Maria-Magdalena Craciun1, Cornelia Ancuta Zara2
1 Department of Nephrology, Theracardia Clinic, Brasov, Romania
2 Department of Cardiology, Theracardia Clinic, Brasov, Romania
Abstract: Introduction – The cardiorenal syndrome (CRS) involves an exchange of roles between the heart and kidneys, both generating acute or chronic primary or secondary disease. Case report – We want, by the following case presentation, to exemplify the potential etiological diagnostic approach of CRS in a multiple comorbidity context. Taking this into consideration, we highlight the case of a 60-year-old male known with diabetes mellitus (DM), hypertension and subject of a surgically revascularized trivascular coronary artery disease (CAD) and chronic kidney disease (CKD), who arrived at Theracardia outpatient clinic for severe decompensate heart failure (HF), a factor which consequently triggered the significant aggravation of the renal pathology. Conclusion – The first particularity of the case is mixed (1 and 2) CRS diagnosis associating DM and anemic syndrome as the main comorbidities. The second particularity is a logistic one: carrying out the case in the ambulatory system by involving an interdisciplinary team consisting of a cardiologist, nephrologist and diabetologist in order to compensate the underlying pathology and the precipitating factors. Outpatient treatment was in agreement with the patient’s option, who refused hospitalization.
Keywords: cardiorenal syndrome, heart failure, chronic kidney disease.
INTRODUCTION
The difficulty of identifying the etiological diagnosis in a CRS context is well known. So, we report a clinical case which gave us the challenging task of searching for the primary pathological sequence, as well as the precipitating factors, which decompensate the cardiac and renal pathologies, that have to be quickly identified and therapeutically solved. The patient was assessed in the ambulatory system and treated at home. These are the objectives of the following clinical case presentation.
CASE PRESENTATION
A 60-year old male patient arrived in February 2016 at the Theracardia clinic, presenting the following symptoms: severe lower limb edema, nocturnal orthopnoea, dyspnoea to minimal efforts, significant decrease in exercise tolerance during the last month. The patient had a history of essential hypertension, DM type 2 on oral antidiabetic medication and atherosclerotic tricoronary disease surgically revascularized in 2005. The patient was known to suffer from CKD, the values associated to renal function amounting to 1.4 mg/dl (serum creatinine). The initial physical examination showed the following data: generalized pallor, orthopnoea associated with peripheral oxygen saturation of 82%, basal bilateral crackles, warm central cyanosis, blood pressure of
