Alina Scridon1,2, Răzvan Constantin Șerban2, Ayman Elkahlout3, Mihaela Opriș2, Dan Dobreanu1,2
1 Department of Physiology, University of Medicine and Pharmacy of Tîrgu Mureș, Romania
2 Department of Cardiology, Emergency Institute for Cardiovascular Diseases and Transplantation Tîrgu Mureș, Romania
3 Laboratory of Cardiac Catheterization, Emergency Institute for Cardiovascular Diseases and Transplantation Tîrgu Mureș, Romania
Contact address:
Mihaela Opriș, MD, PhD
Department of Cardiology, Emergency Institute for Cardiovascular Diseases and Transplantation Tîrgu Mureș, 50, Gheorghe Marinescu Street, 540136, Tîrgu Mureș, Romania
E-mail: m_opris2000@yahoo.com
Abstract: Coronary artery disease is the most frequent cause of heart failure. Accumulating evidence indicate that patients with ischemic cardiomyopathy may benefit from successful coronary revascularization in addition to optimal medical treatment. We report a notable case of very early, highly successful response to percutaneous coronary revascularization in a patient with long history of coronary artery disease and severe myocardial hibernation. In patients with left ventricular dysfunction due to chronic coronary artery disease and high probability of viable myocardium, prompt coronary revascularization should be considered.
Keywords: hibernating myocardium, coronary artery disease, revascularization
Rezumat: Boala coronariană reprezintă cea mai frecventă cauză de insuficiență cardiacă. Rezultatele studiilor clinice indică faptul că revascularizarea coronariană eficientă la pacienții cu cardiomiopatie ischemică poate aduce beneficii suplimentare tratamentului medical optimal. Lucrarea de față prezintă un caz particular de răspuns extrem de favorabil și foarte precoce la terapia de revascularizare coronariană percutană la un pacient cu istoric îndelungat de boală coronariană și hibernare miocardică severă. La pacienții cu disfuncție ventriculară stângă secundară afectării coronariene cronice și la care există o probabilitate mare de miocard viabil, revascularizarea coronariană promptă ar trebui luată în considerare.
Cuvinte cheie: hibernare miocardică, boală coronariană, revascularizare
INTRODUCTION
In more than two thirds of cases, heart failure emanates from cardiac damage due to chronic coronary artery disease1. Left ventricular (LV) dysfunction due to chronic myocardial ischemia has long been considered an irreversible process. The identification of two new entities, myocardial stunning and myocardial hibernation, suggests however that this is not necessarily true2. Myocardial stunning develops in relation with acute transient ischemia, whilst myocardial hibernation arises from chronically reduced coronary blood flow3. More importantly, both settings imply the presence of viable myocardium. Accumulating evidence indicate that patients with ischemic cardiomyopathy may benefit from successful coronary revascularization, displaying improved myocardial function, symptoms, and prognosis, due to functional improvement of the hypoperfused, but viable myocardium2,4,5. However, the time-course and the extent of functional recovery after coronary revascularization seem to be highly dependent on the duration of myocardial hibernation6.
We report a notable case of very early, highly successful response to percutaneous coronary revascularization in a patient with a long history of coronary artery disease and severe myocardial hibernation.
CASE REPORT
A 56-year-old Caucasian male presented for evaluation of New York Heart Association (NYHA) class III heart failure symptoms. At age 45 years, in the absence of any prior symptoms or known cardiac pathology, he was admitted to hospital for a large anterior myocardial infarction. His risk factors included grade II arterial hypertension, dyslipidemia, and grade II obesity. Emergency coronary angiography performed 5 hours after the onset of symptoms revealed proximal subocclusion of the left anterior descending (LAD) artery with TIMI I flow. No other significant coronary lesions were observed at that time. He underwent primary percutaneous coronary intervention with bare metal stent implantation of the LAD, with favorable post-procedural evolution. At discharge, the patient was completely asymptomatic. The ECG revealed persistent ST-segment elevation in leads V2-5, pathological Q waves in V1-3 leads, and negative T waves in lead V6. Echocardiographic assessment showed normal LV function (LV ejection fraction of 60%), with moderate hypokinesia of the apical third of the interventricular septum, and of the anterior and lateral LV walls. He was discharged on double antiplatelet therapy, beta-blocker, angiotensin converting enzyme inhibitor and statin.
