Alina Scridon1,2, C. Gallet2, Moussa M. Arisha3, Valérie Oréa2, B. Chapuis2, C.Barrès2, C. Julien2, Ph. Chevalier2,3
Article received on the 14th February 2012. Article accepted on the 5th March 2012.
1 University of Medicine and Pharmacy of Târgu Mureş, 540000, Romania
2 Unité de Neurocardiologie, Université Lyon 1, F-69008, Lyon, France
3 Hospices Civils de Lyon, Hôpital Louis Pradel, Service de Rythmologie, F-69500, Lyon, France
Dr. Alina Scridon, University of Medicine and Pharmacy of Târgu Mureş, Department of Physiology, 38 Gheorghe Marinescu Street, 540000 Târgu Mureş, Romania. Telephone: 00 40 7 45 30 69 24. Fax: 00 40 2 65 21 04 07
E-mail: alinascridon@yahoo.com
Abstract: Objective – Surgical implantation of radiotelemetry devices is not novel, but its impact on spontaneously hypertensive rats (SHR) has not been previously assessed. This study aimed to evaluate the time necessary for recovery after radiotelemetry device implantation and the quality of ECG signal in various conditions. Methods – Radiotelemetry ECG transmitters were implanted in 8 young and 8 aging SHRs. Morbidity and mortality rates were recorded and the time needed for recovery after implant procedure was assessed based on body weight evolution. The quality of ECG signal was assessed during baseline conditions and various experimental protocols. Results – None of the animals died during or shortly after implant procedure. Within one week all rats regained their initial body weights. All ECG elements were easily recognizable in both baseline conditions and during various experimental protocols. No significant local changes were presented at the moment of device explantation. Conclusion – In our experience, surgery procedures for implanting radiotelemetry ECG devices in SHRs appear to be safe techniques, with short interval of recovery, low morbidity rates and no mortality, regardless the age of animals. Subcutaneous implantation of such devices offers high-quality ECG signal and is very well tolerated, even if long-term keeping in place is needed.
Keywords: radiotelemetry, implant procedure, signal quality, spontaneously hypertensive rat
Rezumat: Obiectiv – Implantarea chirurgicală a dispozitivelor de radiotelemetrie nu este o tehnică nouă, dar impactul ei asupra şobolanilor spontan hipertensivi (SHR) nu a fost încă evaluat. Scopul acestui studiu a fost de a evalua timpul necesar pentru recuperare după implantarea dispozitivelor de telemetrie şi calitatea semnalului ECG în diverse condiţii. Metoda – Dispozitivele de telemetrie au fost implantate la 8 SHR tineri şi 8 bătrâni. Morbiditatea şi mortalitatea au fost înregistrate şi timpul necesar pentru recuperare a fost evaluat pe baza evoluţiei greutăţii corporale. Calitatea semnalului ECG a fost evaluată în condiţii bazale şi în cursul unor protocoale experimentale. Rezultate – Niciun animal nu a murit în timpul sau imediat post-procedural. În decurs de o săptămână toţi şobolanii au recuperat greutatea corporală iniţială. Toate elementele ECG au putut fi uşor recunoscute în condiţii bazale, dar şi în timpul protocoalelor experimentale. La explantare nu au existat modificări locale semnificative. Concluzii – Procedurile chirurgicale de implant a dispozitivelor de radiotelemetrie la SHR par a fi tehnici sigure, cu interval scurt de recuperare, rate scăzute de morbiditate şi mortalitate, indiferent de vârsta animalelor. Implantarea subcutanată a acestor dispozitive oferă un semnal ECG de înaltă calitate şi este bine tolerată, chiar după un interval de timp îndelungat.
Cuvinte cheie: radiotelemetrie, procedura de implant, calitatea semnalului, şobolan spontan hipertensiv
INTRODUCTION
The use of surgically implanted radiotelemetry transmitters that allow monitoring heart rate and ECG has extended a lot lately because of their ability to monitor such functions over several weeks or even months, in conscious, undisturbed animals, without interference with handling or stress. Unlike external ECG monitoring, where animals are restrained by ECG leads that limit their mobility, these devices allow free motion of animals, and manipulation is limited to feeding and cleaning.
