Cristian Mornos1,2, Simina Crisan1,2, Lucian Petrescu1,2, Adina Ionac1,2, Dragos Cozma1
1 „Victor Babes” University of Medicine and Pharmacy, Timisoara, Romania
2 Institute of Cardiovascular Diseases, Timisoara, Romania
Abstract: Recent data have demonstrated that a new tissue Doppler index, E/(E’×S’), index that includes the ratio between early diastolic transmitral and mitral annulus velocity (E/E’), as well as the systolic mitral annulus velocity (S’), has a good accuracy to predict cardiac death in patients with heart failure (HF). We analyzed the prognostic value of the E/(E’×S’) ratio in patients with heart failure with reduced ejection fraction (HFrEF). Methods – Echocardiographic examination was performed in 166 patients hospitalized with the diagnosis of heart failure with reduced ejection fraction, before discharge as well as one month later. Worsening of E/(E’×S’) was defi ned as a value greater than the previous value determined at discharge. The primary event consisted of cardiac death or hospital admission due to HF worsening. Results – During follow-up (34±8.8 months) cardiac events were recorded for 113 patients (68%). The optimal cut-off value for E/(E’×S’) ratio was 1.96 with 84% sensitivity and 80% specifi city. At discharge, 60 patients (36,2%) presented a value of the E/(E’×S’) index ≤1.96 (group I) and 106 patients (63.8%) encountered a value of the E/(E’×S’) index >1.96 (group II). The composite end point was signifi cantly higher in group II than in group I (95 events, 89.6% versus 18 events, 30%, p<0.001). Patients with an E/(E’×S’) ratio >1.96 at discharge and its worsening after one month presented the worst prognosis (p<0.05). Conclusions – In patients with with heart failure with reduced ejection fraction, a value of the E/(E’×S’) index >1.96 at hospital discharge associated with its worsening after one month is an important predictor for future cardiac events
Keywords: Heart failure, hospital readmission, cardiac events, Tissue Doppler imaging.
INTRODUCTION
Since echocardiography is a very useful technique for the diagnostic evaluation of dyspnoeic patients, Doppler imaging is providing useful information regarding the prognosis of patients with heart failure, taking into the fact that the rate of cardiac events after the onset of HF remains high despite recent advances in the management of this condition1. Previous studies have demonstrated that a new tissue Doppler index, E/(E’×S’), has a good accuracy to predict cardiac death in patients with heart failure (HF)2,3. Currently, the main terminology used to describe
HF is historical and is based on the determination of the left ventricular ejection fraction (LVEF)4. This E/(E’×S’) index includes the ratio between early diastolic transmitral and mitral annulus velocity (E/E’), as well as the systolic mitral annulus velocity (S’). Also, from all the patients diagnosed with HF, at least half of the cases are represented by heart failure with reduced ejection fraction (HFrEF), a condition due to the inability of the heart to supply the body’s tissues with an adequate amount of blood under the conditions of normal cardiac filling pressure5. This entity was defined in the 2016 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure as a clinical syndrome characterized by typical symptoms and/or signs associated with LFEF <40%4. Thus, in order to perform a proper assessment of these patients prognosis, a parameter that explores the global LV function is in order. Therefore, we analyzed the prognostic value of an index that associates a marker of diastolic function (E/E’) and a parameter that explores LV systolic performance (S’) in patients with HfrEF: the E/(E’×S’) index. Moreover, we tried to determine if a deterioration of E/(E’×S’) observed at hospital discharge is able to provide complementary prognostic data.
METHODS
Study population
We retrospectively analyzed a group of 400 consecutive patients hospitalized at the Timisoara Institute of Cardiovascular Diseases between January and October 2007 with HF, in sinus rhythm, diagnosed according to the guidelines4. We included adult patients with exacerbation of symptoms of HF with at least 1 New York Heart Association (NYHA) class deterioration, with typical signs of HF and LVEF <40%. The exclusion criteria were represented by: patients with inadequate echocardiographic images, cardiac pacemaker/defibrillator, significant primary valvular heart disease,
acute coronary syndrome at inclusion, coronary revascularization during follow-up, severe pulmonary disease, congenital heart disease, malignant neoplasia or renal failure. Only 166 patients with HFrEF formed our study group. The study has been approved by the local ethics committee and all the patients gave informed consent in agreement with ethics regulations.
