Emilia Goanta1, Ermenegildo de Ruvo2, Leonardo Calò2
1 Institute of Cardiovascular Diseases, Timisoara, Romania
2 Division of Cardiology, Policlinico Casilino, Rome, Italy
Abstract: Implantable cardioverter-defi brillators (ICDs) prevent sudden cardiac death (SCD) in patients at high risk of sudden arrhythmic death. Long-term studies have proven the effi cacy of this system, however, implantation of endocardial leads is associated with signifi cant procedural and long-term complications1,2. To overcome this complications an entirely
subcutaneous implantable cardioverter defi brillator (S-ICD) has been developed. This approach is demonstrated to be safe and effective. The purpose of this review is to assess the current evidence from the clinical trials and future directions of this novel technology.
Keywords: implantable cardioverter defi brillator, ventricular fi brillation, sudden cardiac death, defi brillation threshold, inappropriate shocks.
INTRODUCTION
S-ICD represents a viable option to transvenous implantable defibrillator (TV-ICD) for the prevention of SCD. ICD therapy is well established as a successful treatment strategy. Transvenous leads, however, still remains the weakest part of the system3,4. The complications associated with the TV-ICD lead to the development of an entirely subcutaneous ICD, aiming to provide the same protection of the TV-ICD but with less risk of complications. S-ICD can provide substantial advantages, particularly in young patients with a long life expectancy and an active lifestyle, which are prone to a high risk of lead fracture, and in congenital heart disease with difficult access to the right cardiac chambers. The benefits of avoiding the use of transvenous leads must be weighed against the potential need for pacing. The system has no bradycardia pacing function, only 30 seconds post shock, and no antitachycardia pacing (ATP).
OVERVIEW OF THE SYSTEM
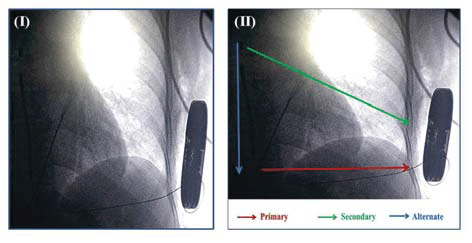
S-ICD detects and treats malignant ventricular arrhythmias without requiring vascular access. The system includes a subcutaneous pulse generator enclosed in a titanium case and a single subcutaneous tripolar electrode with a length of 45 cm (Figure 1 (I)). The lead has polyurethane insulation and is composed of a proximal and distal electrode separated by a shocking coil with a surface area of 750 mm2 and a size of 9F/3 mm. S-ICD system is implanted using anatomical landmarks, after recording a 3-lead surface ECG to assess the surface signals, reducing the need for fl uoroscopy during implant5. The pulse generator is bigger than TV-ICD generator and is placed at the mid-axillary line between the 5th and 6th intercostal spaces. The electrode is positioned
parallel to and 1 to 2 cm to the left sternal midline, through two subcutaneous tunnels, one from the pocket to the xiphoid incision and the second from the xiphoid to the superior incision.
Figure 1. (I) Radiographic image of subcutaneous implantable defibrilator (S-ICD). (II) The three available sensing vector of the S-ICD primary (ring to can), secondary (tip to can) and alternate (tip to ring).
The device senses subcutaneous signals using three available sensing vectors (Figure. 1 (II)): primary (ring to can), secondary (tip to can) and alternate (tip to ring). The system automatically chooses the optimal sense vector for rhythm detection. Four separate algorithms are used to correctly identify the rhythm and prevent oversensing: rate, sinus rhythm template, dynamic morphology and QRS width analysis. For arrhythmia termination two zones are programmable. One is the conditional zone (170-240 bpm, 10 bpm less than shock zone) using rate detection and rhythm discrimination and the other is shock zone (170-250 bpm) using only rate detection. The device testing during implantation should be performed to evaluate proper sensing, detection and charge time, to assess acute energy requirements and to evaluate post shock pacing. Postprocedure the device delivers up to five biphasic shocks per episode of 80 J. Available shock polarity is standard (coil to can) or reverse (can to coil) and can automatically be reversed if initial shock is unsuccessful. Charge time is approximately 14 seconds (sec), with a post-shock pacing at 50 bpm up to 30 sec and no ATP option available.
