introduced technologies, will contribute to establish cryoballoon-assisted ablation as a valuable alternative to RFassisted ablation of paroxysmal AF. However, it still needs to be evaluated whether substrate-based ablation strategies in patients with paroxysmal AF and low-voltage areas may add benefits with respect to rhythm outcome after RF-based ablation techniques.13 In patients with persistent AF, the efficacy of catheterbased PVI using RF current was comparatively assessed with that of PVI plus linear ablation and that of PVI plus complex fractionated atrial electrogram (CFAE) ablation in the STAR AF II study.14 In the 589 study patients randomly assigned to the three study arms according to a 1:4:4 randomization ratio, no differences were found in the proportion of patients who were free from recurrent AF after 18-month followup (59%, 49%, 46%). These results diverge with those reported in a recent meta-analysis15 on limited series showing a 51% relative risk reduction in the incidence of recurrent AF in patients receiving linear ablation in addition to PVI when compared with patients receiving PVI only. The discrepancy of fi ndings between these two studies highlights the value of performing randomized studies in order to validate findings from previous studies using less rigorous methodology. Establishing on a large scale the role of a simpler procedure as the fi rst ablation step in patients with persistent AF may have relevant clinical implications with regard to patient safety. New studies are required to confirm the present findings, investigate new ablation designs and identify the best strategy in patients with persistent AF who failed the first one. The optimal antiarrhythmic management following ablation also still remains to be determined. In the Efficacy of Antiarrhythmic Drugs Short-Term Use After Catheter Ablation for Atrial Fibrillation trial, a total of 2038 patients were randomly assigned to antiarrhythmic drug therapy of control following radiofrequency catheter ablation for paroxysmal, persistent, or longlasting AF.16 The risk of recurrent atrial tachyarrhythmias was reduced in the antiarrhytmic drug therapy group during the treatment period of 3 months, however without an effect on clinical outcomes at later time points. Does catheter ablation of AF have any effect on stroke rate and/or mortality? In a recent nationwide Swedish Patient Register identifying 361 913 patients, Friberg et al. evaluated the possible influence of AF ablation on clinical outcome.17 Using propensity score matching, two cohorts of equal size (2836 patients each) were extracted of which one had received AF ablation and one not. The two cohorts presented similar characteristics in 51 dimensions. After adjustment for known confounders AF ablation was found to be associated with a significantly lower incidence of all-cause mortality (HR = 0.50; 95% CI = 0.37–0.62) and ischemic stroke (HR = 0.69; 95% CI = 0.51–0.93).Reduction in the risk of ischemic stroke by means of AF ablation was most pronounced in sub-groups with CHA2DS2VASc score ≥ 2 (HR = 0.39; 95% CI = 0.19– 0.78) and among patients without a new cardioversion beyond 6 months after ablation (HR = 0.68; 95% CI = 0.48–0.97). These results are encouraging and prompt for the implementation that adequately sized randomized studies may provide to this controversial topic in the next future. Until those trials have arrived and fully reported clinical practice should include continuing life-long anticoagulation after ablation in at risk patients according to the CHADS-VASc Score—a point of view which is strongly supported by the 2016 ESC AF management guidelines.5 The new AF guidelines strengthen a personalized, precision driven approach to patients with atrial fibrillation. Importantly, the role of new AF risk factors and the importance of life style changes for reduction of AF burden and potentially for reduction of AF related risks is intensely described. Moreover, the benefits resulting from integrated AF care, AF heart teams and patient engagement for shared decision-making are presented and specific action is recommended to deliver the best care for AF patients.5
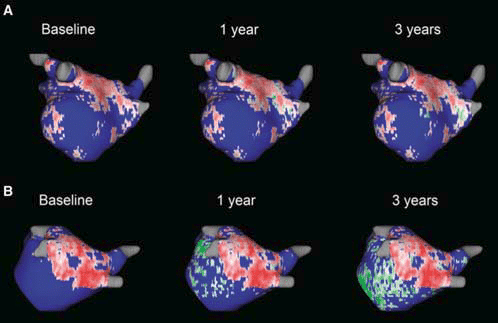
Figure 1. Different dynamics of scar progression with progressive fibrosis over a time period of 3 years in the years after atrial fibrillation ablation. Panel (A) depicts a patient with little to no increase in cardiac fibrosis while panel (B) depicts a patient with massive increase in cardiac fibrosis at 1 year and 3 years (green colour) coinciding with multiple AF recurrences. Reproduced with permission from Gal and Marrouche.4 This Figure has been reprinted by permission of Oxford University Press on behalf of the European Society of Cardiology.
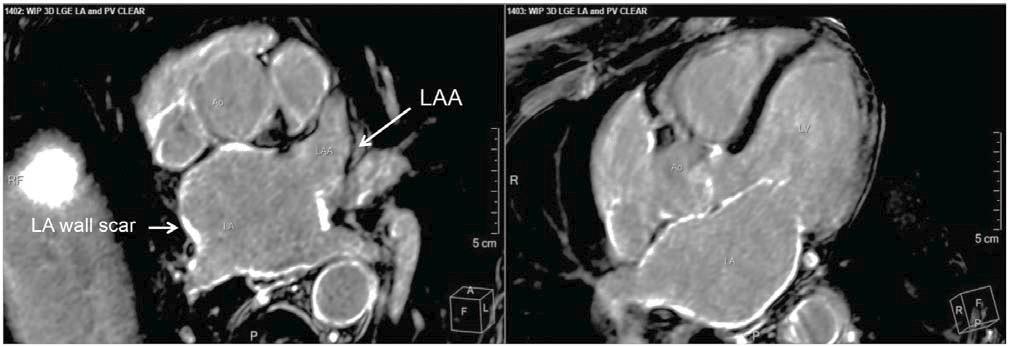
Figure 2. MRI-based imaging of atrial wall fibrosis. MRI cross section at the level of the left atrium (left side) and 5-chamber view (right side). Atrial fibrosis can be detected in various regions of the atrial wall (white spots). Only the left atrial appendage (LAA) is largely free of scar. This Figure has been reprinted by permission of Oxford University Press on behalf of the European Society of Cardiology.