Although surgical techniques used to implant radiotelemetry devices in rats are not novel1-3, and a number of studies have assessed the time needed for recovery following different implant techniques, no such study is available for hypertensive rats, which are prone to present increased morbidity and mortality rates following invasive techniques and general anesthesia.4
This study aims to evaluate morbidity and mortality rates associated with implant procedures of radiotelemetry ECG monitoring devices, and the time needed for recovery after radiotelemetry devices surgical implantation in spontaneously hypertensive rats (SHR).
Radiotelemetry devices only allow analysis in a single ECG lead, which might limit the recognition of ECG elements and complex diagnostics, such as heart rhythm disturbances, mainly during intense animal somatomotor activity.5 Thus, we also thought to assess the quality of the ECG signal obtained during baseline conditions and during various experimental protocols and the ability to recognize different electrocardiographic features.
METHODS
Animals
Male SHRs were purchased from Elevage Janvier (Le Genest Saint Isle, France).
Experiments were conducted on 8 male three months-old SHRs (311.5 ± 7.7 g) and 8 aging (11 moths-old) SHRs (448.1 ± 26.4 g), after one week of accommodation.
All animals were housed in a climate-controlled room (at 21-22°C) with a 12-h light/dark cycle (on 7 AM / off 7 PM) in an accredited animal facility. Before implant procedure rats were housed in groups of 2-3 rats per cage. Once the implant procedure was performed, the rats were housed individually in polycarbonate cages, in order to prevent cannibalism, on standard bedding. All rats were fed standard rat pellets and tap water ad libitum.
All experiments were performed in compliance with the French Ministry of Agriculture and Food and Drug Administration guidelines for animal experimentation and were approved by the local Animal Ethics Committee.
Transmitter implantation procedure
Radiotelemetry ECG transmitters (TA11 CA-F40; Data Sciences International, St. Paul, MN) were implanted under isoflurane anesthesia (2.0 L/min, 4% in air for induction and 0.5 L/min, 2.5% in air for maintenance). Prophylactic injections of penicillin G (50,000 IU s.c.) and ketoprofen (2 mg/kg s.c.) were performed on each animal. Body temperature was maintained at 37°C throughout the procedure, using a heating blanket.
The skin was incised and the body of the transmitter was placed in a dorsal subcutaneous pocket and sutured to the underlying tissue under aseptic conditions. The two ECG leads (bare ends terminating with a small silastic drop to avoid tissue damage) were subcutaneously tunnelized in a lead II configuration. The negative electrode was placed under the right clavicle, and the positive in a latero-basal thoracic position, as illustrated in Figure 1.
Figure 1. Device and lead placement. The body of the transmitter is placed in a dorsal subcutaneous pocket and the two ECG leads are tunnelized in a lead II configuration.
The quality of the signal and correct morphology of ECG elements were evaluated within the procedure. When the quality of the captured ECG signal was satisfactory the distal ends of the leads were secured to the underlying tissue to avoid slipping and the skin layer was sutured.
After the procedure, the rats were allowed to recover under a warm light lamp and they were monitored until they regained their toe-pinch reflex, approximately 10-15 min after the end of the anesthesia, when they were placed in their cages.
All incidents that occurred during the procedure or shortly after were recorded.
Post-implant procedure monitoring
Immediately after the implant procedure the animals were housed individually in polycarbonate cages. They were weighted daily for at least one week after implantation or until body weight has returned to the initial value, measured before the implantation procedure. A single standard electronic scale was used for all weightings throughout the study. The body weight lost because of the surgical procedure and the interval needed for regaining the lost weight were recorded for all animals. Next, weight loss was compared between the two groups, as well as the time needed for regaining the lost weight.
The rats were checked once a day for at least one week after the implant procedure, and twice a week thereafter, for general health, morbidity and mortality.
ECG recording
A unique 24-h continuous ECG recording was performed on unrestrained, conscious rats.
ECG signal capture was accomplished with receivers (RPC-1; Data Sciences International) placed under each experimental cage. Telemetry ECGs were converted to analog signal (Analog ECG Output Adapter R08; Data Sciences International) and routed to a personal computer equipped with a signal acquisition card (NI PCIe-6251; National Instruments, Austin, TX). An acquisition program developed in our laboratory using LabVIEW 2009 software (National Instruments) allowed the signal to be continuously recorded with a 2000 Hz sampling frequency and stored on hard disk.