EVIDENCE
The main concern with any novel technology is proper performance. Several clinical trials have proven the effectiveness of S-ICD in detecting and treating lethal ventricular tachyarrhythmias. Earliest data were published by Bardy et al in 20105. In their study, the best lead confi guration was fi rst identifi ed, chosen from four different confi gurations, selected on the basis
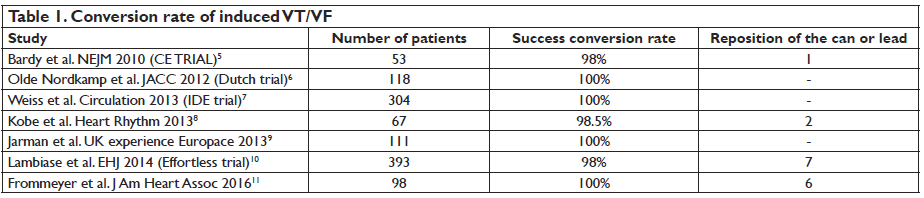
of specific anatomical landmarks. The suitable device configuration was with the pulse generator position left lateral and the electrode coil, of 8mm, at the left parasternal margin. Second, the defi brillation threshold (DFT) of the device was assessed in comparison to that of the conventional ICD. The S-ICD was as effective as TV-ICD for cessation of induced ventricular fibrillation but with a significantly higher energy requirement. Studies that followed this publication supported S-ICD effi cacy and safety in detecting and terminating induced VT/VF episodes, the acute success rate ranging between 98%-100% (Table 1)5-11. Should we check DFT following S-ICD implantation? In the SIMPLE study, TV-ICD implantation without defibrillation testing was non inferior to intraoperative defibrillation testing regarding long-term efficacy of the TV-ICD or total mortality12. This is not the case for S-ICD and the results of two studies underline that DFT intraoperative is currently necessary. One study was published in 2013 by Kobe et al. and assessed the DFT using a step by step protocol. Success conversion rate was quite low using a protocol of 65J, 15J safety margin, with a standard shock polarity, only 89.5%.
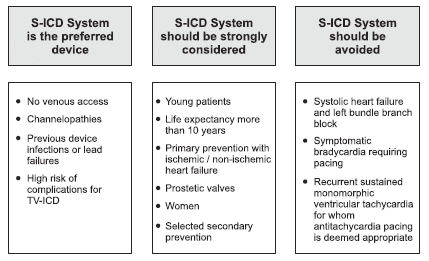
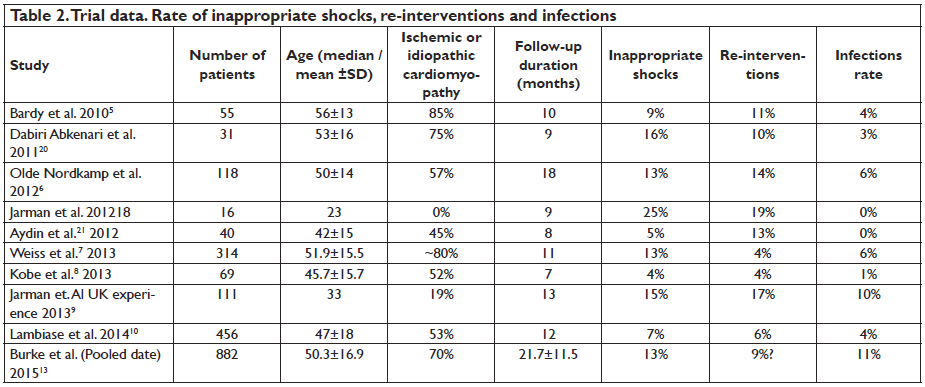
The other cases required change of shock polarity, higher energy or reposition, reaching finally an overall rate of 98%8. In the study published by Frommeyer et al. in 2016 the success rate of acute conversion using a protocol of 65J reached 90%, but 15% of these had a reverse polarity shock. In a small percentage of the cases (4%) a reduce safety margin, less than 10J was accepted. Ineffective repeated shocks droved to reposition of the system in 6 cases11. These results seem anomalous considering the initial evaluation of the lead configuration reported by Bardy which exhibited a DFT mean of 32.5±17 J for the actual S-ICD configuration5. Another aspect to consider is the maximum energy shock of the device, no more than 80J and no other possible interventions to improve shock efficacy that are available in TV-ICD systems, such as additional coil or a subcutaneous array. What about long-term performance? Using pooled data from two large studies, Effortless and IDE trial, on 882 patients, Burke et al. showed that 90.1% of VT/ VF episodes were stopped after one shock and 98.2% were terminated after up to fi ve shocks13. About 37% of VT/VF episodes were self-terminated, due to longer programming time-to-therapy (19.2±5.3sec). Overall, the estimated 3 year rate of inappropriate shocks was 13.1% but this rate was reduced over time. START study results and MADIT-RIT led the operators to change programming of the device14,15. Higher cutoff rate and dual zone shocks programming decreased significantly the incidence of inappropriate shocks. In