Bianca Moise1, Monica Roşca2,3, Daniel Gherasim2, Elena Popa4, Cladiu Stan5, Bogdan A. Popescu2,3, Carmen Ginghină2,3
1 Sanador Hospital, Bucharest
2 „Prof. Dr. C. C. Iliescu” Emergency Institute of Cardiovascular Diseases, Bucharest, Romania
3 „Carol Davila” University of Medicine and Pharmacy, Bucharest, Romania
4 Procord Medical Cabinet, Bucharest, Romania
5 Fundeni Clinical Institute, Bucharest, Romania
Contact address:
Bianca Moise, MD
Sanador Hospital, Bucharest
Abstract: The role of stress testing in patients with atherosclerotic heart disease is well known and new applications have emerged in recent years. Among these, the assessment of hemodynamic changes during stress testing in patients with valvular heart disease aroused a special interest.
Valvular heart disease represents a major public health issue, with an increasing prevalence as the population ages. Considering that curative treatment usually involves surgical replacement of the valve, choosing the optimal timing is often a difficult decision. According to current guidelines, the presence of symptoms in patients with severe lesions is a clear indication for correction2. Typically, symptoms appear during exercise of progressively decreasing intensity, but there are patients who unconsciously limit their activity, leading to a false delaying in the appearance of clinical manifestations and loss of optimal timing of intervention. Moreover, there are patients who remain asymptomatic despite severe lesions and on the other hand there are patients who are symptomatic and investigations reveal only mild to moderate lesions. These categories of patients benefit the most from cardiac stress testing and there are several methods available: electrocardiographic, echocardiographic or nuclear (scintigraphic) stress testing.
To summarize, although not used on a large scale, stress testing can provide important data regarding functional capacity and clinical status (symptomatic or asymptomatic) in patients with valvular heart disease. Imaging stress testing can also assess hemodynamic changes during exercise (transvalvular gradient, regurgitation severity, LV function, and pulmonary pressure). These data can be used for optimal timing of surgical correction or for their predictive value in risk stratification and, in certain subsets of patients, for diagnosis. However, we must also take into account that research in this area is still evolving, that most recommendations are based on expert consensus and not on large clinical trials and thus validation is still needed.
Keywords: valvular heart disease, cardiac stress testing, effort echocardiographiy
Rezumat: Rolul testării la efort în boala cardiacă aterosclerotică este binecunoscut, iar în ultimii ani au început să se contureze noi utilizări. Dintre acestea, evaluarea modificării parametrilor hemodinamici în timpul efortului, la pacienţii cu valvulopatii pare să ofere informaţii prognostice importante.
Valvulopatiile reprezintă o problemă majoră de sănătate publică, cu o prevalenţă în creştere, pe măsura îmbătrânirii populaţiei. Având în vedere că tratamentul adecvat este fie chirurgical, fie intervenţional, stabilirea momentului optim reprezintă, adesea, o decizie dificilă. Conform ghidurilor actuale în vigoare, prezenţa simptomelor la pacienţii cu leziuni severe, constituie indicaţie de corecţie. În mod tipic, simptomatologia apare la efort, de intensitate progresiv scăzută, dar există pacienţi, care, în mod inconştient îşi limitează activitatea, ceea ce duce la întârzierea apariţiei simptomelor. De asemenea, există pacienţi care sunt asimptomatici în ciuda leziunilor severe, iar pe de altă parte sunt pacienţi simptomatici la care sunt descrise leziuni moderate sau chiar uşoare. Aceste două categorii, sunt cei care beneficiază cel mai mult de pe urma testării la efort, fie de natură electrocardiografică, ecocardiografică, sau scintigrafică.
În concluzie, deşi nu este utilizată pe scară largă, la pacienţii cu valvulopatii, testarea de efort poate aduce importante date privind capacitatea funcţională şi obiectiva statusul clinic (simptomatic sau asimptomatic), iar în combinaţie cu metode imagistice permite evaluarea modificărilor hemodinamice care survin în timpul efortului (gradientul transvalvular, gradul regurgitării, funcţia ventriculară, presiunile vasculare). Datele obţinute pot fi utilizate în stabilirea momentului optim pentru intervenţia chirurgicală, dar şi cu rol prognostic, în stratificarea riscului, iar în anumite situaţii, chiar în scop diagnostic. Trebuie ţinut însă cont de faptul că, datele sunt limitate, majoritatea bazându-se pe consensul experţilor şi nu pe studii clinice mari şi necestită validare.