Echocardiography
Before discharge and in a reasonably stable clinical condition (within 24 hour), our patients underwent an echocardiographic examination with an ultrasonographic system (Vivid 7 General Electric, Milwaukee, WI) equipped with multifrequency transducer. LVEF was calculated from apical two- and four-chamber views using a modified Simpson’s rule6 . Left atrial (LA) volume was calculated using the biplane area-length method at the apical four-chamber and apical two-chamber views at ventricular end-systole and was indexed for body surface area6. The regurgitant orifice area (ROA) and the regurgitant volume (RV) of mitral regurgitation were determined7,8. Transmitral fl ow patterns were recorded from apical four-chamber windows with 4-5mm pulsed-sample Doppler volume placed between mitral valve tips in diastole during five consecutive cardiac cycles. Maximal velocities of E and late transmitral fl ow (A) waves were measured during end-expiratory apnea; the velocities were recorded for five consecutive cardiac cycles, and the results were averaged9. The TDI program was set in pulsed-wave Doppler mode10. Motion of mitral annulus was recorded in the apical four-chamber view at a frame rate of 80 to 140 frames per second. A 4-5 mm sample volume was positioned sequentially at the lateral and septal corners of the mitral annulus. The peak early (E’) and late (A’) diastolic mitral annular velocity were determined. The peak mitral annular systolic velocity (S’) was defined as the maximum velocity during systole, excluding the isovolumic contraction. All velocities were recorded for five consecutive cardiac cycles during end-expiratory apnoea, and the results were averaged. All TDI signals were recorded at horizontal time sweep set at 100 mm/s. The average of the velocities from the septal and lateral site of the mitral annulus was calculated. E/E’ and E/(E’×S’) were determined. The same measurements were repeated one month after hospital discharge (30 ± 3 days). Worsening of E/(E’×S’) was defined as a value greater than the previous value determined at discharge.
Clinical variables recorded
At hospital discharge, the following clinical variables were recorded: age, sex, body mass index, heart rate, mean arterial pressures, etiology of HF, NYHA functional class, N-terminal pro-brain natriuretic peptide (NTproBNP) level.
Clinical outcome
The primary event consisted of cardiac death or hospital admission due to HF worsening. Cardiac death was defined as either a death directly related to cardiac disease, mainly congestive HF, or sudden death. The follow-up information was obtained from electronic medical files or by telephone contact with the patients or their family members.
Statistical analysis
Data are expressed as mean ± standard deviation for continuous variables and as proportions for categorical variables. The comparison of continuous variables was performed by an independent sample t-test. For categorical variables, the chi-square test was used. Receiver operating characteristic (ROC) curves for predicting cardiac death were determined for different parameters and area under the ROC curves (AUC) were compared. The cardiac event-free survival rates were calculated using the Kaplan-Meier analysis, and the event rates were compared using the log-rank test.
Patients who died of non-cardiac causes were censored (as non-events) at the time of death. A significance level of 0.05 was assumed for all statistical tests. Statistical analysis was performed using SPSS version 18.0 (SPSS Inc., Chicago, Illinois).