patients with programmed dual zone the incidence of inappropriate shocks at 3 years was signifi cant lower (11.7%) than those with single zone (20.5%). Inappropriate therapy remains a major concern in all ICD system, regardless of whether it is a S-ICD or TV-ICD, and is associated with a decrease in the quality of life and increase in mortality16,17. The rate of inappropriate shocks observed in the S-ICD trials ranges between 4% to 25%, not so different from TV-ICD. These occur, however, through a different mechanism, mostly secondary to T-wave oversensing (Table 2). If we analyze data from the S-ICD trials, the highest rate of inappropriate shocks was reported by Jarman et al. in 201218. This was a small study that included only young patients with a mean age of 20 years, without ischemic or dilated cardiomyopathy. It is curious why this population exhibited the highest rate of inappropriate shocks given the fact that in this group, an S-ICD system should be strongly considered (Figure 2)19. The authors note this could be due to cardiac repolarization, which differs from adult population, although they did not identify any predictor factors for inappropriate shocks, secondary to T-wave oversensing (TWOS). A retrospective study which analyzed patients who received a TV-ICD found that 55% of them at 5 years follow-up would have been suitable for S-ICD. Predictors for S-ICD unsuitability were found to be QRS width, advanced heart failure and secondary prevention22. In a study published by Groh et al. in 2014, 8% of S-ICD candidates had inadequate signals and predictors were T-wave inversions, on standard 12 lead ECG, in DI, DII and aVF23. Another study, published in the same year, found as predictors factors for screening failure patients with hypertrophic cardiomyopathy, heavy weight, prolonged QRS duration and R:T ratio less than 3 in the ECG lead with the largest T wave24.
Figure 2. Recommendations for S-ICD implantation19.
This aspect should underline the importance of preimplantation screening to identify the suitable patients for S-ICD. On the other hand, a study from Zeb et al. in 2015 found less specifi c the S-ICD screening tool, especially in patients with complex congenital heart disease25. Templates acquired during exercise testing post-implant procedure can prevent TWOS, leading also in choosing the best sensing vector confi guration26. New algorithms developed for TWOS may help prevent inappropriate shocks without affecting ventricular sensing arrhythmias27. Other points of concern are the rate of re-interventions and infections associated with S-ICD system (Table 2). Rate of re-interventions was up to 19%, being higher in the young population and mainly due to pocket erosions18. Rate of infections was up to 11%, higher in the pooled data published by Burke and al 13. In their data on 882 patients, only 1.7% of infections required extraction. The majority of superficial wounds responded well to antibiotic treatment. Nevertheless, the incidence declined overtime and this may be related to initial inexperience of the operators with the surgical technique of implantation. Providing a rigorous procedural preparation and improving the implantation technique by using 2-incision technique instead of 3-incision technique may have helped28.