Cuvinte cheie: valvulopatii, testarea la efort, ecocardiografia de efort
INTRODUCTION
The role of stress testing in patients with atherosclerotic heart disease is well known and new applications have emerged in recent years. Among these, the assessment of hemodynamic changes during stress testing in patients with valvular heart disease aroused a special interest.
Valvular heart disease represents a major public health issue, with an increasing prevalence as the population ages1. Considering that curative treatment usually involves surgical replacement of the valve, choosing the optimal timing is often a difficult decision. According to current guidelines, the presence of symptoms in patients with severe lesions is a clear indication for correction2. Typically, symptoms appear during exercise of progressively decreasing intensity, but there are patients who unconsciously limit their activity, leading to a false delaying in the appearance of clinical manifestations and loss of optimal timing of intervention. Moreover, there are patients who remain asymptomatic despite severe lesions and on the other hand there are patients who are symptomatic and investigations reveal only mild to moderate lesions. These categories of patients benefit the most from cardiac stress testing3 and there are several methods available: electrocardiographic, echocardiographic or nuclear (scintigraphic) stress testing.
MITRAL STENOSIS
Mitral valve stenosis is currently mostly due to rheumatic disease1. The narrowing of the mitral orifice leads to an increased pressure gradient between the left atrium and left ventricle during diastole, with passive backward transmission of the pressure elevation to the pulmonary vascular system. The first consequence is post-capillary pulmonary congestion and later an arterial component is added to pulmonary hypertension. The transvalvular pressure gradient is dependent on the transvalvular flow rate and the duration of diastole. An increase in flow or shortening of diastole, during exercise or in presence of arrhythmias, lead to increased transvalvular gradient, higher pulmonary pressure and clinical symptoms4,5. Gorlin was the first to evaluate hemodynamic changes during stress in patients with mitral stenosis. He used a bicycle in semi-supine position during heart catheterization6. Other invasive studies followed7, confirming the pressure increase in the left atrium and the simultaneous increase in pulmonary artery pressure or an inadequate increase in cardiac output during exercise. Stress echocardiography was introduced much later and showed good correlation with invasive methods8,9.
Current recommendations for stress echocardiography in mitral stenosis can only be found in the American guidelines for the management of patients with valvular heart disease, where it is recommended if there is a discrepancy between clinical and echocardiographic data. Stress echocardiography is useful for assessing hemodynamic changes during exercise, such as an increase in mean transmitral gradient and in pulmonary artery systolic pressure (PASP) (class I recommendation, level of evidence C). The threshold for recommending percutaneous valvotomy is an increase in mean transmitral gradient to more than 15 mmHg or in PASP to more than 60 mmHg10. Although the latest European guidelines mention stress echocardiography as an assessment tool for asymptomatic patients or for those with equivocal symptoms, it is not stated as a recommendation, considering that further studies are necessary2.
Besides its role in establishing the optimal timing for surgical correction, there are some data suggesting the prognostic value of stress echocardiography, although this is not completely validated yet. Thus, exercise capacity is inversely related to PASP measured at peak exercise11. Moreover, duration of exercise is related to PASP at rest in patients with low mitral valve compliance, whereas in patients with normal mitral compliance it is related to post-exercise stroke-volume12. Furthermore, it has been shown that patients with a quick increase in mean transmitral gradient and in PASP (during the first stages of exercise testing) have a worse prognosis than patients with the same hemodynamic changes at peak exercise13. Mitral stenosis severity is also related to an inadequate increase in stroke-volume during exercise14,15.
It has been shown that dyspnoea during stress testing is related to decreased exercise capacity and higher increase in PASP at peak exercise compared to fatigue or no symptoms during exercise13, although these conclusions have not been consistent throughout studies. These results need further validation.
On the other hand, the role of non-imaging stress testing is generally limited, being useful for asymptomatic patients or patients with equivocal symptoms to provide objective evidence of exercise performance and symptoms. An excessive increase in heart rate at low stage exercise, a decrease in cardiac output during exercise as shown by a decrease in blood pressure and the onset of chest pain (due to low cardiac output or pulmonary hypertension (PH) could favour early intervention, although there are no clear recommendations in this area4,10.