To ensure that the signal from one transmitter was not received and recorded by another receiver, the cages were placed at least 45 cm apart from each other, as recommended by the producer.
ECG analysis
ECG data were analyzed using a program recently developed in our laboratory using LabVIEW 2010 software (National Instruments) to automatically detect R waves and measure RR intervals. All ECG tracings were visually assessed and artifactual periods were discarded prior to analysis. ECG analysis was visually performed by two independent cardiologists. All ECG elements were assessed.
Experimental protocols
Emotional stress protocol in conscious rats
A mild emotional stress protocol elicited by means of a jet of air blown into the cage for 20 min was applied to each animal.6,7 Baseline recordings of approximately 30 min were performed for each animal before the onset of the protocol. The quality of the ECG signal was assessed based on ECG recordings obtained during stress protocol.
Subcutaneous injection of carbamylcholine in conscious rats
A baseline recording was obtained for each animal before a 0.4 mg/kg dose of carbamylcholine was injected subcutaneously into all SHRs. A 10-min period initiated 5 min after carbamylcholine injection was analyzed, and the quality of the ECG signal was assessed based on ECG recordings obtained during this interval.
Subcutaneous injection of isoprenaline in conscious rats
A baseline recording of at least 30 min was obtained for each animal before a 0.5 mg/kg dose of isoprenaline was injected subcutaneously into 4 of the 8 young SHRs. Experimental protocol was discontinued after 3 of the 4 SHRs having received isoprenaline died shortly after drug injection. A 10-min period initiated 5 min after isoprenaline injection was analyzed and the quality of the ECG signal was assessed based on ECG recordings obtained during this interval.
Local macroscopic structural assessment at explantation
Devices were kept in place for 4 months in aging SHRs and 8 months respectively in young SHRs. At the end of these intervals the devices were explanted and local macroscopic evaluation of the device, the two leads and surrounding tissues was visually performed.
Statistics
All data are expressed as mean ± SD. Between-group comparisons were performed using the Student test for paired or unpaired determinations, as appropriate. Differences within a group were determined by analysis of variance for repeated measures. A p value of less than 0.05 was considered statistically significant. Statistical analyses were undertaken using GraphPad Prism® software (GraphPad Software; San Diego, CA).
RESULTS
Post-implant evolution
At the moment of implant procedures, aging SHRs had higher body weight compared to young SHRs (448.1 ± 26.4 g for aging SHRs vs. 311.5 ± 7.7 g for young SHRs, p < 0.0001).
The day after the procedure mean body weight of young SHRs decreased by 6.1 ± 1.8 g (from 311.5 ± 7.7 g to 305.4 ± 8.3 g, p = 0.003) (Figure 2). In aging SHRs a mean weight loss of 7.1 ± 6.7 g was observed (from 448.1 ± 26.4 g before the procedure to 441.0 ± 23.9 g the day after the procedure, p = 0.03) (Figure 3). The two groups did not present significantly different initial weight losses (6.1 ± 1.8 g for young SHRs vs. 7.1 ± 6.7 g for aging SHRs, p = 0.59).
In young SHRs, the initial weight loss maintained until the sixth day after the procedure (D6) (p < 0.0001 compared to pre-procedure weight, repeated-measures ANOVA), but there was no significant difference between pre-procedural weight and the weight measured in the seventh day after the implant procedure (D7) (311.5 ± 7.7 g before the implant procedure vs. 309.5 ± 9.3 g in D7, p = 0.78). The tenth day after the procedure (D10) animal’s weights (323.6 ± 10.4 g) exceeded those measured before the implant procedure (p < 0.001) (Figure 2). In four of the 8 young SHRs the maximum weight loss was recorded the day after the procedure (D1), in 3 SHRs the minimum body weight was obtained in D2, while one rat had a minimum weight in D3.
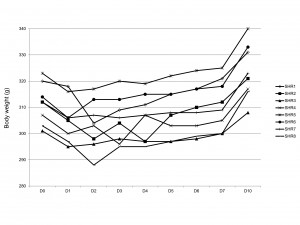
Figure 2. Body weight evolution in young SHRs. Counting of days starts with day 0 (D0), corresponding with the day of implantation. For D0 body weight was determined before the implant procedure.