Stroke prevention
An interesting finding referred to as the ‘obesity paradox’ was recently reported in a sub-analysis from the ARISTOTLE trial.18 Out of 17 913 patients enrolled in this study, 7159 were categorized as obese, 6702 overweight and 4052 normal. During 1.8 years followup, higher body masses were associated with a lower risk of all-cause mortality (overweight, HR = 0.67; 95% CI = 0.59–0.78; obese, HR = 0.63; 95% CI = 0.54– 0.74). Such benefit extended to the risk of stroke in the female (P = 0.048), but not in the male gender. No measure of adiposity was associated with a different risk of bleeding. Among possible explanations for this finding are an earlier more rigorous use of co-medications and life-style modification19 and better metabolic reserve,20 which may ultimately affect intermediateterm prognosis in obese patients. Another interesting fi nding was observed in a recent subanalysis from the Engage AF-TIMI 48 trial.21 In this study, a higher degree of protection from all-cause mortality vs. vitamin K antagonist (VKA) therapy was found in the edoxaban 30 mg arm (HR = 0.87; 95% CI = 0.79–0.96, P = 0.006) than in the The year in cardiology 2016: arrhythmias and cardiac implantable electronic devices edoxaban 60 mg arm (HR = 0.92; 95% CI = 0.83–1.01, P = 0.08). This benefi t occurred in spite of an evident increased risk of ischemic stroke (HR = 1.41; 95% CI = 1.19–1.67, P < 0.001) at the lower edoxaban dose, which was not found at the higher dose (HR = 1.00; 95% CI = 0.83–1.19, P = 0.97). The fewer total deaths observed with edoxaban were predominantly due to a signifi cantly lower rate of fatal bleeding in the edoxaban groups and particularly in the low dose group. These fi ndings raise our attention on the delicate balance between risk and benefi t associated with administration or oral anticoagulants and shift the objective of their use from thromboembolic events to cardiovascular morbidity as a whole. In addition, further subgroup analyses were able to demonstrate a consistent net clinical effi cacy and safety of edoxaban in other high risk subgroups such as the elderly22 and patients at increased risk of falls,23 hence establishing the drug as a valuable alternative in our armamentarium for stroke prevention in AF. A recent randomized controlled study (Ensure AF)24 showed that oral edoxaban 60 mg once daily presented similar effi cacy and safety outcomes as VKAs when administered during the peri-procedural phase on cardioversion of atrial fi brillation. In the 30 days following cardioversion using either an early or delayed strategy, 1095 patients assigned to edoxaban presented a 0.5% incidence of aggregate stroke, myocardial infarction, peripheral embolism or cardiovascular death vs. a 1.0% observed in 1104 patients assigned to VKA therapy (OR 0.46; 95% CI = 0.12–1.43). Similarly low incidences of periprocedural major bleeding (0.3% and 0.5%) were observed in the two arms (OR 0.61; 95% CI = 0.09–3.13). These results are similar to those recently reported by Cappato et al. in the XVeRT trial investigating oral rivaroxaban vs. VKA therapy in the same clinical setting.25 Both trials were not numerous enough to test a non-inferiority hypothesis. However, the high reproducibility of primary effi cacy and safety outcomes in the two studies make these NOACs a valuable alternative to VKAs in these patients. After the authorization for market release of three of the four novel oral anti-coagulants (NOACs) previously investigated in large phase III trials, a number of post-authorization studies have been published providing real-life evidence for effi cacy and safety of these new drugs. In a previous registry investigating the real-life effi cacy and safety of rivaroxaban, Camm et al. had shown that during about 1-year follow-up, the incidences of major bleeding (2.1 per 100 patientyears) and stroke events (0.7 per 100 patient-years) were low and superimposable to those observed in Rocket AF.26,27 Most recently, the results from three studies using claims database as data source were reported. 28-30 In the REVISIT-US registry,28 a measure of net clinical benefi t was inferred by the aggregate estimate of ischemic stroke and intracranial haemorrhage reported in the investigated populations. Real life treatment with rivaroxaban and apixaban was associated with a 39% (HR 0.61; 95% CI = 0.45–0.82) and a 37% (HR 0.63; 95% CI = 0.35–1.12) risk reduction in the aggregate incidence of ischemic stroke and intracranial haemorrhage as calculated in 22 822 patients and in 8166 patients, respectively. More recently, results showing a similar benefi t of dabigatran vs. VKA were presented by the same authors. Another real world analysis performed a propensitymatched analysis comparing apixaban (15 390 patient), dabigatran (28 614 patients), and rivaroxaban (32 350 patients) each with warfarin in OptumLabs Data Warehouse (OLDW).29 They found a similar risk for ischemic stroke for dabigatran vs. warfarin (HR 0.98, 95% CI 0.76–1.26, P = 0.98) and for rivaroxaban vs. warfarin (HR 0.93, 95% CI 0.72–1.19, P = 0.56), and a lower risk for apixaban vs. warfarin (HR 0.67, 95% CI 0.46–0.98, P = 0.04). The risk of major bleeding was similar for rivaroxaban vs. warfarin (HR 1.04, 95% CI 0.90–1.20], P = 0.60), and lower for dabigatran vs. warfarin (HR 0.79, 95% CI 0.67–0.94, P < 0.01) as well as apixaban vs. warfarin (HR 0.45, 95% CI 0.34–0.59, P < 0.001). Finally, a very recent FDA analysis in 52 240 dabigatranand 66 651 rivaroxaban-treated elderly (≥ 65 years). Medicare patients revealed a trend for lower risk of thromboembolic stroke with rivaroxaban compared with dabigatran (HR, 0.81; 95% CI, 0.65–1.01; P = 0.07).30 At the same time, however, intracranial haemorrhage (HR, 1.65; 95% CI, 1.20–2.26; P = 0.002) as well as major extracranial bleeding (HR, 1.48; 95% CI, 1.32–1.67; P < 0.001) were increased with rivaroxaban compared with dabigatran, with a trend towards an increased all-cause mortality (HR, 1.15; 95% CI, 1.00–1.32; P = 0.051). While comparisons between large phase III study and postauthorization outcome measures are recommended, statistics on ‘head-to-head’ comparison among NOACs should clearly be discouraged. The available evidence, in fact, demonstrates that any ‘real world’ analysis equally comes with a number of possible limitations, including residual confounding, short follow-up, selected patient populations, inconsistency of outcome measures (i.e. major bleeding defi nition), lack of external adjudication, and incomplete follow-up, hence limiting the generalizability of such comparative data. The primary—and likely the only— conclusion that can be drawn from data from postauthorization studies is that their fi ndings are consistent with the safety and effi cacy of NOACs observed in the large-scale randomized clinical trials after their adoption in daily practice by large segments of the medical community across the world. As such, the current 2016 guidelines for the management of atrial fi brillation recommend the use of NOACs as fi rst line therapy in patients who newly start anticoagulation treatment for AF, with a Class I recommendation, level of evidence A.5 In contrast, the use of aspirin newly received a class III recommendation (possible harm) given its limited efficacy and frequently underestimated bleeding risk.