The patient did well for 8 years, when he was readmitted for chest pain and heart failure symptoms at moderate exertion. He admitted having abandoned his treatment 6 years earlier. The ECG displayed a persistent pattern (Figure 1). Echocardiography revealed 50% LV ejection fraction, hypertrophied and slightly dilated LV (LV end-diastolic diameter 57 mm), akinesia of the apex and of the apical third of the interventricular septum, hypokinesia of the apical third of the anterior and lateral walls and of the middle third of the interventricular septum, and mild aortic and mitral valve regurgitation. Coronary angiography was performed, revealing no restenosis in the proximal LAD stent, a 30% stenosis followed by severe stenosis of the mid segment of the circumflex artery (ACx), and 30% stenosis in the proximal segment of the right coronary artery (RCA) (Figure 2A, B). Successful coronary angioplasty of the ACx with bare metal stent implantation was performed (Figure 2C). The patient was discharged free from angina, on double antiplatelet therapy, beta-blocker, angiotensin conversion enzyme inhibitor, statin, and calcium channel blocker.
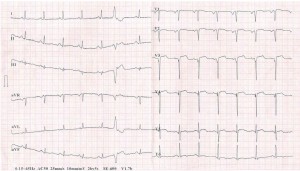
Figure 1. ECG tracing depicting ST-segment elevation in leads V2-5, pathological Q waves in V1-3 leads, and negative T waves in lead V6.
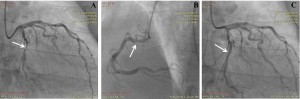
Figure 2. Coronary angiograms. (A) Right-anterior-oblique caudal view showing severe stenosis of the mid segment of the circumflex artery (ACx) (arrow). (B) Left-anterior-oblique cranial view showing 30% stenosis in the proximal segment of the right coronary artery (arrow). (C) Right-anterior-oblique caudal view showing the final angiographic outcome of coronary angioplasty of the ACx with bare metal stent implantation (arrow).
Three years later, the patient was readmitted with one month history of dyspnea and fatigue at mild exertion. Again, the patient admitted having abandoned his treatment one year earlier. Physical examination revealed rales on pulmonary auscultation, and mild systolic and diastolic murmurs on the mitral and aortic points, respectively. ECG findings were similar to the previous tracings. Compared to the prior examination, echocardiography revealed severely impaired LV systolic function (LV ejection fraction 27%) with preserved LV walls thickness (Figure 3A), and the presence of an apical LV thrombus (Figure 3B), for which the patient was started on anticoagulation. Coronarographic examination revealed 90% stenoses of the first diagonal (Figure 4A), of the ACx proximal to the stent (Figure 4B), and of the first segment of the RCA (Figure 4C). Percutaneous transluminal angioplasty and primary stenting of the 90% stenoses of the ACx (Figure 4D) and RCA (Figure 4E) with bare metal stents was performed, with successful procedural outcome. Three days after the procedure, echocardiographic examination revealed significant recovery of LV systolic function, with >60% basal (Figure 5) and 45% global ejection fraction. The patient’s symptoms were relieved, and he was discharged on oral anticoagulation with vitamin K antagonists, dual antiplatelet therapy, beta-blocker, angiotensin converting enzyme inhibitor, calcium channel blocker, low-dose loop diuretic, statin, and gastric protection.