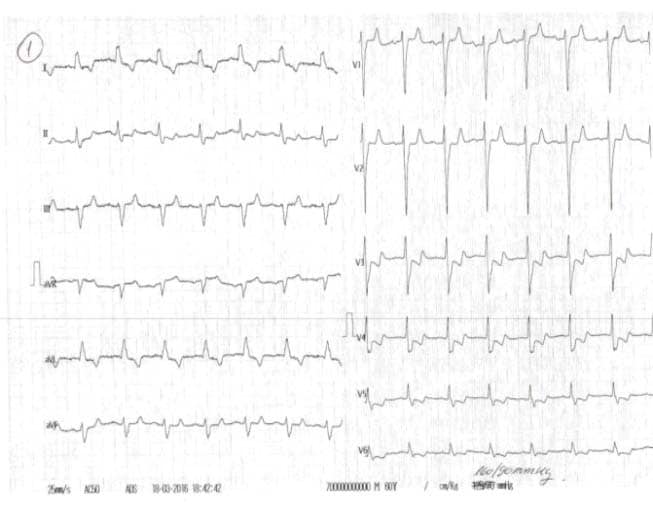
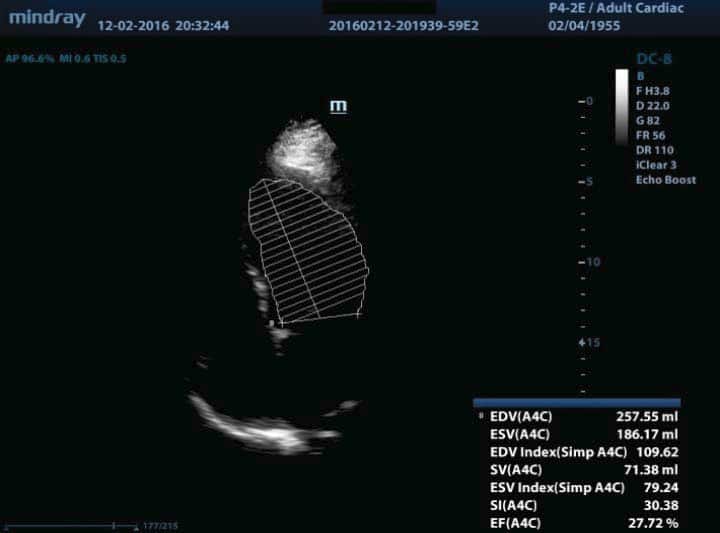
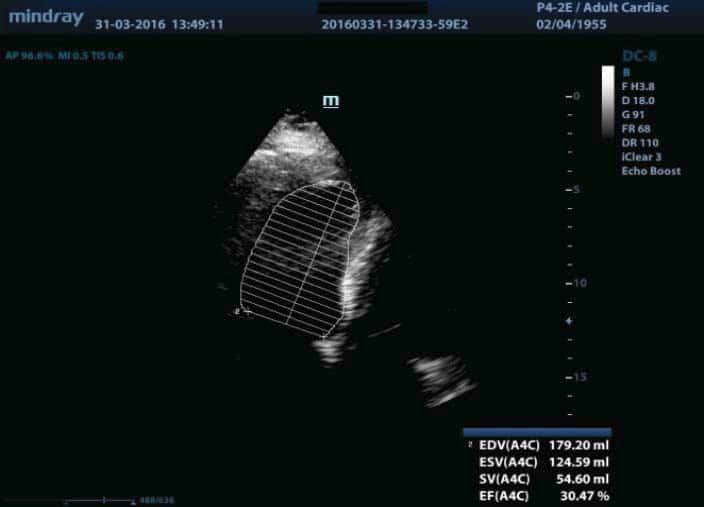
180/90 mmHg, pulse of 110 bpm, gallop rhythm, jugular turgor, hepatomegaly, ascites, severe edema in the upper third of the thigh, abdominal obesity and body weight of 114 kg (body index mass of 39.4 kg/m2). The laboratory indicated the following: leukocytosis (leukocytes 12000/μl), anemia (hemoglobin 9.3 g/dl, mean corpuscular volume of 98fl , serum iron of 35 mg/dl, transferrin saturation of 15%, ferritin of 75 ng/ml, peripheral smear with normochromic, normocytic appearance, positive Adler reaction for occult bleeding), glycosylated hemoglobin 7.9%, NT pro BNP 20000 pg/ml, nitrogen retention (serum creatinine of 3.1 mg/ dl, urea nitrogen of 152 mg/dl), hyperkalemia (K 6.3 mmol/l). We also found normal values for serum Na (145 mmol/l, urinary Na 120 mmol/24 hours, urinary albumin/ creatinine ratio (ACR) of 20 mg/g, normal lipid profi le under lipid-lowering treatment and negative urine culture. The chest X-ray examination showed a cardio-pulmonary cardiothoracic index of 65, hilar density and pulmonary stasis. The electrocardiogram (EKG) revealed a tachycardic sinus rhythm of 110/min, QRS axis – 20, subepicardic lesion (1 mm horizontal ST segment depression in V2-V5), subepicardic anterolateral ischemia (negative T in DI and aVL) (Figure 1). The transthoracic echocardiography revealed increased left ventricle (LV) volumes, significant segmental wall motion abnormalities with severely altered global systolic function (ejection fraction (EF) of 27% calculated with the biplane Simpson’s method), moderate secondary mitral regurgitation, elevated LV filling pressures (E/Ea> 12), secondary tricuspid regurgitation with possible pulmonary hypertension (PASP estimated to 56 mmHg) (Figure 2). Based on clinical, biological and imaging data, we established the diagnosis of decompensated chronic HF class III NYHA with severe LV systolic dysfunction, ischemic dilated cardiomyopathy (DCM), metabolically unbalanced DM type 2, macrovascular complicated, stage 3 high risk essential hypertension, balanced dyslipidemia on statin, obesity stage 2, CKD 3A KDOQI stage – glomerular filtration rate (GFR) 55ml/min/1,73 m2, hyperkalemia, multifactorial anemia (secondary to CKD and iron deficiency). The patient opted for ambulatory assessment in our