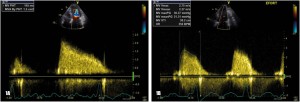
Figure 1. Transthoracic echocardiography – apical 4 chamber view, transmitral continuous wave (CW) Doppler recording: A. At rest: by tracing the velocity flow curve the mean transmitral gradient is derived: 8.5 mmHg; the pressure half-time (PHT) method is used to derive effective mitral valve area: 1,5 cm2;
B. During exercise a significant increase in mean transmitral gradient is recorded (from 8.5 to 21 mmHg)
MITRAL REGURGITATION
The incidence of mitral regurgitation is rising, being currently the second most frequent valvular heart disease after aortic stenosis. The causes of mitral regurgitation are very diverse1.
The role of stress echocardiography in organic mitral regurgitation is to assess: mitral regurgitation severity, PASP, the change in left ventricle (LV) volumes and in ejection fraction (EF). According to current guidelines for the management of patients with valvular heart disease, exercise echocardiography is not recommended for patients with clear indication for surgical correction, such as symptomatic patients with severe regurgitation or asymptomatic patients with LV dysfunction2. On the other hand, asymptomatic patients with severe regurgitation and preserved LV EF could benefit from stress testing, which might reveal a latent LV dysfunction3, considering that a normal or near-normal EF at rest could be the consequence of decreased afterload in this setting16 and could lead to the delay of surgical intervention.
LV subclinical dysfunction is defined as the incapacity to increase LV EF by >4% during exercise. The increase in LV end-systolic volume during exercise also represents a decreased contractile reserve. Moreover, the assessment of contractile reserve does not only allow the assessment of LV subclinical dysfunction preoperatively, but also accurately predicts LV function early postoperatively. From this point of view, stress echocardiographic parameters (indexed LV end-systolic volume during exercise, LV EF at peak exercise, the increase in LV EF post-exercise) are superior to resting parameters17. Exercise time-velocity integral at the level of LV outflow tract has been proposed as a surrogate marker of contractile reserve, since it is easier to measure than LV EF, but validation is still lacking15.
Furthermore, another study has shown that subclinical contractile dysfunction is also related to clinical cardiac events (congestive heart failure and recent onset of atrial fibrillation)18 occurring both in operated patients and in patients treated conservatively16. These data suggest that exercise echocardiographic parameters can contribute in certain situations to the indication for surgical correction, even if the patient is asymptomatic and without overt echocardiographic LV dysfunction at rest, in order to decrease the risk of late clinical and hemodynamic complications.
Stress echocardiography can also be used to assess mitral regurgitation severity during exercise, as shown in several studies that included patients with varying degrees and several etiologies of mitral regurgitation.
Thus, it has been shown that in degenerative mitral regurgitation a significant proportion of patients with moderate or severe regurgitation has a dynamic component, similar to functional mitral regurgitation, with a significant increase in mitral regurgitation severity during exercise. This dynamic component is due to an increase in the degree of prolapse and in the dilation of mitral annulus and these changes are related to an increase in PASP at peak exercise and to a shorter time interval until the onset of symptoms19. Furthermore, up to one third of patients with mitral valve prolapse and without mitral regurgitation at rest can develop different degrees of mitral regurgitation during exercise, most likely also due to the dilation of mitral annulus during end-systole, which impairs valve coaptation and increases the degree of prolapse. These patients are at a higher risk for heart failure, syncope or for developing progressive mitral regurgitation with indication for surgical correction20. This study adds a possible diagnostic role of stress echocardiography in mitral regurgitation to the predictive role discussed previously.
Data regarding rheumatic mitral regurgitation are limited, considering that its incidence is decreasing in developed countries and most studies of this pathology have been conducted before stress echocardiography became available on a larger scale. However, one study that included patients with mild rheumatic mitral valve disease (both regurgitation and stenosis) has shown a dramatic increase in mitral regurgitation severity during exercise, with no obvious explanation21. It is unclear whether these data can be extrapolated to patients with isolated mitral regurgitation.