RESULTS
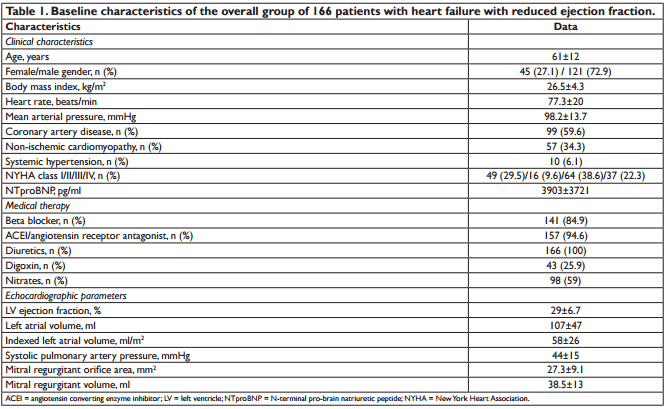
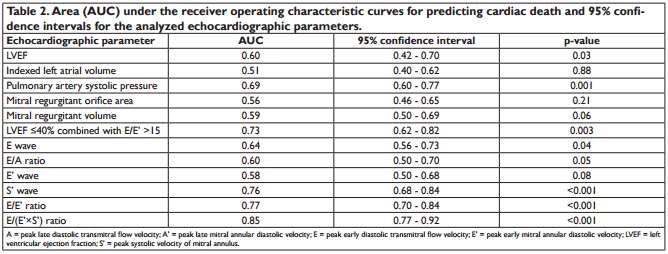
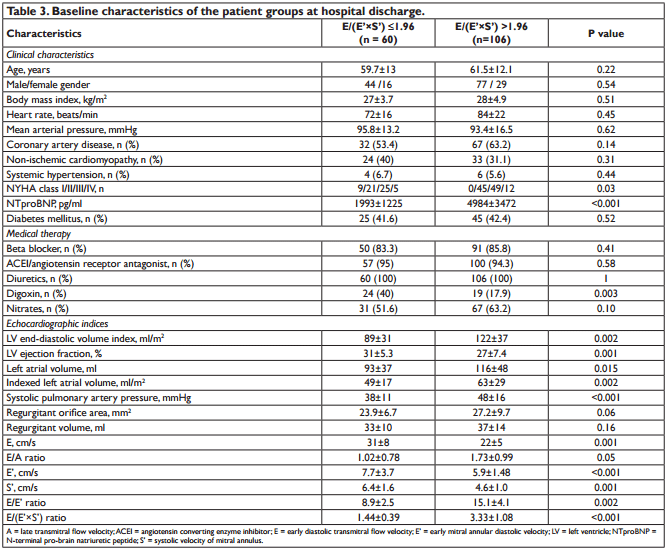
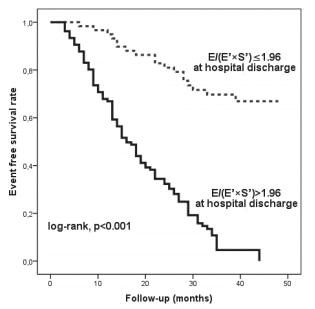
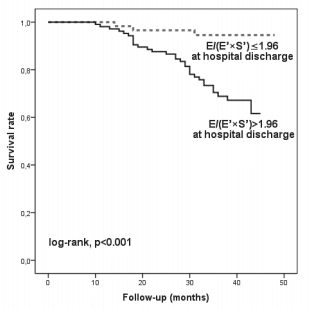
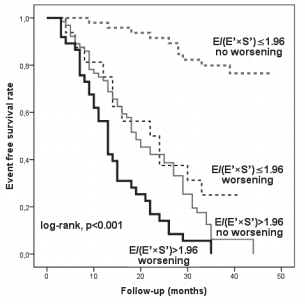
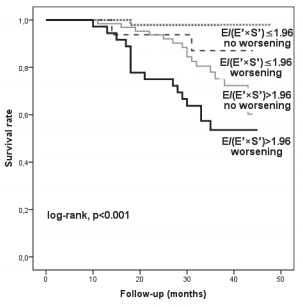
We analysed 166 consecutive hospitalized patients (60 ± 13 years; 121 men), with HF, in sinus rhythm. The mean LVEF was 29 ± 6.7% and mitral annular velocities from TDI were recordable at both sites in all patients. The aetiology of HF was coronary artery disease (99 patients), non-ischemic cardiomyopathy (57 patients) and systemic hypertension (10 patients). Coronary artery disease was assessed by angiocoronarography for all the 99 patients of the coronary artery disease group. Baseline characteristics of the overall group are presented in Table 1. During the follow-up period (34 ± 8.8 months) cardiac events occurred in 113 patients (68%). The fi rst cardiac event was represented by cardiac death in 6 patients (3.6%) and readmission for HF in 107 patients (64.4%). By analyzing echocardiographic parameters at hospital discharge, the ROC curves to predict cardiac events identifi ed the greatest AUC for E/(E’×S’) (AUC = 0.85, p <0.001) followed by E/E’ (AUC = 0.77, p <0.001) and S’ (AUC = 0.76, p <0.001) (Table 2). The optimal cut-off value for E/(E’×S’) ratio was 1.96 with 84% sensitivity and 80% specificity. Patients were divided into 2 groups according to E/(E’×S’) at discharge: group I consisted of patients with E/(E’×S’) ≤1.96 (60 patients, 36.2%) and group II with E/(E’×S’) >1.96 (106 patients, 63.8%). Table 3 outlines the characteristics of patient groups. Patients of group II had significantly greater NYHA functional class, higher NTproBNP and pulmonary artery systolic pressures (PASP), larger LV and LA, higher values for E, E/E’ and E/(E’×S’), lower LVEF, E’ and S’. Age, gender, body mass index, heart rate, E/A, ROA, and RV, did not differ between the groups. The composite end point was signifi cantly higher in group II than in group I (95 events, 89.6% versus 18 events, 30%, p<0.001). Kaplan–Meier analysis showed that cardiac event-free survival rate during follow-up was signifi cantly higher in group I than in group II (log rank, p<0.001) (Fig. 1). During the entire follow-up period, cardiac death occurred in 34 patients (20.4%) and was signifi cantly higher in group II (31 deaths, 29.2%) than in group I (3 deaths, 5.