In aging SHRs, the initial weight loss also maintained until the sixth day after the procedure (D6) (p < 0.001 compared to pre-procedure weight, repeated-measures ANOVA), but there was no significant difference between pre-procedural weights and the weights measured in the seventh day after the implant procedure (D7) (448.1 ± 26.4 g before the implant procedure vs. 444.0 ± 29.7 g in D7, p = 0.21) (Figure 3). The body weight measured the tenth day after the procedure (D10) was not significantly different compared to values obtained before the implant procedure (p = 0.35). In 3 of the 8 aging SHRs maximum weight loss was recorded the third day after the procedure (D3), while in the remaining 5 minimum body weight was obtained in D4. Body weight distribution was more heterogeneous in aging SHRs compared to young SHRs.
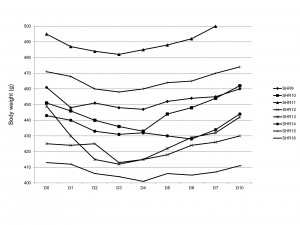
Figure 3. Body weight evolution in aging SHRs. Counting of days starts with day 0 (D0), corresponding with the day of implantation. For D0 body weight was determined before the implant procedure.
Already the day after the surgery (D1), all rats, regardless their age, seemed to have no effects on general health due to surgery. One week after the implant procedure (D7), all animals exhibited normal behavior and overall appearance, and did not show any consequences of surgery. No animal died during or shortly after the implantation procedure.
ECG analysis in conscious, unrestrained rats
High-quality ECG recordings were obtained in all animals in unrestrained, baseline conditions, starting one week after the implant procedure.
All ECG elements were easily recognizable on surface ECG in baseline conditions (Figure 4).
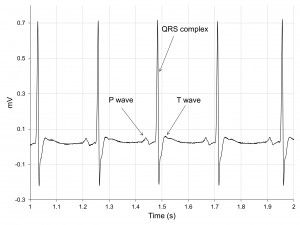
Figure 4. Baseline ECG tracing in a SHR. All elements are easily recognizable – P wave, QRS complex, T wave. Of note, the absence of the ST segment in rats.
Rhythm analysis was possible in all animals and various types of rhythm and conduction disturbances could be diagnosed based on surface ECG in baseline conditions (Figure 5).
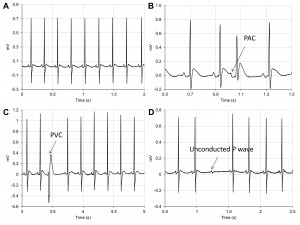
Figure 5. Baseline ECG tracings in SHRs showing – (A) normal sinus rhythm; (B) premature atrial contraction (PAC); (C) premature ventricular contraction (PVC) and (D) unconducted P wave.
Twenty-four-hour ECG monitoring indicated that aging SHRs were more bradycardic (278 ± 4 bpm) than young SHRs (326 ± 6 bpm, p < 0.01).
Quality of the ECG signal during emotional stress in conscious rats
The emotional stress induced significant increase in heart rate in both groups (from 282 ± 17 bpm to 412 ± 28 bpm in young SHRs, p < 0.001, and from 273 ± 26 bpm to 363 ± 20 bpm in aging SHRs, p < 0.001).
The quality of the ECG signal remained satisfactory during emotional stress, all ECG elements being recognizable throughout the duration of the protocol, despite sinus tachycardia (Figure 6).
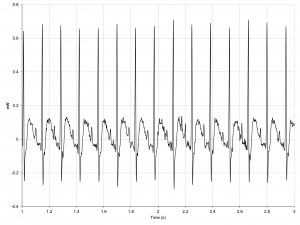
Figure 6. ECG tracing in a SHR during emotional stress protocol. All ECG elements remain easily recognizable, despite sinus tachycardia.
Quality of the ECG signal after carbamylcholine administration in conscious rats
The quality of the ECG signal remained satisfactory after carbamylcholine was injected, all ECG elements being recognizable on surface ECG tracings. Soon after the drug was injected SHRs presented frequent episodes of atrial tachyarrhythmia (Figure 7).