At the moment, the only hemodynamic parameter derived from stress testing that has direct impact on indicating surgical correction is the value of PASP. PASP increase above 60 mmHg during stress testing is a class IIa indication for intervention, in the American guidelines and a class IIb indication in the European guidelines, both with a C level of evidence2,10. There are a few studies regarding the relationship between exercise-induced PH and the onset of symptoms, mitral regurgitation severity, LV and right ventricle (RV) function, as well as the potential predictive value of PH in patients with degenerative mitral regurgitation. It has been shown that PH during exercise defined as PASP higher than 60 mmHg has a stronger predictive value than PH at rest in asymptomatic patients with mitral regurgitation and is related to a significant shorter asymptomatic time interval. Patients with exercise-induced PH also show a significant increase in mitral regurgitation severity during exercise and significant changes in regurgitant orifice area and regurgitant volume as compared to patients without exercise-induced PH22.
Functional (secondary) mitral regurgitation is prevalent and denotes abnormal function of a structurally normal mitral valve in the setting of ischemic heart disease, non-ischemic dilated cardiomyopathy or other diseases which may evolve towards LV dysfunction and heart failure. While for ischemic mitral regurgitation, rest and exercise parameters and their prognostic significance and relation to symptoms have been extensively studied, data for non-ischemic etiologies are scarce and it is unclear whether conclusions reached for patients with coronary heart disease can be extrapolated to those without. The mechanism of functional mitral regurgitation is based on mitral annulus dilation, tethering of the leaflets and reduced LV contractility23. Considering that functional mitral regurgitation has an important dynamic component, stress testing plays a key role in its evaluation. It has been shown that even mild mitral regurgitation alters prognosis24, finding that could be explained by significant worsening of regurgitation severity during exercise which was strongly related to impaired exercise capacity25. There is also evidence that in patients with functional mitral regurgitation PASP increases significantly during exercise and this is related to an increase in regurgitant volume26, but the clinical and prognostic value of this finding is not clearly defined.
Objective evidence of exercise performance provided by stress testing brings significant functional and prognostic data in many cardiac diseases. Although there are not many studies regarding non-ischemic mitral regurgitation, it seems that in severe types of regurgitation without indication for surgical correction exercise duration is the only parameter with predictive value regarding clinical cardiac events (sudden cardiac death, heart failure, atrial fibrillation and LV dysfunction). The annual risk for cardiac events is five times lower in patients with exercise duration >15 minutes as compared to patients with impaired exercise tolerance and exercise duration <15 minutes27.
Besides stress echocardiography, the role of cardiopulmonary exercise testing has been recently assessed in patients with mitral regurgitation. This test provides information regarding functional capacity, peak oxygen consumption (VO2) and exercise duration being strongly related. Independent predictors of decreased peak VO2 are E/e’ ratio, decreased aortic stroke-volume and atrial fibrillation, whereas mitral regurgitation severity is weakly related to peak VO2. Patients with impaired functional capacity have a higher rate of surgical correction and clinical events as compared to patients with normal functional capacity. Although the severely impaired functional capacity is related with an increased incidence of clinical events in patients treated conservatively28, the potential role of cardiopulmonary exercise testing in the timing of surgical correction is still under debate.
At the moment there are no data regarding the relation of blood pressure response to increased mitral regurgitation severity during exercise and decreased functional capacity15.
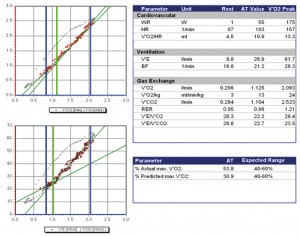
Figure 2. Cardiopulmonary exercise testing in a patient with severe organic mitral regurgitation: peak VO2 = 2.093 l/min or 24 ml/min/kg (57% of predicted value). First ventilatory threshold at 1.125 l/min (53% of actual peak VO2, 30% of predicted peak VO2) reached at heart rate = 103 bpm and 55W load. Circulatory power = 4272 mmHg*ml/min. VE/VCO2 slope = 23. Half-time (time interval during which VO2 declines to half its peak value during recovery) = 76 sec. VO2 curve with continual rise.