FUTURE DIRECTIONS
The S-ICD represents a major advancement in ICD technology. Since it was introduced on the market the system continues to evolve. Maybe, for the next generations of devices, current limitation will be addressed such as device longevity, dimension and shape, lower DFT and pacing ability. Integration of an S-ICD system with a leadless pacing could play an important role and would widen the implant indications in those with bradycardia or in patients requiring ATP-pacing. Recently, a study published by Tjong et.al investigated the feasibility of an S-ICD with a leadless pacing device29. The study was first conducted on animals, and later, on human subjects. They concluded there was no interference on performance between devices, without dislodgment
of the leadless pacing system after repeated shocks, from either S-ICD or external defi brillator. This same author published, shortly after, the fi rst concept study of a combined leadless pacing prototype in a patient with an preexisting S-ICD, manufactured by the same company30. Ventricular tachycardia was successfully detected by the S-ICD and communication to the leadless
pacing trigger with success the ATP pacing with interruption of the tachycardia. Promising results also come from some recent studies using substernal leads placement. Lead implants were done using percutaneous sub-xiphoid approach, under fluoroscopic guidance with a peelable sheath into the substernal space. Preliminary data have shown that this approach requires lower DFT than S-ICD, providing the opportunity for greater device longevity and smaller device size31. Furthermore, it seems that from this extravascular space pacing was possible, overcoming the limitations of current S-ICD, without the need to integrate a leadless pacing system in the majority of patients32. This could mean the beginning of a new era of devices, but further studies are needed to assess the feasibility and safety of these systems.
Conflict of interest: none declared.
References
1. Goldenberg I, Gillespie J, Moss AJ, Hall WJ, Klein H, McNitt S, Brown MW, Cygankiewicz I, Zareba W, Executive Committee of the Multicenter Automatic Defi brillator Implantation Trial II. Long-term benefi t of primary prevention with an implantable cardioverter-defi brillator: an extended 8-year follow-up study of the Multicenter Automatic Defi brillator Implantation Trial II. Circulation 2010;122:1265–1271.
2. van der Heijden AC, Borleffs CJ, Buiten MS, Thijssen J, van Rees JB, Cannegieter SC, Schalij MJ, van Erven L. The clinical course of patients with implantable defi brillators: Extended experience on clinical outcome, device replacements, and device-related complications. Heart Rhythm 2015;12:1169–1176.
3. Maisel WH. Transvenous implantable cardioverter-defi brillator leads: the weakest link. Circulation. 2007; 115(19): 2461-3.
4. Maisel WH, Kramer DB. Implantable cardioverter-defi brillator lead performance. Circulation 2008; 117(21): 2721-3
5. Bardy GH, Smith WM, Hood MA, Crozier IG, Melton IC, Jordaens L, Theuns D, Park RE, Wright DJ, Connelly DT, Fynn SP, Murgatroyd FD, Sperzel J, Neuzner J, Spitzer SG, Ardashev AV, Oduro A, Boersma L, Maass AH, Van Gelder IC, Wilde AA, van Dessel PF, Knops RE, Barr CS, Lupo P, Cappato R, Grace AA (2010) An entirely subcutaneous implantable cardioverter-defi brillator. N Engl J Med 363(1):36–44
6. Olde Nordkamp LR, Dabiri Abkenari L, Boersma LV, Maass AH, de Groot JR, van Oostrom AJ, Theuns DA, Jordaens LJ, Wilde AA, Knops RE. The entirely subcutaneous implantable cardioverter-defibrillator: initial clinical experience in a large Dutch cohort. J Am Coll Cardiol. 2012;60:1933–1939.
7. Weiss R, Knight BP, Gold MR, Leon AR, Herre JM, Hood M, Rashtian M, Kremers M, Crozier I, Lee KL, Smith W, Burke MC (2013) Safety and effi cacy of a totally subcutaneous implantable-cardioverter defibrillator. Circulation 128(9):944–953
8. Kobe J, Reinke F, Meyer C, Shin DI, Martens E, Kaab S, Loher A, Amler S, Lichtenberg A, Winter J, Eckardt L. Implantation and follow-up of totally subcutaneous versus conventional implantable cardioverterdefibrillators: a multicenter case-control study. Heart Rhythm 2013; 10:29–36.
9. Jarman JW, Todd DM. United Kingdom national experience of entirely subcutaneous implantable cardioverter-defi brillator technology: important lessons to learn. Europace. 2013;15:1158–1165.