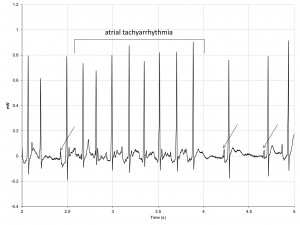
Figure 7. ECG tracing in a SHR after carbamylcholine administration showing frequent episodes of atrial tachyarrhythmia.
Quality of the ECG signal after isoprenaline administration in conscious rats
In young SHRs, significant initial increase in heart rate was observed immediately after isoprenaline was injected (from 296 ± 24 bpm to 450 ± 5 bpm, p = 0.001). Soon after the drug was injected, all SHRs presented episodes of torsades de pointes-like polymorphic ventricular tachycardia (Figure 8A). In three of the four young SHRs this arrhythmia degenerated into ventricular fibrillation (Figure 8B) and led to the death of these animals. In the fourth animal the arrhythmia terminated spontaneously, with restoration of sinus rhythm.
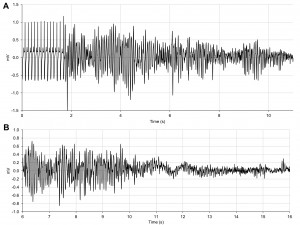
Figure 8. ECG tracing in a SHR after isoprenaline administration showing (A) torsades de pointes-like polymorphic ventricular tachycardia which (B) degenerates into ventricular fibrillation.
Local changes due to the presence of the device
At the moment of explantation all devices were in place. In one young SHR the negative ECG lead has slipped approximately 0.5 cm compared to its initial position. In all other animals the leads remained at their initial placement. No signs of infection were observed neither in the pocket, at the site of ECG leads placement, nor in the subcutaneous tunnels used for ECG leads placement. Little and thin adherences were present in two aging SHRs and in one young SHR, while no adherences were noticed in the other animals.
DISCUSSION
The main findings of the present study are that i) hypertensive rats, regarding their age, regained their initial weight within one week after subcutaneous radiotelemetry device implantation; ii) the initial weight loss after surgical procedure was similar in young and aging SHRs; iii) subcutaneous radiotelemetry device implantation was associated with small morbidity and no deaths were recorded during or shortly after these procedures; iv) high-quality ECG recordings were obtained in all animals and all ECG elements were easily recognizable on surface ECG in both baseline conditions and after various experimental protocols; v) radiotelemetry devices induced small or no local changes even after long periods of time.
Usefulness of radiotelemetry monitoring
It is generally accepted that, with the exception of studies of anesthetic drugs, special interest should be granted to achieving data from conscious, unrestrained animals, because such conditions optimally mimic physiological or pathophysiological conditions and the results of such measurements have the highest chances of being correctly extrapolated into humans. An important issue when performing experimental studies is the ability to measure various physiological parameters without the interference of external stressors such as handling of animals before or during measurements, which could lead to serious inconsistencies and might affect the reproducibility of the method.
Although wireless telemetry technology for experimental uses has existed for more than 60 years8,9, these devices had become largely used only in the last 20 years. Thus, radiotelemetry ECG monitoring is a relatively innovative technique, useful for studying pathophysiological mechanisms of various cardiovascular diseases.
Compared to non-invasive techniques, radiotelemetry device implantation offers the advantages of reducing stress, handling, restraint, anesthesia.1 Moreover, given that decreased animal stress also diminishes inter-individual variability10, the use of radiotelemetry devices also allows reducing the number of animals used in single or multiple studies.11,12
Timecourse of recovery after surgical implantation of radiotelemetry transmitters
Spontaneously hypertensive rats are one of the most frequently used strains for cardiovascular experimental studies, and judging by the number of publications, SHRs are the most studied model of hypertension.13 Since the use of radiotelemetry transmitters becomes more and more used, there is much interest in knowing the response of SHRs to implant procedures of such devices. Before planning a long-term study for cardiovascular assessment of SHRs by telemetry monitoring, data regarding the time required for recovery after radiotelemetry transmitter surgical implantation are needed. Especially since SHRs are known to be prone to increased morbidity and mortality rates following invasive techniques and general anesthesia.4 This is the first study to report the time needed for recovery and thus the time required prior to recordings onset in SHRs of different ages, based on body weight evolution. This parameter has been previously shown to be one of the most sensitive markers of recovery after surgical procedures.14
In consistency with previous studies,15 aging SHRs had higher baseline body weights compared to young SHRs. In our study, the weight of rats subjected to surgery decreased slightly the first day after the procedure, and the weight loss maintained for the next six days, regardless the age of the animals. This could be due to stress induced by anesthesia and subsequent surgery, as previously reported.16,17 Young SHRs had a maximum weight loss within the first 3 days, while for aging SHRs the maximum weight loss was recorded within the first 4 days after the procedure.