AORTIC STENOSIS
According to the European Heart Survey, aortic stenosis has become the most prevalent valvular heart disease. Its etiology is mostly degenerative and less frequently congenital or rheumatic1. Considering the dramatic natural history of this disease, the optimal timing of surgical correction is very important. If for symptomatic patients with severe aortic stenosis or for patients with LV dysfunction (LV EF <50%) surgical correction is firmly indicated, for asymptomatic patients with severe aortic stenosis and preserved LV EF, the evidence is less clear2.
The role of stress testing is best defined for aortic stenosis as compared to other valvular heart diseases, being firmly contraindicated in symptomatic patients2,10. On the other hand, for asymptomatic patients stress testing is recommended in several settings: for severe degenerative aortic stenosis, to reveal symptoms that may be unreported due to significant auto-limitation of exercise level, for young patients with moderate-severe congenital aortic stenosis to recommend the level of activity that is safe for the patient and for patients with discordant clinical and echocardiographic data29,30.
Stress testing (including ECG stress testing) can provide data regarding exercise capacity, blood pressure response or onset of exercise-induced symptoms10 and it is used not only for optimal timing of surgical correction, but also for its predictive value in risk stratification31. According to the European guidelines on the management of valvular heart disease, valve replacement is recommended for asymptomatic patients with exercise-induced symptoms which are clearly related to aortic stenosis as a class I recommendation with C level of evidence2. For patients with comorbidities that could be partially or totally responsible for exercise-induced dyspnea, cardiopulmonary exercise testing could provide significant additional data regarding the cardiac, pulmonary or peripheric cause of breathlessness32, but evidence is still scarce and further studies are necessary. The second indication for valve replacement in severe aortic stenosis based on stress testing is an abnormal blood pressure response with blood pressure decreasing during exercise below the value at rest2 as a class IIa recommendation with C level of evidence in the European guidelines. The American guidelines feature both indications, exercise-induced symptoms and abnormal blood pressure response during exercise, as class IIb recommendations with C level of evidence10. Regarding the prognostic value of exercise testing it has been shown that a normal clinical response to stress testing is significantly related to absent cardiac events (sudden cardiac death, acute heart failure or symptoms which lead to surgical correction) during the first year after stress testing31. Another study has shown that only 19% of patients with exercise-induced symptoms or an increase in systolic blood pressure <20 mmHg have remained asymptomatic during a 2-year follow-up as compared to 85% of patients with normal response to stress testing33.
Besides data provided by ECG stress testing, echocardiographic stress testing can provide information on hemodynamic changes and their impact on LV function. The parameters assessed during exercise are related to the aortic valve (aortic valve area, mean transvalvular gradient), the LV function (systolic and diastolic function), as well as the onset or worsening of mitral regurgitation and the increase in PASP32,34.
It has been shown that an increase in mean transvalvular gradient with more than 18 mmHg during exercise has a stronger negative predictive value than echocardiographic parameters at rest or other parameters during stress testing35. Thus it has been shown that patients with an increase in mean transvalvular gradient >20 mmHg during exercise have a 3.8-fold higher risk of clinical cardiac events35. No relation was found between the value of mean gradient at rest and during exercise36. The increase in mean transvalvular gradient during exercise could be determined by a markedly reduced and rigid valve area, with reduced valve compliance that leads to a minimal or absent increase in aortic valve area as the flow rate increases. These patients have decreased exercise capacity and worse prognosis than patients with preserved valve compliance, with reduced valve area at rest that increases during exercise37. The increase in mean transvalvular gradient >20 mmHg during exercise is a class IIb recommendation with C level of evidence for valve replacement2.
LV EF is another echocardiographic parameter with prognostic value that should be assessed during stress testing. Normally LV EF should increase during exercise. However there are patients with aortic stenosis and normal LV EF at rest that does not increase or even decreases during exercise. This has been shown first by radionuclide angiography38 and then by echocardiography39,40. The physiopathological explanation implies that the pressure overload determined by aortic stenosis exceeds the compensatory mechanisms of the LV, leading to an inadequate increase of EF during exercise36. These patients have a worse prognosis, with shorter asymptomatic period compared to those with LV contractile reserve shown by an increase in EF during exercise39,40. It has been shown recently that there are patients with severe aortic stenosis, asymptomatic at rest, with a biphasic response to exercise: during the first stages of stress testing there is an increase in mean transvalvular gradient and longitudinal myocardial deformation, whereas at peak exercise the values of these parameters decrease. This finding can be explained by a mismatch between after-load and contractility, as a result of decreased coronary flow reserve even in patients with normal epicardial coronary arteries, especially if LV hypertrophy is present41.