Ventricular arrhythmias and sudden cardiac death
Catheter ablation of ventricular tachycardia (VT) is an important technique to manage patients with recurrent VT (Figure 3).31 However, randomized clinical trials evaluating the potential benefi ts of catheter ablation as compared with antiarrhythmic drug therapy are scarce. The recently published VANISH trial randomized patients with drug refractory ventricular tachycardia in the setting of ischemic cardiomyopathy and defi brillator protection to VT catheter ablation with continuation of baseline antiarrhythmic medications vs. escalated antiarrhythmic drug therapy.32 In the latter group, amiodarone was initiated if another drug had been used previously. The dose of amiodarone was increased up to 300 mg/day and mexiletine was added thereafter, if clinically required. During 27-month follow-up, signifi cantly more deaths, VT storm events or appropriate ICD shocks were reported in the 127 patients assigned to the escalated therapy arm than in the 132 patients assigned to the ablation arm (69% vs. 59%; HR = 0.72; 95% CI = 0.53–0.98). However, although such benefi cial effects on VT recurrence could be observed in the ablation group there was no difference in overall survival indicating that additional factors such as progression of structural heart disease and progressive heart failure may also play an important role for the prognosis of these patients. Recurrent ventricular tachycardia in patients with repaired tetralogy of Fallot is a significant risk factor for sudden cardiac death. Treatment with catheter ablation is diffi cult due to the complex anatomy after surgical repair. However, as recently shown detailed electroanatomical reconstruction and mapping of the conduction properties in the operated areas effectively identifi es critical conduction isthmus that promotes VT.33 In one of the largest patient series of Fallot patients with VT reported so far it could be shown that discrete ablation of the isthmus results in VT termination and rendered VT noninducible in the majority of patients. In patients with effective ablation VT recurrence was very low proving the benefi ts of this approach. In a recent study, Kudenchuck et al. compared parenteral amiodarone, lidocaine, and saline placebo, along with standard of care, in adults with out of- hospital cardiac arrest, shock refractory ventricular fi brillation (VF) or pulseless VT after at least one shock.34 Of 3026 enrolled patients, 974 were assigned to amiodarone, 993 to lidocaine and 1059 to placebo. No differences in survival to hospital discharge (24%, 24% and 21%, respectively) or neurologic outcome were found among the three groups. Interestingly, active drug administration was associated with a higher survival rate among patients with by-stander witnessed cardiac arrest (P = 0.05), but not among those with unwitnessed cardiac arrest. These fi ndings offer a serious argument against the administration of intravenous antiarrhythmic drugs in unwitnessed out of- hospital cardiac arrest victims, but leave the door open for their possible use in by-stander witnessed victims.
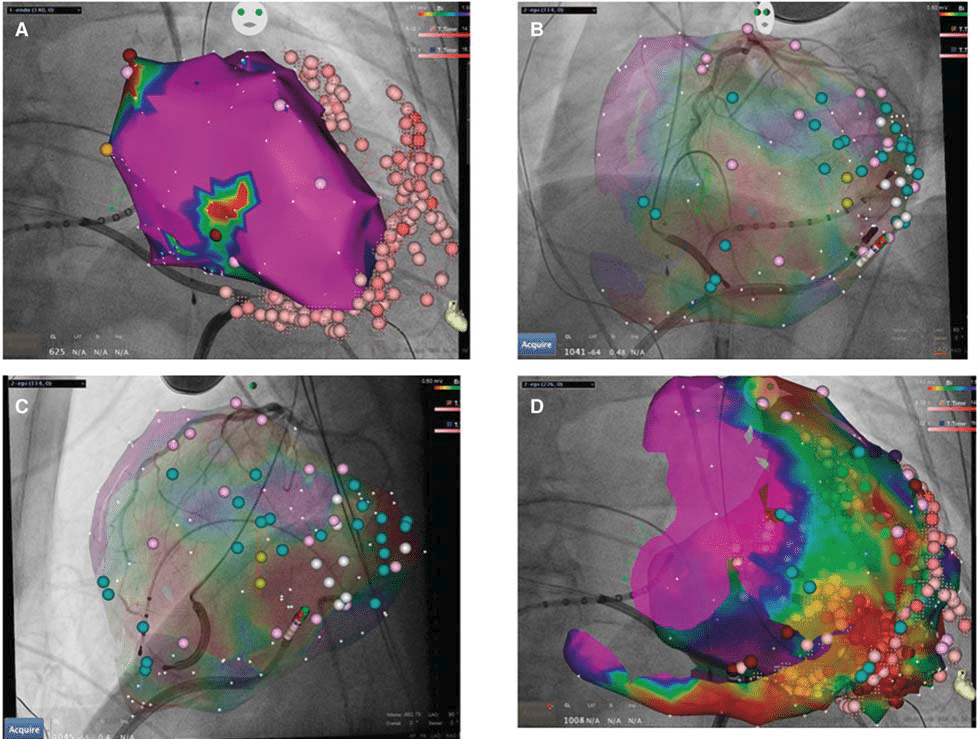
Figure 3. Endo- and epicardial VT ablation. Three-dimensional mapping of the left ventricular endocardial surface (A) as well as the epicardium (B–D), with superimposed coronary angiograms to detect the epicardially located coronary arteries. While only a small area of low-voltage is detectable on the endocardium (A, red area), the epicardial surface shows extensive fibrosis (D). Pink points denote sites of ablation. This Figure has been reprinted by permission of Oxford University Press on behalf of the European Society of Cardiology.