10. Lambiase PD, Barr C, Theuns DA, Knops R, Neuzil P, Johansen JB, Hood M, Pedersen S, Kaab S, Murgatroyd F, Reeve HL, Carter N, Boersma L. Worldwide experience with a totally subcutaneous implantable defibrillator: early results from the EFFORTLESS S-ICD Registry. Eur Heart J. 2014;35:1657–1665.
11. Frommeyer G, Zumhagen S, Dechering DG, Larbig R, Bettin M, Löher A, Köbe J, Reinke F, Eckardt L. Intraoperative Defi brillation Testing of Subcutaneous Implantable Cardioverter-Defi brillator Systems-A Simple Issue? J Am Heart Assoc. 2016 Mar 15 ;5(3):e003181.
12. Healey JS, Hohnloser SH, Glikson M, Neuzner J, Mabo P, Vinolas X, Kautzner J, O’Hara G, VanErven L, Gadler F, Pogue J, Appl U, Gilkerson J, Pochet T, Stein KM, Merkely B, Chrolavicius S, Meeks B, Foldesi C, Thibault B, Connolly SJ; Shockless IEi. Cardioverter defi brillator implantation without induction of ventricular fi brillation: a singleblind, non-inferiority, randomised controlled trial (SIMPLE). Lancet. 2015;385:785–791.
13. Burke MC, Gold MR, Knight BP, Barr CS, Theuns DA, Boersma LV, Knops RE, Weiss R, Leon AR, Herre JM, Husby M, Stein KM, Lambiase PD. Safety and effi cacy of the totally subcutaneous implantable defibrillator: 2-year results from a pooled analysis of the IDE Study and EFFORTLESS Registry. J Am Coll Cardiol. 2015;65:1605–1615.
14. Gold MR, Theuns DA, Knight BP, Sturdivant JL, Sanghera R, Ellenbogen KA, Wood MA, Burke MC. Head-to-head comparison of arrhythmia discrimination performance of subcutaneous and transvenous
ICD arrhythmia detection algorithms: the START study. J Cardiovasc Electrophysiol. 2012 Apr;23(4):359-66.
15. Moss AJ, Schuger C, Beck CA, Brown MW, Cannom DS, Daubert JP, Estes NA 3rd, Greenberg H, Hall WJ, Huang DT, Kautzner J, Klein H, McNitt S, Olshansky B, Shoda M, Wilber D, Zareba W; MADIT- RIT Trial Investigators. Reduction in inappropriate therapy and mortality through ICD programming. N Engl J Med. 2012 Dec 13;367(24):2275-83.
16. Kamphuis HC, de Leeuw JR, Derksen R, Hauer RN, Winnubst JA. Implantable cardioverter defibrillator recipients: quality of life in recipients with and without ICD shock delivery: a prospective study. Europace 2003;5:381–389.
17. Poole JE, JohnsonGW, Hellkamp AS, Anderson J, Callans DJ, Raitt MH, Reddy RK, Marchlinski FE, Yee R, Guarnieri T, Talajic M, Wilber DJ, Fishbein DP, Packer DL, Mark DB, Lee KL, Bardy GH. Prognostic importance of defibrillator shocks in patients with heart failure. N Engl J Med 2008;359:1009–1017.
18. Jarman JW, Lascelles K, Wong T, Markides V, Clague JR, Till J. Clinical experience of entirely subcutaneous implantable cardioverter-defi – brillators in children and adults: cause for caution. Eur Heart J. 2012 Jun;33(11):1351-9.
19. Poole, J and Gold, M. Who Should Receive the Subcutaneous Implanted Defibrillator? The Subcutaneous Implantable Cardioverter Defi brillator Should be Considered in all ICD patients Who Do not Require Pacing. Circulation 2013; 6: 1236-1245.
20. Dabiri Abkenari L, Theuns DA, Valk SD, Van Belle Y, de Groot NM, Haitsma D, Muskens-Heemskerk A, Szili-Torok T, Jordaens L. Clinical experience with a novel subcutaneous implantable defibrillator system in a single center. Clin Res Cardiol. 2011 Sep;100(9):737-44.