%), p<0.001. Kaplan–Meier analysis showed that in patients with HFrEF survival rate was signifi cantly higher in group I than in group II (Fig. 2). One month after hospital discharge we identifi ed worsening of E/(E’×S’) ratio in 53 patients (31.9%). Thirty seven of this patients (22.2% of our overall group) presented an initial value of E/(E’×S’) ratio greater than 1.96. However, as shown in Figure 3, the worsening of E/(E’×S’) was associated with lower cardiac event-free survival rate, regardless of the E/(E’×S’) value at inclusion in the study (2.7% versus 14%, p = 0.002 in patients with the initial E/(E’×S’) >1.96, and 25% vs. 79.5%, p <0.001 in those with E/(E’×S’) ≤1.96 at hospital discharge, respectively). The subgroup of patients with an initial E/(E’×S’) ratio >1.96 and its worsening after one month presented the worst prognosis in the overall population (Fig. 3). The same subgroup presented the higher rate of cardiac death (Fig. 4).
Figure 1. Kaplan-Meier curves of cardiac event-free survival in patients with heart failure with reduced ejection fraction according to E/(E’×S’) ratio at hospital discharge below and above 1.96. E = early diastolic transmitral velocity; E’ = early mitral annular diastolic velocity; S’ = systolic mitral annular velocity
Figure 2. Kaplan-Meier survival curves in patients with heart failure with reduced ejection fraction according to E/(E’×S’) ratio at hospital discharge below and above 1.96. E = early diastolic transmitral velocity; E’ = early mitral annular diastolic velocity; S’ = systolic mitral annular velocity.
Figure 3. Kaplan-Meier event free survival curves of patients classifi ed according to the initial E/(E’×S’) value and to worsening of E/(E’×S’) one month after hospital discharge. E = maximal early diastolic transmitral velocity; E’ = maximal early mitral annular diastolic velocity using the average of the medial and lateral site of mitral annulus; S’ = maximal systolic mitral annular velocity using the average of the medial and lateral site of mitral annulus.
Figure 4. Kaplan-Meier survival curves of patients classifi ed according to the initial E/(E’×S’) value and to worsening of E/(E’×S’) one month after hospital discharge. E = maximal early diastolic transmitral velocity; E’ = maximal early mitral annular diastolic velocity using the average of the medial and lateral site of mitral annulus; S’ = maximal systolic mitral annular velocity using the average of the medial and lateral site of mitral annulus.