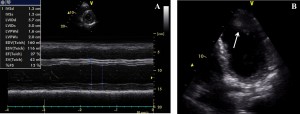
Figure 3. Echocardiographic images. (A) M-mode of the left ventricle (LV) in left parasternal short axis view demonstrating hypertrophied and slightly dilated LV, and severely impaired LV systolic function (LV ejection fraction 27%). (B) B-mode in apical view showing the presence of an apical LV thrombus (arrow).
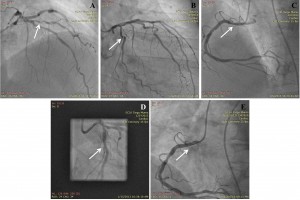
Figure 4. Coronary angiograms. (A) Right-anterior-oblique cranial view showing severe stenosis of the first diagonal artery (arrow). (B) Right-anterior-oblique caudal view showing severe stenosis of the circumflex artery (ACx) proximal to the stent (arrow). (C) Left-anterior-oblique cranial view showing severe stenosis of the first segment of the right coronary artery (RCA) (arrow). (D) Right-anterior-oblique caudal view showing the final angiographic outcome of coronary angioplasty of the ACx with bare metal stent implantation (arrow). (E) Left-anterior-oblique cranial view showing the final angiographic outcome of coronary angioplasty of the RCA with bare metal stent implantation (arrow).
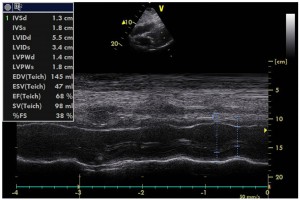
Figure 5. M-mode echocardiographic image of the left ventricle (LV) in left parasternal long axis view demonstrating significant recovery of LV systolic function post-coronary angioplasty, with >60% basal LV ejection fraction.
At 6-months follow-up, the patient continued to do well, with no further chest pain or dyspnea on exertion. Echocardiographic findings were similar to pre-discharge results, showing 45% LV ejection fraction and no intra-ventricular thrombosis.
DISCUSSION
Heart failure affects 1% to 3% of the general population, associating 5-year mortality rates as high as 75% following a first hospital admission7. Although optimal pharmacological therapy significantly reduces mortality among heart failure patients, this population continues to display high mortality rates8.
More than two thirds of heart failure cases are related to chronic coronary artery disease1. Until recently, LV dysfunction in these patients has been considered an irreversible process. However, in the past decades, myocardial hibernation, which can be demonstrated in up to one third of patients with chronic coronary artery disease and impaired LV function9, has been recognized as an aggravating factor for LV dysfunction in this population2. Unlike tissue necrosis, myocardial hibernation implies the presence of hypoperfused but viable myocardium10, and, more importantly, appears to be partially or even completely reversible upon surgical or percutaneous coronary revascularization3. This observation could explain the significantly higher survival rates associated with the use of revascularization strategies in addition to optimal pharmacological strategies11. Indeed, accumulating evidence indicate that patients with heart failure due to chronically reduced coronary blood flow may benefit from coronary revascularization through improved myocardial function, symptoms, and prognosis2,4,5. Furthermore, benefit from revascularization may also be attributable to decreased propensity to ventricular arrhythmias and reduction of subsequent ischemic events due to restored coronary blood flow12,13.
Differentiation between LV dysfunction caused by myocardial necrosis and scar tissue formation versus myocardial hibernation may thus have important clinical consequences14. In our patient, preserved thickness of LV walls despite severely impaired ejection fraction, indicating preserved myocardial viability15, prompted us to perform coronary angiography and stent angioplasty of significant coronary lesions. Given that the time-course and the extent of functional recovery after coronary revascularization seem to be highly dependent on the duration of the hibernating status6, the short history of heart failure symptoms in our patient could explain the very early significant recovery of myocardial function after successful coronary revascularization.
Cases of significant early recovery following percutaneous coronary revascularization have already been reported. However, this usually happens in patients with stunned myocardium and LV dysfunction following acute coronary events16, whilst in patients with hibernating myocardium recovery usually takes longer17. Furthermore, early recovery of myocardial function upon percutaneous coronary revascularization in patients with hibernating myocardium is usually less important and occurs in patients with less severe LV impairment18.