21. Aydin A, Hartel F, Schlüter M, Butter C, Kobe J, Seifert M, Gosau N, Hoffmann B, Hoffmann M, Vettorazzi E, Wilke I, Wegscheider K, Reichenspurner H, Eckardt L, Steven D, Willems S. Shock effi cacy of subcutaneous implantable cardioverter-defi brillator for prevention of sudden cardiac death: initial multicentre experience. Circ Arrhythm Electrophysiol 2012;5:913–9.
22. de Bie MK, Thijssen J, van Rees JB, Putter H, van der Velde ET, Schalij MJ, van Erven L. Suitability for subcutaneous defi brillator implantation: results based on data from routine clinical practice. Heart. 2013 Jul;99(14):1018-23.
23. Groh CA, Sharma S, Pelchovitz DJ, Bhave PD, Rhyner J, Verma N, Arora R, Chicos AB, Kim SS, Lin AC, Passman RS, Knight BP. Use of an electrocardiographic screening tool to determine candidacy for a subcutaneous implantable cardioverter-defi brillator. Heart Rhythm. 2014 Aug;11(8):1361-6.
24. Olde Nordkamp LR, Warnaars JL, Kooiman KM, de Groot JR, Rosenmoller BR, Wilde AA, Knops RE. Which patients are not suitable for a subcutaneous ICD: incidence and predictors of failed QRST-
wave morphology screening. J Cardiovasc Electrophysiol. 2014 May;25(5):494-9.
25. M. Zeb, N. Curzen, V. Allavatam, Wilson D, Yue A, Roberts P, Morgan. Sensitivity and specifi city of the subcutaneous implantable cardioverter defibrillator pre-implant screening tool Int J Cardiol, 195 (2015), pp. 205–209.
26. Kooiman KM, Knops RE, Olde Nordkamp L, Wilde AA, de Groot JR. Inappropriate subcutaneous implantable cardioverter-defi brillator shocks due to T-wave oversensing can be prevented: implications for management. Heart Rhythm. 2014 Mar;11(3):426-34.
27. Brisben AJ, Burke MC, Knight BP, Hahn SJ, Herrmann KL, Allavatam V, Mahajan D, Sanghera R, Gold MR. A new algorithm to reduce inappropriate therapy in the S-ICD system. J Cardiovasc Electrophysiol 2015;26:417–23.
28. Knops RE, Olde Nordkamp LR, de Groot JR, Wilde AA. Two-incision technique for implantation of the subcutaneous implantable cardioverter- defibrillator. Heart Rhythm 2013;10: 1240–3.
29. Tjong FV, Brouwer TF, Smeding L, Kooiman KM, de Groot JR, Ligon D, Sanghera R, Schalij MJ, Wilde AA, Knops RE. Combined leadless pacemaker and subcutaneous implantable defibrillator therapy: feasibility, safety, and performance. Europace. 2016 Nov;18(11):1740-1747. Epub 2016 Mar 3.
30. Tjong FV, Brouwer TF, Kooiman KM, Smeding L, Koop B, Soltis B, Shuros A, Wilde AA, Burke M, Knops RE. Communicating antitachycardia pacing-enabled leadless pacemaker and subcutaneous implantable defibrillator. J Am Coll Cardiol. 2016 Apr 19;67(15):1865-6.
31. Joseph Y.S Chan, Jacek Lelakowski, Francis Murgatroyd, Lucas Boersma, Jian Cao, Vladimir Nikolski, Griet Wouters, and Mark Hall Novel Extravascular Defi brillation Configuration With A Coil In The Substernal Space. Heart Rhythm May 6, 2016. Oral abstract AB26-02.
32. Darius Sholevar, Stankey Tung, Vikas Kuriachan, Peter Leong-Sit, Henri Roukoz, Gregory Engel, Steven P. Kutalek, Devender Akula, Mellisa G. Christie, Marina Ostanniy, Amy Thompson and Franck Molin. Feasibility of extravascular pacing with a novel substernal electrode confi guration: results from the multi-center substernal pacing acute clinical evaluation (SPACE) trial. Heart Rhythm May 6, 2016. AB27-02.
 This work is licensed under a
This work is licensed under a