One week after the implant procedure all animals, regardless their age, regained their initial weights. Thus, we concluded that approximately one week is needed for SHRs to recover after subcutaneous surgical implantation of radiotelemetry devices, regardless their age. However, young SHRs tended to gain weight more rapidly after the procedure compared to aging SHRs. The tenth day after the implant procedure young SHRs already started to gain weight, while no such tendency was observed in aging SHRs and this could be due to flattened weight curve in SHRs with age.18
Morbidity and mortality related to telemetry implant procedures have been shown to be highly variable according to various features of the technique used for implantation,19,20 mainly its invasiveness, but also to the operator’s experience. In our study, small morbidity and no deaths were recorded during or after these procedures in either young or aging SHRs. Thus, subcutaneous implantation of ECG radiotelemetry devices under isoflurane anesthesia appears to be a safe technique in SHRs, regardless of their age.
Telemetry devices were well tolerated, even after long periods of time. Neither young nor aging SHRs presented serious local side effects due to the presence of the device. Local reactions with formation of adherences were rare and discrete. No signs of inflammation or infection were observed at the site of implantation. Thus, these devices seem to be well tolerated, allowing long-term recordings, with potentially important application in long-term pharmacological studies.
ECG signal quality
In our study, ECG signals were sampled at 2000 Hz sampling frequency, giving a high-resolution activity, and the resolution was of 16-bit. The quality of the acquired signal was very good, except for some brief periods when animals presented intense movement. Such periods should be discarded prior to analysis, especially if heart rate variability, or arrhythmia assessment, are the main purposes of the study. The programs developed in our laboratory for recording and analysis of ECG data allowed easy recognition of all ECG elements in both baseline and experimental conditions.
Determination of heart rate is crucial in most cardiovascular experimental protocols. Handling of animals before or during experimental protocols is a major source of error when assessing this parameter.1 Radiotelemetry monitoring allows heart rate, as well as other parameters, to be recorded in stress-free conditions. Moreover, this technique allows the possibility of long-term monitoring of such parameters, also allowing more complex evaluations, like circadian rhythm, which are not possible with standard external measurement devices.
Heart rate analysis indicated that aging SHRs were more bradycardic compared to young SHRs. In this strain, rise in blood pressure has been shown to begin around 5-6 weeks of age and systolic pressure may reach values between 180 and 200 mmHg in the adult.21 Long-term hypertension appears to force the autonomic nervous system to produce a higher vagal and lower sympathetic tone in order to compensate the increased blood pressure. This hypothesis is further sustained by previous studies showing that after the age of 4-5 weeks mean arterial pressure presents rapid increase, in association with progressive bradycardia.22
Conclusions
In our experience, surgery procedures for implanting radiotelemetry ECG devices in SHRs appear to be safe techniques, with short interval of recovery, low morbidity rates and no mortality, regardless the age of animals. Subcutaneous implantation of such devices offers high-quality ECG signal and is very well tolerated, even if long-term keeping in place is needed.
Conflict of interest: none declared
References
1. Kramer K, Kinter LB. Evaluation and applications of radio telemetry in small laboratory animals. Physiol Genomics, 2003; 13: 197–205.
2. Brockway BP, Mills PA, Azar SH. A new method for continuous chronic measurement and recording of blood pressure, heart rate and activity in the rat via radio telemetry. Clin Exp Hypertens A, 1991; 13: 885–95.
3. Deveney AM, Kjellström Å, Forsberg T, Jackson DM. A pharmacological validation of radiotelmetry in conscious, freely moving rats. J Pharmacol Toxicol Methods, 1998; 40: 87–93.