CONCLUSIONS
This case report illustrates a very early, highly successful response to revascularization in a patient with a long history of coronary artery disease and severe myocardial hibernation. In patients with LV dysfunction due to chronic coronary artery disease and high probability of viable myocardium, prompt coronary revascularization should be considered.
Conflicts of interests: none declared.
References
1. Gheorghiade M, Sopko G, De Luca L et al. Navigating the crossroads of coronary artery disease and heart failure. Circulation, 2006; 114: 1202-13.
2. Camici PG, Rimoldi OE. The contribution of hibernation to heart failure. Ann Med, 2004; 36: 440-447.
3. Rahimtoola SH. The hibernating myocardium. Am Heart J, 1989; 117: 211-21.
4. Beller GA. More evidence for the survival benefit of coronary revascularization versus medical therapy in patients with ischemic cardiomyopathy and hibernating myocardium. Circ Cardiovasc Imaging, 2013; 6(3): 355-7.
5. Henderson RA, Timmis AD. Almanac 2011: stable coronary artery disease. An editorial overview of selected research that has driven recent advances in clinical cardiology. Romanian Journal of Cardiology, 2012; 22(1): 15-25.
6. Rahimtoola SH, La Canna G, Ferrari R. Hibernating myocardium: another piece of the puzzle falls into place. J Am Coll Cardiol, 2006; 47: 978-80.
7. McMurray JJV, Stewart S. The burden of heart failure. Eur Heart J Suppl, 2002; 4: D50-8.
8. Khand A, Gemmel I, Clark AL et al. Is the prognosis of heart failure improving? J Am Coll Cardiol, 2000; 36: 2284-6.
9. Elsässer A, Schlepper M, Klövekorn WP et al. Hibernating myocardium: an incomplete adaptation to ischemia. Circulation, 1997; 96: 2920-31.
10. Lupaşcu L, Popescu BA, Ginghină C. Viabilitatea miocardică – diagnostic şi implicaţii terapeutice. Revista Română de Cardiologie, 2010; 25(4): 248-53.
11. Di Carli MF, Hachamovitch R. New technology for noninvasive evaluation of coronary artery disease. Circulation, 2007; 115: 1464-80.
12. Canty JM Jr, Suzuki G, Banas MD et al. Hibernating myocardium: chronically adapted to ischemia but vulnerable to sudden death. Circ Res, 2004; 94: 1142-9.
13. Wissner E, Mookadam F. Thirty-four years of hibernating myocardium: a case report. J Nucl Cardiol, 2007; 14(5): 745-9.
14. Buckley O, Di Carli M. Predicting benefit from revascularization in patients with ischemic heart failure: imaging of myocardial ischemia and viability. Circulation, 2011; 123(4): 444-50.
15. Cwajg J, Cwajg E, Nagueh SF et al. End-diastolic wall thickness as predictor of recovery of function in myocardial hibernation. Relation to rest-redistribution Tl-201 tomography and dobutamine stress echocardiography. J Am Coll Cardiol, 2000; 35: 1152-61.
16. Sharafi A, Kassaian SE, Sharif AY et al. Significant improvement in severely stunned left ventricle after percutaneous coronary intervention. J Teh Univ Heart Ctr 3, 2008; 173-5.
17. Bax JJ, Visser FC, Poldermans D et al. Time course of functional recovery of stunned and hibernating segments after surgical revascularization. Circulation, 2001; 104(12 Suppl 1): I314-8.
18. Schinkel AF, Poldermans D, Vanoverschelde JL et al. Incidence of recovery of contractile function following revascularization in patients with ischemic left ventricular dysfunction. Am J Cardiol, 2004; 93(1): 14-7.
 This work is licensed under a
This work is licensed under a