CARDIAC ELECTRONIC DEVICES
Implantable defibrillator therapy
Who benefits from an ICD and who does not? The final jury is not out on this ever moving target. In a study of 1116 patients with symptomatic systolic heart failure not caused by coronary artery disease (the DANISH trial), Kober et al recently showed that implantable cardioverter defi brillator (ICD) therapy in addition to usual care did not confer a significant protection from all-cause mortality as compared with usual care only during long-term follow-up (68 months).35 In this study, the 50% (highly significant) relative reduction of sudden death risk in patients assigned to an ICD was offset by a larger proportion of patients in this same group presenting with deaths caused by other cardiovascular causes and, above all, by non-cardiovascular death. All time-to-event curves tended to diverge in favour of the ICD population during the fi rst 5 years of follow-up and then to converge. These results contribute signifi cantly to the ongoing debate on the usefulness of ICD therapy for the primary prophylaxis of patients with non-ischemic cardiomyopathy.36,37 The relatively old age at entry (64 years) and the long duration from time to diagnosis of heart failure to enrolment (19 years) make the investigated population of this study a highly selected one and one with a relatively low life-expectancy (ejection fraction at entry, 0.25). Indeed, a subgroup analysis focusing on patient age revealed a signifi cant statistical interaction, with younger patients (< 59 years old) deriving a benefi t from ICD in terms of all-cause mortality which was not evident in the elderly patients. In addition, the variety of reported cardiomyopathies makes the investigated population rather heterogeneous. Further studies are needed to evaluate the protective efficacy of ICD therapy in patients in whom a non-ischemic dilated cardiomyopathy is diagnosed at a younger age and whose eligibility for primary prophylaxis is raised at a relatively short time interval from diagnosis of heart failure. Previous studies investigating such a population were not numerous enough to test a superiority hypothesis by the ICD system vs. pharmacological therapy only.38 In summary, the results of the DANISH trial are important, but in the end reinforce clinical practice which should ideally already be in place—i.e. to take into consideration competing risk and modes of death, particularly non-cardiovascular as well as pump failure, when deciding to opt for ICD implantation, especially in patients with nonischemic heart disease. These results are in line with a recent large prospective, multicentre registry of patients with cardiac resynchronization therapy (CRT). In a total of 1705 consecutive patients implanted with either a CRT-P (535 patients) or CRT-D (1170 patients), the adjusted morality hazard 1.54 in CRT-P vs. CRT-D (CI 1.07– 2.21, P = 0.0209).39 However, 95% of the excess mortality in CRT-P recipients was due to an increase in non-sudden cardiac death, hence re-iterating the importance of an individualized ‘competing risk’ analysis prior to the right device for each patient. In a recent report, Vehmemeijer et al. performed a comprehensive review and meta-analysis on the indications, effi cacy and safety of ICD therapy in adults with congenital heart disease.40 Overall, 2162 patients (66% males) with a mean age of 37 years at implant were included from 24 studies. The devices were implanted for primary prevention in 53% of patients (95% CI = 43.5–62.7%), with non-sustained VT representing the most frequent indication, followed by impaired LV function, inducible VT, syncope, and palpitations or presyncope. The most frequent substrate was tetralogy of Fallot, followed by transposition of great arteries, congenitally corrected transposition of great arteries, ventricular or atrial septal defects and others. During 3.6-year follow-up, 24% of patients received an appropriate and 22% an inappropriate ICD intervention, inclusive of shock and/or anti-tachycardia pacing. All-cause mortality occurred in 10% of patients. These data offer the rationale for a thoughtful decision process concerning the relatively high rate of complications and inappropriate ICD therapy in these patients.
Subcutaneous implantable cardioverter defibrillators
In a recent study, Friedman et al. evaluated the trends and in-hospital outcomes associated with early adoption of the S-ICD in USA.41 Out of 393 734 ICD implants reported to the National Cardiovascular Data Registry ICD Registry between September 2012 (US Food and Drug Administration S-ICD approval date) and March 2015, the investigators performed a 1:1:1 propensity-matched analysis of 5760 patients to compare in-hospital outcomes among patients with S-ICD with those of patients with single chamber (SC)-ICD and dual chamber (DC)-ICD. The proportion of patients receiving an S-ICD among all ICD patients during the investigated period was 0.9%. Compared with SCICD and DC-ICD, patients receiving an S-ICD were younger, more prevalently female, black, undergoing dialysis and survivors of cardiac arrest. Interestingly, many patients presented with a high number of comorbidities. DFT testing resulted in a successful defibrillation in 99.7% of 2629 patients undergoing induction of ventricular arrhythmias at time of implant. In-hospital complication rates associated with an S-ICD were low (1.1%), similar to those associated with a SC-ICD (1.0%), and lower than those associated with a DCICD (1.2, P < 0.001). These fi gures provide an initial perspective of the impact of S-ICD in daily practice and offer an encouraging view on their safety at implant. Another, preliminary report on the use of a subcutaneous ICD in a limited population of young patients (mean age, 34 years) with congenital heart disease recently showed a 100% success rate of device implant, and a 100% conversion rate with ≤ 80J of induced arrhythmias.42 Randomized trials are required to confirm these results and evaluate the clinical impact of S-ICD during long-term follow-up.43 The still young technology of the S-ICD is at the same time evolving rapidly. A novel high pass fi lter (SmartPass, available for Gen 2 and Gen 2.5 of the EMBLEM S-ICD) was introduced this year designed to reduce the risk of T-wave oversensing in S-ICD patients (Theuns et al., presented at HRS 2016). Modelling of inappropriate shock episodes recorded in the large EFFORTLESS registry demonstrated a reduction in inappropriate shocks by 81% compared with the fi rst generation SICD. One of the (perceived) major limitations of current S-ICD systems is the lack of pacing capability, hence limiting its use in patients with known monomorphic VT or an indication for bradycardia pacing. This year it was demonstrated for the fi rst time in an animal model that communication of an S-ICD with a leadless cardiac pacemaker is possible, resulting in adequate termination of a monomorphic VT as well as in normal VVI functionality of the leadless pacer.44 These data are highly encouraging on the way to a further improvement of the current S-ICD system.