4. Howell SJ, Sear JW, Foëx P. Hypertension, hypertensive heart disease and perioperative cardiac risk. Br J Anaest, 2004; 92(4): 570–83.
5. Sgoifo A, Stilli D, Medici D, et al. Electrode Positioning for Reliable Telemetry ECG Recordings During Social Stress in Unrestrained Rats. Physiol Behav, 1996; 60(6): 1397–401.
6. Burke SL, Head GA. Cardiac and renal baroreflex control during stress in conscious renovascular hypertensive rabbits: effect of rilmenidine. J Hypertens, 2009; 27(1): 132–41.
7. Kanbar R, Oréa V, Barrès C, Julien C. Baroreflex control of renal sympathetic nerve activity during air-jet stress in rats. Am J Physiol Regul Integr Comp Physiol, 2007; 292(1): R362–7.
8. Brockway BP, Hassler CR. Application of radio-telemetry to cardiovascular measurements in pharmacology and toxicology. In New Technologies and Concepts for Reducing Drug Toxicity. Eds: Salem H, Baskin SI. Boca Raton: CRC, 1993, 109–32.
9. Kramer K, Kinter L, Brockway BP, et al. The use of radiotelemetry in small laboratory animals: recent advances. Contemp Top Lab Anim Sci, 2001; 40: 8–16.
10. Schnell CR, Gerber P. Training and remote monitoring of cardiovascular parameters in non-human primates. Prim Rep, 1997; 49: 61–70.
11. van Acker SA, Kramer K, Grimbergen JA, et al. Doxorubicin-induced cardiotoxicity monitored by ECG in freely moving mice. A new model to test potential protectors. Cancer Chemother Pharmacol, 1996; 38: 95–101.
12. Kinter LB. Cardiovascular telemetry and laboratory animals welfare: New reduction and refinement alternatives. In: General Pharmacology/Safety Pharmacology Meeting. Philadelphia, PA: 1996.
13. Pinto YM, Paul M, Ganten D. Lessons from rat models of hypertension: from Goldblatt to genetic engineering. Cardiovasc Res, 1998; 39: 77–88.
14. Greene AN, Clapp SL, Alper RH. Timecourse of recovery after surgical intraperitoneal implantation of radiotelemetry transmitters in rats. J Pharmacol Toxicol Methods, 2007; 56(2): 218–22.
15. Labat C, Cunha RSA, Challande P, Safar ME, Lacolley P. Respective contribution of age, mean arterial pressure, and body weight on central arterial distensibility in SHR. Am J Physiol Heart Circ Physiol, 2006; 290(4): H1534–9.
16. Kuntz C, Wunsch A, Bay F, et al. Prospective randomized study of stress and immune response after laparoscopic vs conventional colonic resection. Surg Endosc, 1998; 12(7): 963–7.
17. O’Neil PJ, Kaufman LN. Effects of indwelling arterial catheters or physical restraint on food consumption and growth patterns of rats: Advantages of noninvasive blood pressure measurements techniques. Lab Anim Sci, 1990; 40: 641–3.
18. Ritz MF, Fluri F, Engelter ST, Schaeren-Wiemers N, Lyrer PA. Cortical and Putamen Age-Related Changes in the Microvessel Density and Astrocyte Deficiency in Spontaneously Hypertensive and Stroke-Prone Spontaneously Hypertensive Rats. Curr Neurovasc Res, 2009; 6, 279–87.
19. Kramer K, van Acker SABE, Voss HP, et al. Use of telemetry to record electrocardiogram and heart rate in freely moving mice. J Pharmacol Toxicol Methods, 1993; 30(4): 209–15.
20. Moran MM, Roy RR, Wade CE, Corbin BJ, Grindeland RE. Size constraints of telemeters in rats. J Appl Physiol, 1998; 85(4): 1564–71.
21. Kundu S, Rao JP. The story of spontaneously hypertensive rat (SHR): A Review. Al Ameen J Med Sci, 2008; 1(1): 65–6.
22. Minami N, Imai Y, Munakata M, et al. Age-related changes in blood pressure, heart rate and baroreflex sensitivity in SHR. Clin Exp Pharmacol Physiol Suppl, 1989; 15: 85–7.
 This work is licensed under a
This work is licensed under a