clinic, treatment at home and refused hospitalization. The clinical, biological and ultrasound control carried out during the next 4 weeks under optimal medical treatment for HF (furosemidum 60mg/day, carvedilolum 12.5mg/day, ivabradinum 10mg/day, trimetazidinum 70mg/day, candesartan cilexetil 8mg/day), acidum acetylsalicylicum 75mg/day, rosuvastatinum-ezetimibum 10/10mg/day, electrolytic balance and oxygenation confirmed a positive evolution. We mention that the patient was already on treatment with angiotensin II receptor blocker for two years under stable renal function and we chose to remain on this therapy for its effect on left ventricular function. We also started insulinum glarginum 22UI/day and treated anemia with darbepoetinum alfa 30 micrograms/week and iron therapy with iron hydroxide (III) sucrose complex 100mg/5ml/week, 4 doses, as recommended by the nephrologist and a diabetologist. The 8 kg weight loss, the absence of peripheral edema, dyspnoea correction both at rest and at minimal efforts, 6MWT 100 m, constant diuresis, optimal blood pressure and heart rate are the factors confirming cardiorespiratory compensation. From a paraclinic point of view, the biological control revealed a decrease in nitrogen retention (serum creatinine of 2.2mg/dl, urea nitrogen of 92 mg/dl), a decrease of the Nt-proBNP (11295 pg/ml) and normal ionic profile. Three months after the first assessment, the nitrogen retention was restored to the average value (serum creatinine of 1.4 mg /dl). In terms of cardiac compensation, the ultrasound showed a decrease in the diastolic and systolic volumes of the LV, a slight improvement of the EF, the lowering of the degree of mitral regurgitation and of the LV filling pressures (Figure 3).
Figure 1. The electrocardiogram (EKG) at presentation shows a tachycardic sinus rhythm of 110 bp/min, QRS axis-20, previous subepicardic injury (1 mm horizontal ST segment depression in V2-V5), subepicardic anterolateral ischemia (negative T in DI and aVL).
Figure 2. The initial transthoracic echocardiography shows increased left ventricle (LV) volumes, significant segmental wall kinetic abnormalities, ejection fraction (EF) of 27%, moderate secondary mitral regurgitation, elevated LV filling pressures, secondary tricuspid regurgitation with possible pulmonary hypertension (PASP estimated to 56 mmHg).
Figure 3. The transthoracic echocardiography after treatment shows a decrease in the diastolic and systolic volumes of the LV, a slight improvement of the EF -30,47%, the lowering of the degree of mitral regurgitation and of the LV filling pressures.