Leadless pacemaker
Leadless pacing has taken centre stage in the field of bradycardia pacing for the last years, and important new data surfaced during the year 2016. The primary results of the Micra experience in 725 patients, published in print early in the year,45 demonstrated favourable electrical values (threshold, sensing, impedance) in 292 of 297 patients with paired 6-month data. About 28 major complications occurred in 25 of 725 patients (4.0%), including 11 (1.9%) cardiac perforation or effusion and 1 death (0.1%). These positive results were reinforced by additional follow-up which were presented at Cardiostim, with an average follow-up duration of 7.7 ± 3.9 months. There was no signal apparent with very few additional clinical events; most importantly, no macro dislodgement and no embolization occurred. With now over 2000 Micra pacemakers implanted, the latter is also mirrored in the ‘real world’ outside the clinical trial, hence reinforcing particularly the safety of the device.
Wearable cardioverter defibrillators
Several studies have documented the efficacy and safety of wearable cardioverter defi brillators.46-48 In a large German registry, 94 patients (1.6%) were treated by the WCD due to ventricular tachyarrhythmias, an incidence of 8.4 (95% confi dence interval, 6.8–10.2) per 100 patient-years (German life vest Circulation 2016). About 112 of the 120 (93%) shocked patients survived 24 h after treatment, whereas asystole was observed in two patients (0.03%) with one resulting death. Taking together the available data, a recent science advisory from the American Heart Association,49 suggested a list of conditions for which this therapy may be recommended, which is in great parts similar to the ESC guidelines for the prevention of sudden cardiac death.50 Among them are the following circumstances: (i) as a bridging therapy in situations associated with risk of death in which ICDs have been shown to reduce sudden cardiac death but not overall mortality such as within 40 days of myocardial infarction; (ii) when there is a clear indication for an implantable device accompanied by a transient contraindication or need for interruption in ICD care such as infection; (iii) when there is concern about a heightened risk of sudden cardiac death that may resolve over time or with treatment of left ventricular dysfunction, e.g. in ischemic heart disease with recent revascularization, newly diagnosed non-ischemic dilated cardiomyopathy in a patient starting guideline-directed medical therapy, or secondary cardiomyopathy (tachycardia mediated and thyroid mediated) in which the underlying cause is potentially reversible; (iv) as a bridge to more defi nitive therapy such as cardiac transplantation. In light of the non-defi nitive nature of the studies conducted in this fi eld, the authors recognize that their document provides a tentative framework to assist in decision-making of an increasingly used therapy for the protection from sudden cardiac death during a transient clinical phase, but further studies are required to support these recommendations.
Conflict of interest: R.C. has acted as a consultant to Abbott, Bayer, Biosense Webster, Boehringer Ingelheim, Boston Scientifi c, Daiichi Sankyo, ELA Sorin, Medtronic, Pfi zer and St. Jude; participated in speakers’ bureaus for Abbot, BARD, Bayer, Biosense Webster, Boehringer Ingelheim, Boston Scientifi c, Medtronic, Sanofi and St. Jude; acted as a study investigator for Abbott, BARD, Bayer, Biosense Webster, Cameron Health, Medtronic, Pfi zer and Sanofi ; received grants from BARD, Biosense Webster, Boston Scientifi c, ELA Sorin, Medtronic, St. Jude; and holds equity and intellectual property rights in Cameron Health. G.H. Research grants from Biotronik, Boston Scientifi c and
St. Jude Medical through the University Leipzig/Heart Center. J.S. has received consultant and/or speaker fees from Amgen, Astra-Zeneca, Atricure, Bayer, Biosense Webster, Biotronik, Boehringer-Ingelheim, Boston Scientifi c, Bristol-Myers Squibb, Cook Medical, Daiichi Sankyo, Medtronic, Novartis, Pfi zer, Roche, Sanofi -Aventis, Sorin, St. Jude Medical and Zoll. J.S. is co-director of CorXL. He has received grant support through his institution from Bayer Healthcare, Biosense Webster, Biotronik, Boston Scientific, Daiichi Sankyo, Medtronic, und St. Jude Medical.
Drug and material disclaimer: The mention of trade names, commercial products organizations, and the inclusion of advertisements in the journal does not imply endorsement by the European Heart Journal, the editors, the editorial board, Oxford University Press or the organization to which the authors are affi liated. The editors and publishers have taken all reasonable precautions to verify drug names and doses, the results of experimental work and clinical fi ndings published in the journal. The ultimate responsibility for the use and dosage of drugs mentioned in the journal and in interpretation of published material lies with the medical practitioner, and the editors and publisher cannot accept liability for damages arising from any error or omissions in the journal. Please inform the editors of any errors. The opinions expressed in the European Heart Journal are those of the authors and contributors, and do not necessarily refl ect those of the European Society of Cardiology, the editors, the editorial board, Oxford University Press or the organization to which the authors are affi liated. OUP and the ESC are not responsible or in any way liable for the accuracy of the translation, for any errors, omissions or inaccuracies, or for any consequences arising therefore. Sandro Brusich, Ivica Premužić Meštrović and Hrvoje Vražić are solely responsible for the translation published in this reprint. Translation edited by: Mario Ivanuš a. Language editing: Tomislav Salopek.