DISCUSSION
The main fi nding of our study is that E/(E’×S’) ratio is useful in order to predict the worsening of HF as well as cardiac death in patients with HFrEF in sinus rhythm. An E/(E’×S’) index >1.96 at hospital discharge was the best predictor for future cardiac events when compared to several other echocardiographic parameters and NTproBNP level. The subgroup of patients with an initial E/(E’×S’) index >1.96 associated with its worsening after one month encountered the worst prognosis. Predicting the prognosis of patients with LV dysfunction is a matter of great clinical importance. Previous studies in the fi eld of conventional echocardiographic imaging have suggested that LA size11,12, LV volumes indices11, E/A13 and LVEF11 were robust predictors of
outcome in HF patients. RV and ROA are also accepted as prognostic markers for cardiac events14. A high value of RV and ROA would probably lead to an increase of the transmitral E wave and consecutively of the E/(E’×S’) ratio. Consequently, E/(E’×S’) ratio includes the prognostic value of the ROA and RV. In our study, conventional echocardiographic parameters like PASP,
LVEF, LAVI, E/A, E, RV or ROA registered a lower accuracy in predicting cardiac events in comparison with E/(E’×S’). The superiority of E/(E’×S’) index could be explained by the dependence of the mitral fl ow to the volemic status, LA pressure, age and myocardial relaxation. Nowadays, TDI is widely available on echocardiographic equipment of various manufacturers. One of the advantages of this new technique is that it does not require the tracing of endocardial contours, unlike LV volumes and LVEF11. In the last years, a handful of studies have evaluated the prognostic value of TDI parameters in order to predict cardiac events15-20. Wang et al15. have demonstrated that both S’ and E’ were predictors of cardiac mortality on univariate analysis, but E’ was marginally superior on multivariate analysis. Other studies identified E/E’11,18,21,22 and S’18,21 as strong predictors of outcome in HF patients. Møller et al23. reported that E/E’ ≥15 was an independent predictor of cardiac outcome after first myocardial infarction and Hirata and colleagues20 showed that a combined index including LVEF ≤40% and E/E’ >15 permitted identification of patients at higher risk of cardiac outcome in patients with HF. Although LVEF ≤40% combined with E/E’ >15 offered a good accuracy for the prediction of future cardiac events, E/(E’×S’) was a better predictor. Because of the time-dependent changes in S’ and E/E’, we tried to determine whether our new index obtained at the time of the fi rst TDI examination after
hospital discharge is able to provide complementary prognostic data. TDI measurements were repeated one month after hospital discharge at the patient’s fi rst visit requested by the outpatient clinical program of HF control. The subgroup of patients with a high E/ (E’×S’) ratio at hospital discharge and its worsening after one month presented the worst prognosis in the overall population The utility of NTproBNP to predict future cardiac events was demonstrated by a number of previous studies analyzing patients with reduced systolic function17,24, patients presenting to the emergency department with dyspnea25 or consecutive patients with acute or chronic HF4, demonstrated. Echocardiography as well as NTproBNP determination at hospital discharge, after appropriate medical treatment, were performed. Statistical analysis of our data supports the observation that NTproBNP has prognostic value but it is inferior to E/(E’×S’), despite the good relationship between these two parameters26. Lim et al.27 showed also the higher sensitivity of an abnormal echocardiogram to predict outcome compared with NTproBNP. Coronary artery disease was highly prevalent in the present series and one cannot rule out the occurrence of ischemic events contributing to the death of the patients. One possible explanation for the ability of E/
(E’×S’) ratio to predict future cardiac deaths is that we are detecting occult myocardial ischemia. When coronary disease causes regional hibernation of the myocardium, the E’ and S’ velocities drop. Those who went on to have coronary events may, therefore, have had a higher E/(E’×S’) ratio due to regional changes in myocardium, caused by subclinical coronary disease. The final mechanism of different cardiac diseases is probably the same, that is, ischemia of LV subendocardial fibers, as suggested by Wang et al.15. TDI velocities derived from the mitral annulus primarily refl ect longitudinal motion, due to the longitudinally directed fibers, which are found in the subendocardium. This may explain why E/(E’×S’) ratio is so useful for the assessment of the consequences of ischemia, to which the subendocardium is particularly sensitive. Our new TDI parameter associates an index of diastolic function (E/E’) and a marker that explores LV
systolic performance (S’) and therefore may provide supplementary prognostic information compared to each index alone. In a recent study, E/(E’ × S’) was the only independent predictor with an incremental prognostic value over the Meta-analysis Global Group in Chronic Heart Failure score and was superior to the E/E’ ratio and longitudinal LV strain in patients with HFrEF28. No bidimensional speckle tracking-derived parameter remained significant in multivariable models in HFrEF.The reproducibility and simplicity, together with the prognostic value of our new index, made it important to use in the routine procedure. TDI recordings should be given high priority in routine echocardiographic examinations of patients with HFrEF29. Our study has a number of limitations. First, the number of patients in this study was relatively small; however, we were able to reach several significant observations. We deliberately did not use more sophisticated
Doppler parameters that are difficult to record and thus are not suitable for daily practice. We have limited TDI measurements at two sites (medial and lateral mitral annulus) and we did not examine anterior and posterior velocities that might have provided additional information. The study centre functioned as a tertiary invasive centre and therefore the study population may not reflect a general population of patients with HF. Our study is a single-center study and its reproduction in other centers or by multicenter studies would argue for its validity. Future studies are also necessary in order to compare the prognostic value of E/(E’×S’) ratio with that of the newer parameters analyzing myocardial deformation, like LV longitudinal strain, strain rate and/or torsion determined by twoor three-dimensional echocardiography. Another limitation of our study is the one that, for the coronary artery disease group, the patients with basal wall motion abnormalities were not excluded from the analysis. However, we took into consideration this specific category of patients, considering the observations of Price et al., who suggested that the velocity of an individual myocardial segment may be influenced by the motion of the adjacent muscle; consequently an akinetic basal segment interrogated from the apical view may have near normal values when influenced by a hyperkinetic near segment30.