DISCUSSION
CRS is defined as a complex pathophysiological disorder of the heart and kidneys, given that acute or chronic dysfunction of one organ may induce acute or chronic dysfunction of the other organ1. The different cardiorenal interactions generated 5 types of CRS, as proposed by Ronco and his collaborators2. As suggested in the mentioned source, CRS 1 refers to the acute deterioration of the cardiac function, leading to acute renal injury. Conversely, CRS 2 consists in the chronic cardiac dysfunction which causes chronic progressive renal disease. CRS 3 is linked to the primary sudden deterioration of the renal function, leading to acute cardiac dysfunction, whereas CRS 4 consists in the primary chronic kidney disease that contributes to cardiac dysfunction. Lastly, CRS 5 includes an acute or chronic systemic pathology, resulting in cardiorenal mixed dysfunction. Prior to taking into account the diagnosis of CRS, we had excluded the hepato-renal syndrome, given the following reasons: the absence of an acute or chronic liver pathology (invalidated by the biological control and the ultrasound), normal urinary volume, the absence of hyponatremia, ascites (the only element that might have been associated with the hepato-renal syndrome), being interpreted in the context of HF decompensation. We also excluded the diabetic nephropathy, considering the fact that the DM started to develop two years before, and there was no albuminuria in laboratory tests (normal ACR). The two types of CRS (1 and 2) are illustrated in our case presentation. The case therefore highlights a patient with known cardiac and renal pathologies, whose renal function has been aggravated in the context of severe HF decompensation, severe LV systolic dysfunction and ischemic DCM. The chronic renal pathology might be considered as ischemic nephropathy due to a prolonged and persistent low renal perfusion pressure in a chronic heart failure patient. Regarding this mechanism, Schrier and his collaborators showed that both high output cardiac failure and low output cardiac failure by different pathways induce a low fullness of the arterial circulation and consequently high sympathetic nervous system activity, high vasopresin release and activated renin-angiotensin-aldosterone system, mechanisms which lead to diminished renal hemodynamics, including low renal perfusion pressure, along with low renal sodium and water excretion3. These diagnostic features presented above thus evidently indicate the mixed (1 and 2) CRS. Another aspect worth mentioning would be the fact that many studies confirm that the immune systems, inflammation, oxidative stress and apoptosis may contribute to the final nonhemodynamic pathways of the organ dysfunction in the heart-kidney crosstalk. Cytokines may be produced by various tissues and cleared by the kidney. The inability to metabolize or remove cytokines in case of acute renal failure and CRS 1 may increase infl ammation and a global dysfunction4. A few cardiorenal risk factors mentioned in various studies are found in our patient increasing the potential poor outcome of CRS. These are: type 2 DM, hypertension, obesity, dyslipidemia and anemic syndrome5,6. We highlight that dyslipidemia is involved in increasing both cardiovascular risk and progression of renal injury. According to this, it is well established that statines reduce cardiovascular morbidity and mortality6. Among the comorbidities mentioned above, we consider the anemic syndrome as a particularity of the case, given the multifactorial etiology and its various implications. On the one hand, laboratory findings as low hemoglobin, as well as normochromic, normocytic appearance in the context of CKD 3A KDOQI stage, indicate renal anemia. On the other hand, low serum iron, low transferrin saturation and low ferritin levels sustain associated iron deficiency. We also found positive Adler reaction, which indicated colonoscopy as further investigation in order to find a possible source of occult bleeding. Anemia is prevalent in patients with CKD, as well as in those with HF according to studies7,8. As we know from various studies, the etiology of anemia in the context of this heart-kidneys crosstalk is multifactorial9. Anemia is associated with increased mortality, morbidity and the worsening of renal function10. The literature discusses the so-called cardiorenal anemia11. It was reported that the cardiorenal anemia syndrome is a risk factor for higher mortality in patients with chronic HF and a reduced EF11,12,13. Anemia with a hemoglobin level ≤12 g/dl was observed to 53.6% of CKD patients with an estimated GFR of 15-30 ml/min/1.73 m2 and in approximately 50% of patients with HF7,8. Please also note that iron deficiency is common in HF patients, as it is with other chronic illnesses, including CKD and it can lead to anemia and/or skeletal muscle dysfunction without anemia14. Within an HF population, iron deficiency is associated with a worse prognosis15,16 Recently, it has been suggested that the correction of iron deficiency and anemia in patients with HF and renal dysfunction may prevent the progression of both conditions13.
CONCLUSIONS
The particularities of this case are: the CRS type 1 and CRS type 2 association on top of a severe HF decompensated patient, mutual aggravation of both renal and cardiac function and difficulty to manage it, taking into account the complexity of the case. On the other hand, pointing out the precipitating reversible factors, such as fl uid and electrolyte imbalance, anemia or glycemic disorders and treating them properly can restore the normal clinical, biological and echographic parameters. Multiple comorbidities could be an obstacle in managing such a complex CRS patient, but an interdisciplinary outpatient team may find the best way to compensate the patient who refuses hospitalization, lowering the costs for both the patient and the health care system.
Conflict of interest: none declared.