References
1. Appelboam A, Reuben A, Mann C, Gagg J, Ewings P, Barton A, et al; REVERT trial collaborators. Postural modification to the standard Valsalva manoeuvre for emergency treatment of supraventricular tachycardias (REVERT): a randomised controlled trial. Lancet. 2015 Oct 31;386(10005):1747-53. https://doi.org/10.1016/S0140- 6736(15)61485-4
2. Haemers P, Hamdi H, Guedj K, Suffee N, Farahmand P, Popovic N, et al. Atrial fi brillation is associated with the fibrotic remodelling of adipose tissue in the subepicardium of human and sheep atria. Eur Heart J. 2017 Jan 1;38(1):53-61. https://doi.org/10.1093/eurheartj/ehv625
3. Nalliah CJ, Sanders P, Kottkamp H, Kalman JM. The role of obesity in atrial fi brillation. Eur Heart J. 2016 May 21;37(20):1565-72. https://doi.org/10.1093/eurheartj/ehv486
4. Gal P, Marrouche NF. Magnetic resonance imaging of atrial fi brosis: redefi ning atrial fi brillation to a syndrome. Eur Heart J. 2017 Jan 1;38(1):14-19. https://doi.org/10.1093/eurheartj/ehv514
5. Kirchhof P, Benussi S, Kotecha D, Ahlsson A, Atar D, Casadei B, et al. 2016 ESC Guidelines for the management of atrial fi brillation developed in collaboration with EACTS. Eur Heart J. 2016 Oct 7;37(38):2893-2962. https://doi.org/10.1093/eurheartj/ehw210
6. Chao TF, Liu CJ, Tuan TC, Chen SJ, Wang KL, Lin YJ, et al. Ratecontrol treatment and mortality in atrial fibrillation. Circulation. 2015 Oct 27;132(17):1604-12. https://doi.org/10.1161/CIRCULATIONAHA. 114.013709
7. Kotecha D, Holmes J, Krum H, Altman DG, Manzano L, Cleland JG, et al; Beta-Blockers in Heart Failure Collaborative Group. Efficacy of blockers in patients with heart failure plus atrial fi brillation: an individual-patient data meta-analysis. Lancet 2014;384:2235-2243. https://doi.org/10.1016/S0140-6736(14)61373-8
8. Tarantini G, Mojoli M, Urena M, Vahanian A. Atrial fi brillation in patients undergoing transcatheter aortic valve implantation: epidemiology, timing, predictors, and outcome. Eur Heart J. 2017 May 1;38(17):1285-1293. https://doi.org/10.1093/eurheartj/ehw456
9. Breithardt G, Baumgartner H. Valvular heart disease among nonvalvular atrial fi brillation: a misnomer, in search of a new term. Eur Heart J. 2015 Jul 21;36(28):1794-7. https://doi.org/10.1093/eurheartj/ehv193
10. Kuck KH, Brugada J, Fürnkranz A, Metzner A, Ouyang F, Chun KR, et al; FIRE AND ICE Investigators. Cryoballoon or Radiofrequency Ablation for Paroxysmal Atrial Fibrillation. N Engl J Med. 2016 Jun 9;374(23):2235-45. https://doi.org/10.1056/NEJMoa1602014
11. Kuck KH, Fürnkranz A, Chun KR, Metzner A, Ouyang F, Schlüter M, et al; FIRE AND ICE Investigators. Cryoballoon or radiofrequency ablation for symptomatic paroxysmal atrial fi brillation: reintervention, rehospitalization, and quality-of-life outcomes in the FIRE AND ICE trial. Eur Heart J. 2016 Oct 7;37(38):2858-2865. https://doi.org/10.1093/eurheartj/ehw285
12. Luik A, Radzewitz A, Kieser M, Walter M, Bramlage P, Hörmann P, et al. Cryoballoon Versus Open Irrigated Radiofrequency Ablation in Patients With Paroxysmal Atrial Fibrillation: The Prospective, Randomized, Controlled, Noninferiority FreezeAF Study. Circulation. 2015 Oct 6;132(14):1311-9. https://doi.org/10.1161/CIRCULATIONAHA.115.016871
13. Rolf S, Kircher S, Arya A, Eitel C, Sommer P, Richter S, et al. Tailored atrial substrate modifi cation based on low-voltage areas in catheter ablation of atrial fi brillation. Circ Arrhythm Electrophysiol. 2014 Oct;7(5):825-33. https://doi.org/10.1161/CIRCEP.113.001251
14. Verma A, Jiang CY, Betts TR, Chen J, Deisenhofer I, Mantovan R, et al; STAR AF II Investigators. Approaches to catheter ablation for persistent atrial fi brillation. N Engl J Med. 2015 May 7;372(19):1812- 22. https://doi.org/10.1056/NEJMoa1408288
15. Wynn GJ, Das M, Bonnett LJ, Panikker S, Wong T, Gupta D. Efficacy of catheter ablation for persistent atrial fibrillation: a systematic review and meta-analysis of evidence from randomized and nonrandomized controlled trials. Circ Arrhythm Electrophysiol. 2014 Oct;7(5):841-52. https://doi.org/10.1161/CIRCEP.114.001759
16. Kaitani K, Inoue K, Kobori A, Nakazawa Y, Ozawa T, Kurotobi T, et al; EAST-AF Trial Investigators. Effi cacy of Antiarrhythmic Drugs Short-Term Use After Catheter Ablation for Atrial Fibrillation (EAST-AF) trial. Eur Heart J. 2016 Feb 14;37(7):610-8. https://doi.org/10.1093/eurheartj/ehv501
17. Friberg L, Tabrizi F, Englund A. Catheter ablation for atrial fibrillation is associated with lower incidence of stroke and death: data from Swedish health registries. Eur Heart J. 2016 Aug;37(31):2478- 87. https://doi.org/10.1093/eurheartj/ehw087
18. Sandhu RK, Ezekowitz J, Andersson U, Alexander JH, Granger CB, Halvorsen S, et al. The ‘obesity paradox’ in atrial fi brillation: observations from the ARISTOTLE (Apixaban for Reduction in Stroke and Other Thromboembolic Events in Atrial Fibrillation) trial. Eur Heart J. 2016 Oct 7;37(38):2869-2878. https://doi.org/10.1093/eurheartj/ehw124