CONCLUSIONS
The results of our study indicate that in patients with HF in sinus rhythm, the novel TDI derived index, E/(E’×S’), is an important independent long-term prognostic parameter of cardiac death or hospitalization due to HF worsening. E/(E’×S’) >1.96 at hospital discharge can identify patients at high risk of future cardiac events, particularly if it is associated with worsening
after one month.
Acknowledgements: This work was supported by CNCSIS–UEFISCU, project number PN II/RU code PD526/2010 and TD530/2007.
References
1. Zile MR, Brutsaert DL. New concepts in diastolic dysfunction and diastolic heart failure: Part I: diagnosis, prognosis, and measurements of diastolic function. Circulation 2002; 105:1387–1393
2. Mornos C, Petrescu L, Cozma D, Ionac A. A new tissue Doppler index to predict cardiac death in patients with heart failure. Arq Bras Cardiol. 2014 Jan;102(1):19-29.
3. Mornos C, Petrescu L, Pescariu S, Dan R, Cozma D. Prognostic value of septal E/(E’×S’) ratio in predicting cardiac death in patients with heart failure. Kardiol Pol. 2014;72(2):166-74.
4. Ponikowski P, Voors AA, Anker SD, Bueno H, Cleland JG, Coats AJ, Falk V, González-Juanatey JR, Harjola VP, Jankowska EA, Jessup M, Linde C, Nihoyannopoulos P, Parissis JT, Pieske B, Riley JP, Rosano GM, Ruilope LM, Ruschitzka F, Rutten FH, van der Meer P; Authors/Task Force Members.; Document Reviewers. 2016 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure: The Task Force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC). Developed with the special contribution of the Heart Failure Association (HFA) of the ESC. Eur J Heart Fail. 2016;18(8):891-975
5. Travessa AM, de Menezes Falcão LF.Treatment of Heart Failure With Reduced Ejection Fraction-Recent Developments. Am J Ther. 2016;23(2):e531-49.
6. Lang RM, Bierig M, Devereux RB, et al (2005) Recommendations for chamber quantifi cation: a report from the American Society of Echocardiography’s Guidelines and Standards Committee and the Chamber Quantification Writing Group, developed in conjunction with the European Association of Echocardiography, a branch of the European Society of Cardiology. J Am Soc Echocardiogr 18(12):1440-1463
7. Otto CM (2000) Valvular regurgitation: diagnosis, quantitation and clinical approach. In: Otto CM, ed. Textbook of clinical echocardiography, W.B. Saunders, Philadelphia, pp 265-300
8. Zoghbi WA, Enriquez-Sarano M, Foster E, et al (2003) Recommendations for evaluation of the severity of native valvular regurgitation with two-dimensional and Doppler echocardiography. J Am Soc Echocardiogr 16(7):777-802
9. Quiñones MA, Otto CM, Stoddard M, et al (2002) Recommendations for quantification of Doppler echocardiography: a report from the Doppler Quantification Task Force of the Nomenclature and Standards Committee of the American Society of Echocardiography. J Am Soc Echocardiogr 15:167–184
10. Oki T, Tabata T, Yamada H, et al (1997) Clinical application of pulsed Doppler tissue imaging for assessing abnormal left ventricular function. Am J Cardiol 79:921–928
11. Nikitin NP, Loh PH, Silva R, et al (2006) Prognostic value of systolic mitral annular velocity measured with Doppler tissue imaging in patients with chronic heart failure caused by left ventricular systolic dysfunction. Heart 92:775–779
12. Leung DY, Boyd A, Ng AA, et al (2008) Echocardiographic evaluation of left atrial size and function: current understanding, pathophysiologic correlates, and prognostic implications. Am Heart J 156(6):1056-1064
13. Seo Y, Ishizu T, Kawano S et al. Combined approach with Doppler echocardiography and B-type natriuretic peptide to stratify prognosis of patients with decompensated systolic heart failure. J Cardiol 2008;52(3):224-31.