References
1. Ronco C, McCullough P, Anker SD, Anand I, Aspromonte N, Bayshow SM, Bellomo R, Berl T, Bobek I, Cruz DN, Daliento L, Davenport A, Haapio M, Hillege H, House AA, Katz M, Maisel A, Mankaad S, Zanco P, Nebaza A, Palazzuoli A, Ronco F, Shaw A, Sheinfeld G, Soni S, Vescovo G, Zamperetti N, Ponikowski P. Acute Dialysis Quality Initiative (ADQI) Consensus Group: Cardio-renal syndromes: report from the consensus conference of the Acute Dialysis Quality Initiative. Eur Heart J 2010;31:703-11.
2. Ronco C, Haapio M, House AA, Anavekar N, Bellomo R. Cardiorenal syndrome. J Am Coll Cardiol 2008;52:1527-39.
3. Schrier RW, Abraham WT. Hormones and hemodynamics in heart failure. N Engl J Med 1999;34:577-85.
4. Virzi GM, Clementi A, Brocca A, De Cal M, Vescovo G, Granata A, Ronco C. The hemodynamic and nonhemodynamic Crosstalk in cardiorenal syndrome type 1. Cardiorenal Med 2014;4:103-12.
5. Sahasranam KV. Cardiorenal Syndrome.BMH Medical Journal 2014; 1(4):72-6
6. Seliger SL, Weiss NS, Gillen DL, Kestenbaum B, Ball A, Sherrard DJ, Stehman-Breen CO. HMG-Coa reductase inhibitors are associated with reduced mortality in ESRD patients. Kidney Int 2002;61: 297-4.
7. McClellan W, Aronoff SL, Bolton WK, Hood S, Lorber DL, Tang KL, Tse TF, Wasserman B, Leiserowitz M. The prevalence of anemia in patients with chronic kidney disease. Curr Med Res Opin 2004;20:1501-10.
8. Young JB, Abraham WT, Albert NM, Gattis Stough W, Gheorghiade M, Greenberg BH, O’Connor CM, She L, Sun JL, Yancy CW, Fonarow GC. Relation of low hemoglobin and anemia to morbidity and mortality in patients hospitalized with heart failure (insight from the OPTIMIZE-HF registry). Am J Cardiol 2008;101:223-30.
9. Parfrey PS, Harnett JD, Foley RN, Kent GM, Murray DC, Barre PE, Guttmann RD. Impact of renal transplantation on uremic cardiomiopathy. Transplantation 1995;60(9):908-14.
10. Silverberg DS. The role of erythropoiesis stimulating agents and intravenous (IV) iron in the cardio renal anemia syndrome. Heart Fail Rev 2011;16:609-14.
11. Silverberg DS, Wexler D, Iaina A. The importance of anemia and its correction in the management of severe congestive heart failure. Eur J Heart Fail 2002;4:681-86.
12. Kazory A, Ross EA. Anemia: The point of convergence or divergence for kidney disease and heart failure? J Am Coll Cardiol 2009;53:639-47.
13. Scrutinio D, Passantino A, Santoro D, Catanzaro R. The cardiorenal anaemia syndrome in systolic heart failure: Prevalence, clinical correlates, and long-term survival. Eur J Heart Fail 2011;13:61-7.
14. Jankowska EA, von Haehling S, Anker SD, Macdougall IC, Ponikowsi P. Iron deficiency and heart failure: diagnostic dilemmas and therapeutic perspectives. Eur Heart J 2013;34:816-26.
15. Jankowska EA, Kasztura M, Sokolski M, Bronisz M, Nawrocka S, Oleskowska-Florek W, Zymlinski R, Biegus J, Siwolowski P, Banasiak W, Anker SD, Filippatos G, Cleland JGF, Ponikowski P. Iron deficiency defined as depleted iron stores accompanied by unmet cellular iron requirements identifies patients at the highest risk of death after an episode of acute heart failure. Eur Heart J 2014;35:2468-76.
16. Jankowska EA, Malyszko J, Ardehali H, Koc-Zorawska E, Banasiak W, von Haehling S, Macdougall IC, Weiss G, McMurray JJV, Anker SD, Gheorghiade M, Ponikowski P. Iron status in patients with chronic heart failure. Eur Heart J 2013;34:827-34.
 This work is licensed under a
This work is licensed under a