19. Wang J, Yang YM, Zhu J, Zhang H, Shao XH, Tian L, et al. Overweight is associated with improved survival and outcomes in patients with atrial fi brillation. Clin Res Cardiol. 2014 Jul;103(7):533- 42. https://doi.org/10.1007/s00392-014-0681-7
20. Nieuwlaat R, Prins MH, Le Heuzey JY, Vardas PE, Aliot E, Santini M, et al. Prognosis, disease progression, and treatment of atrial fi brillation patients during 1 year: follow-up of the Euro Heart Survey on atrial fi brillation. Eur Heart J. 2008 May;29(9):1181-9. https://doi.org/10.1093/eurheartj/ehn139
21. Giugliano RP, Ruff CT, Wiviott SD, Nordio F, Murphy SA, Kappelhof JA, et al. Mortality in Patients with Atrial Fibrillation Randomized to Edoxaban or Warfarin: Insights from the ENGAGE AF-TIMI 48 Trial. Am J Med. 2016 Aug;129(8):850-857.e2. https://doi.org/10.1016/j.amjmed.2016.02.028
22. Kato ET, Giugliano RP, Ruff CT, Koretsune Y, Yamashita T, Kiss RG, et al. Effi cacy and Safety of Edoxaban in Elderly Patients With Atrial Fibrillation in the ENGAGE AF-TIMI 48 Trial. J Am Heart Assoc. 2016;5:e003432. https://doi.org/10.1161/JAHA.116.003432
23. Steffel J, Giugliano RP, Braunwald E, Murphy SA, Mercuri M, Choi Y, et al. Edoxaban Versus Warfarin in Atrial Fibrillation Patients at Risk of Falling: ENGAGE AF-TIMI 48 Analysis. J Am Coll Cardiol. 2016 Sep 13;68(11):1169-78. https://doi.org/10.1016/j.jacc.2016.06.034
24. Goette A, Merino JL, Ezekowitz MD, Zamoryakhin D, Melino M, Jin J, et al; ENSURE-AF investigators. Edoxaban versus enoxaparinwarfarin in patients undergoing cardioversion of atrial fi brillation (ENSURE-AF): a randomised, open-label, phase 3b trial. Lancet. 2016 Oct 22;388(10055):1995-2003. https://doi.org/10.1016/S0140-6736(16)31474-X
25. Cappato R, Ezekowitz MD, Klein AL, Camm AJ, Ma CS, Le Heuzey JY, et al; X-VeRT Investigators. Rivaroxaban vs. vitamin K antagonists for cardioversion in atrial fi brillation. Eur Heart J. 2014 Dec 14;35(47):3346-55. https://doi.org/10.1093/eurheartj/ehu367
26. Camm AJ, Amarenco P, Haas S, Hess S, Kirchhof P, Kuhls S, et al; XANTUS Investigators. XANTUS: a real-world, prospective, observational study of patients treated with rivaroxaban for stroke prevention in atrial fi brillation. Eur Heart J. 2016 Apr 7;37(14):1145-53. https://doi.org/10.1093/eurheartj/ehv466
27. Patel MR, Mahaffey KW, Garg J, Pan G, Singer DE, Hacke W, et al; ROCKET AF Investigators. Rivaroxaban versus warfarin in nonvalvular atrial fi brillation. N Engl J Med. 2011 Sep 8;365(10):883-91. https://doi.org/10.1056/NEJMoa1009638
28. Coleman CI, Antz M, Bowrin K, Evers T, Simard EP, Bonnemeier H, et al. Real-world evidence of stroke prevention in patients with nonvalvular atrial fi brillation in the United States: the REVISIT-US study. Curr Med Res Opin. 2016 Dec;32(12):2047-2053. https://doi.org/10.1080/03007995.2016.1237937
29. Yao X, Abraham NS, Sangaralingham LR, Bellolio MF, McBane RD, Shah ND, et al. Effectiveness and Safety of Dabigatran, Rivaroxaban, and Apixaban Versus Warfarin in Nonvalvular Atrial Fibrillation. J Am Heart Assoc. 2016;5:e003725. https://doi.org/10.1161/JAHA.116.003725
30. Graham DJ, Reichman ME, Wernecke M, Hsueh YH, Izem R, Southworth MR, et al. Stroke, Bleeding, and Mortality Risks in Elderly Medicare Benefi ciaries Treated With Dabigatran or Rivaroxaban for Nonvalvular Atrial Fibrillation. JAMA Intern Med. 2016 Nov 1;176(11):1662-1671. https://doi.org/10.1001/jamainternmed.2016.5954
31. Chen J, Todd DM, Proclemer A, Sciaraffi a E, Estner HL, Broadhurst P, et al; Conducted by the Scientifi c Initiative Committee, European Heart Rhythm Association; Conducted by the Scientifi c Initiative Committee European Heart Rhythm Association. Management of patients with ventricular tachycardia in Europe: results of the European Heart Rhythm Association survey. Europace. 2015 Aug;17(8):1294-9. https://doi.org/10.1093/europace/euv255
32. Sapp JL, Wells GA, Parkash R, Stevenson WG, Blier L, Sarrazin JF, et al. Ventricular Tachycardia Ablation versus Escalation of Antiarrhythmic Drugs. N Engl J Med. 2016 Jul 14;375(2):111-21. https://doi.org/10.1056/NEJMoa1513614
33. Kapel GF, Sacher F, Dekkers OM, Watanabe M, Blom NA, Thambo JB, et al. Arrhythmogenic anatomical isthmuses identifi ed by electroanatomical mapping are the substrate for ventricular tachycardia in repaired Tetralogy of Fallot. Eur Heart J. 2017 Jan 21;38(4):268-276. https://doi.org/10.1093/eurheartj/ehw202
34. Kudenchuk PJ, Brown SP, Daya M, Rea T, Nichol G, Morrison LJ, et al; Resuscitation Outcomes Consortium Investigators. Amiodarone, Lidocaine, or Placebo in Out-of-Hospital Cardiac Arrest. N Engl J Med. 2016 May 5;374(18):1711-22. https://doi.org/10.1056/NEJMoa1514204
35. Køber L, Thune JJ, Nielsen JC, Haarbo J, Videbæk L, Korup E, et al; DANISH Investigators. Defi brillator Implantation in Patients with Nonischemic Systolic Heart Failure. N Engl J Med. 2016 Sep 29;375(13):1221-30. https://doi.org/10.1056/NEJMoa1608029