14. Enriquez-Sarano M, Akins CW, Vahanian A (2009) Mitral regurgitation. Lancet 373(9672):1382-1394
15. Wang M, Yip GW, Wang AY, et al (2003) Peak early diastolic mitral annulus velocity by tissue Doppler imaging adds independent and incremental prognostic value. J Am Coll Cardiol 41:820–826
16. Dokainish H, Zoghbi WA, Lakkis NM, et al (2005) Incremental predictive power of B-type natriuretic peptide and tissue Doppler echocardiography in the prognosis of patients with congestive heart failure. J Am Coll Cardiol 45:1223-1226
17. Bruch C, Rothenburger M, Gotzmann M, et al (2006) Risk stratification in chronic heart failure: independent and incremental prognostic value of echocardiography and brain natriuretic peptide and its Nterminal fragment. J Am Soc Echocardiogr 19:522-528
18. Yamamoto T, Oki T, Yamada H, et al (2003) Prognostic value of the atrial systolic mitral annular motion velocity in patients with left ventricular systolic dysfunction. J Am Soc Echocardiogr 16:333-339
19. Yu CM, Sanderson JE, Marwick TH, et al (2007) Tissue Doppler imaging a new prognosticator for cardiovascular diseases. J Am Coll Cardiol 49(19):1903-1914
20. Hirata K, Hyodo E, Hozumi T, et al (2009) Usefulness of a combination of systolic function by left ventricular ejection fraction and diastolic function by E/E’ to predict prognosis in patients with heart failure. Am J Cardiol 103(9):1275-1279
21. Acil T, Wichter T, Stypmann J, et al (2005) Prognostic value of tissue Doppler imaging in patients with chronic congestive heart failure. Int J Cardiol 103:175–181
22. Olson JM, Samad BA, Alam M (2008) Prognostic value of pulse-wave tissue Doppler parameters in patients with systolic heart failure. Am J Cardiol 102(6):722-725
23. Møller JE, Pellikka PA, Hillis GS, et al (2006) Prognostic importance of diastolic function and fi lling pressure in patients with acute myocardial infarction. Circulation 114(5):438-444
24. Dini FL, Conti U, Fontanive P, et al (2008) Prognostic value of Nterminal pro-type-B natriuretic peptide and Doppler left ventricular diastolic variables in patients with chronic systolic heart failure stabilized by therapy. Am J Cardiol 102(4):463-468
25. Berni A, Cappelli F, Bitossi L, et al (2009) Non-invasive tissue Doppler imaging pulmonary capillary wedge pressure measurement improves NT-proBNP prognostic value in heart failure. Acta Cardiol 64(2):213- 218
26. Mornos C, Ionac A, Cozma D, et al (2008) The relationship between tissue Doppler imaging and seric NTproBNP levels in sinus rhythm patients: a prospective study. Int J Cardiovasc Imaging 24:399–407
27. Lim TK, Hayat SA, Gaze D, et al (2007) Independent value of echocardiography and N-terminal pro-natriuretic peptide for the prediction of major outcomes in patients with suspected heart failure. Am J Cardiol 100(5):870-875
28. Obokata M, Takeuchi M, Negishi K, Ohte N, Izumo M, Yamashita E, Ebato M, Yuda S, Kurabayashi M, Nakatani S. Relation Between Echocardiogram-Based Cardiac Parameters and Outcome in Heart Failure With Preserved and Reduced Ejection Fraction. Am J Cardiol. 2016;118(9):1356-1362.
29. Kadappu KK, Thomas L. Tissue Doppler imaging in echocardiography: value and limitations. Heart Lung Circ. 2015;24(3):224-33.
30. Price DJ, Wallbridge DR, Stewart MJ. Tissue Doppler imaging: current and potential clinical applications. Heart. 2000 Nov;84 Suppl 2:II11-8.
 This work is licensed under a
This work is licensed under a