36. Bardy GH, Lee KL, Mark DB, Poole JE, Packer DL, Boineau R, et al; Sudden Cardiac Death in Heart Failure Trial (SCD-HeFT) Investigators. Amiodarone or an implantable cardioverterdefi brillator for congestive heart failure. N Engl J Med. 2005 Jan 20;352(3):225-37. https://doi.org/10.1056/NEJMoa043399
37. Desai AS, Fang JC, Maisel WH, Baughman KL. Implantable defibrillators for the prevention of mortality in patients with nonischemic cardiomyopathy: a meta-analysis of randomized controlled trials. JAMA. 2004 Dec 15;292(23):2874-9. https://doi.org/10.1001/jama.292.23.2874
38. Bänsch D, Antz M, Boczor S, Volkmer M, Tebbenjohanns J, Seidl K, et al. Primary prevention of sudden cardiac death in idiopathic dilated cardiomyopathy: the Cardiomyopathy Trial (CAT). Circulation. 2002 Mar 26;105(12):1453-8. https://doi.org/10.1161/01.CIR.0000012350.99718.AD
39. Marijon E, Leclercq C, Narayanan K, Boveda S, Klug D, Lacaze-Gadonneix J, et al; CeRtiTuDe Investigators. Causes-of-death analysis of patients with cardiac resynchronization therapy: an analysis of the CeRtiTuDe cohort study. Eur Heart J. 2015 Nov 1;36(41):2767-76. https://doi.org/10.1093/eurheartj/ehv455
40. Vehmeijer JT, Brouwer TF, Limpens J, Knops RE, Bouma BJ, Mulder BJ, et al. Implantable cardioverter-defi brillators in adults with congenital heart disease: a systematic review and meta-analysis. Eur Heart J. 2016 May 7;37(18):1439-48. https://doi.org/10.1093/eurheartj/ehv735
41. Friedman DJ, Parzynski CS, Varosy PD, Prutkin JM, Patton KK, Mithani A, et al. Trends and In-Hospital Outcomes Associated With Adoption of the Subcutaneous Implantable Cardioverter Defi brillator in the United States. JAMA Cardiol. 2016 Nov 1;1(8):900-911. https://doi.org/10.1001/jamacardio.2016.2782
42. Moore JP, Mondésert B, Lloyd MS, Cook SC, Zaidi AN, Pass RH, et al; Alliance for Adult Research in Congenital Cardiology (AARCC). Clinical Experience With the Subcutaneous Implantable Cardioverter- Defi brillator in Adults With Congenital Heart Disease. Circ Arrhythm Electrophysiol. 2016;9:e004338. https://doi.org/10.1161/CIRCEP.116.004338
43. Olde Nordkamp LR, Knops RE, Bardy GH, Blaauw Y, Boersma LV, Bos JS, et al. Rationale and design of the PRAETORIAN trial: a Prospective, RAndomizEd comparison of subcuTaneOus and tRansvenous ImplANtable cardioverter-defi brillator therapy. Am Heart J. 2012 May;163(5):753-760.e2. https://doi.org/10.1016/j.ahj.2012.02.012
44. Tjong FV, Brouwer TF, Kooiman KM, Smeding L, Koop B, Soltis B, et al. Communicating Antitachycardia Pacing-Enabled Leadless Pacemaker and Subcutaneous Implantable Defi brillator. J Am Coll Cardiol. 2016 Apr 19;67(15):1865-6. https://doi.org/10.1016/j.jacc.2016.02.039
45. Reynolds D, Duray GZ, Omar R, Soejima K, Neuzil P, Zhang S, et al P; Micra Transcatheter Pacing Study Group. A Leadless Intracardiac Transcatheter Pacing System. N Engl J Med. 2016 Feb 11;374(6):533- 41. https://doi.org/10.1056/NEJMoa1511643
46. Klein HU, Meltendorf U, Reek S, Smid J, Kuss S, Cygankiewicz I, et al. Bridging a temporary high risk of sudden arrhythmic death. Experience with the wearable cardioverter defi brillator (WCD). Pacing Clin Electrophysiol. 2010 Mar;33(3):353-67. https://doi.org/10.1111/j.1540-8159.2009.02590.x
47. Feldman AM, Klein H, Tchou P, Murali S, Hall WJ, Mancini D, et al; WEARIT investigators and coordinators; BIROAD investigators and coordinators. Use of a wearable defi brillator in terminating tachyarrhythmias in patients at high risk for sudden death: results of the WEARIT/BIROAD. Pacing Clin Electrophysiol. 2004 Jan;27(1):4-9.
https://doi.org/10.1111/j.1540-8159.2004.00378.x
48. Wäßnig NK, Günther M, Quick S, Pfl uecke C, Rottstädt F, Szymkiewicz SJ, et al. Experience With the Wearable Cardioverter-Defibrillator in Patients at High Risk for Sudden Cardiac Death. Circulation.
2016 Aug 30;134(9):635-43. https://doi.org/10.1161/CIRCULATIONAHA.115.019124
49. Piccini JP Sr, Allen LA, Kudenchuk PJ, Page RL, Patel MR, Turakhia MP; American Heart Association Electrocardiography and Arrhythmias Committee of the Council on Clinical Cardiology and Council on Cardiovascular and Stroke Nursing. Wearable Cardioverter- Defi brillator Therapy for the Prevention of Sudden Cardiac Death: A Science Advisory From the American Heart Association. Circulation. 2016 Apr 26;133(17):1715-27. https://doi.org/10.1161/CIR.0000000000000394
50. Priori SG, Blomström-Lundqvist C, Mazzanti A, Blom N, Borggrefe M, Camm J, et al. 2015 ESC Guidelines for the management of patients with ventricular arrhythmias and the prevention of sudden cardiac death: The Task Force for the Management of Patients with Ventricular Arrhythmias and the Prevention of Sudden Cardiac Death of the European Society of Cardiology (ESC). Endorsed by: Association for European Paediatric and Congenital Cardiology (AEPC). Eur Heart J. 2015 Nov 1;36(41):2793-867. https://doi.org/10.1093/eurheartj/ehv316.
 This work is licensed under a
This work is licensed under a