Impaired prognosis is predicted not only by an inadequate increase in LV EF during exercise, but also by more subtle signs of myocardial dysfunction, which can be assessed only by special echocardiographic techniques like speckle-tracking echocardiography (STE). It has been shown that there is a relation between an impaired response to stress testing (exercise testing limited by the onset of symptoms or inadequate increase in blood pressure) and longitudinal myocardial dysfunction, as proven by decreased LV global longitudinal strain and decreased basal longitudinal strain42. At the moment these findings have been reported in a small number of studies and further research is needed.
Diastolic dysfunction occurs early in the evolution of aortic stenosis and leads to increased LV filling pressures, which could explain the onset of exercise dyspnoea in patients with severe aortic stenosis43. While the E/e’ ratio allows an accurate estimation of LV filling pressures at rest and is related to the pulmonary capillary wedge pressure (PCWP) assessed invasively44, a recent study has shown that during exercise both E and e’ increase and the E/e’ ratio remains unchanged45. The authors concluded that a better estimate of PCWP increase during exercise is provided by the ratio between the value of E-wave at rest and the value of e’ wave during exercise45.
Regarding the increase of PASP during stress testing in patients with aortic stenosis and its predictive value, data are scarce and further studies are required.
In aortic stenosis with LV systolic dysfunction (LV EF <40%) with aortic valve area <1 cm2, but with mean transvalvular gradient <40 mmHg, dobutamine stress echocardiography is recommended to assess LV contractile reserve and aortic stenosis severity2.
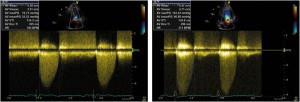
Figure 3. Transthoracic echocardiography – apical 5 chamber view, CW Doppler recording at the level of the aortic valve: A. At rest: mean transvalvular gradient = 52 mmHg; B. During exercise, the mean transvalvular gradient is derived by tracing the velocity flow curve: 96 mmHg.
AORTIC REGURGITATION
The incidence and severity of aortic regurgitation increase with age46 and its causes are very diverse, ranging from congenital to acquired valve disease or diseases of the aortic root10. In the vast majority of cases, aortic regurgitation has a slow evolution with a long asymptomatic period, during which the LV adapts to volume overload through a series of compensatory mechanisms involving the increase in end-diastolic volume and hypertrophy10,47.
According to current guidelines the main indications for surgical correction are the onset of symptoms, LV dysfunction or significant LV dilation in patients with severe aortic regurgitation2,10.
Current European guidelines do not recommend stress testing for the optimal timing of surgical correction2, whereas in the American guidelines stress testing has a class IIa recommendation with B level of evidence for assessing functional capacity and symptomatic response in patients with equivocal symptoms and with C level of evidence before participation in athletic activities.
The additional role of imaging stress testing in patients with aortic regurgitation has not been clearly defined. There are several studies regarding the role of radionuclide ventriculography48,49 or stress echocardiography50 in this setting. Exercise stress testing with radionuclide angiography is recommended in the American guidelines for the assessment of LV function in asymptomatic or symptomatic patients with chronic aortic regurgitation as a class IIb recommendation with C level of evidence10.
Aside from assessing symptomatic status, stress testing can also provide data regarding subclinical myocardial dysfunction with possible prognostic value. Thus, it has been shown that the lack of contractile reserve (defined as the absence of increase in LV EF immediately after exercise as compared to the value at rest) in patients with asymptomatic aortic regurgitation and preserved LV EF at rest predicts progressive LV dysfunction in conservatively treated patients50. On the other hand, the same study showed that normal contractile reserve is related to an improvement in LV function after valve replacement50.
Exercise testing with radionuclide angiography can identify patients at risk for abnormal LV function response to postoperative stress testing. Moreover, preoperative stress testing predicts the type of response of LV EF (increase, decrease or no change) to postoperative stress testing48. Radionuclide angiography stress testing can also provide data regarding right ventricle function and the quantification of ventricular volumes and regurgitant fraction51.
Besides LV contractile reserve, other determinants of LV EF and stroke-volume changes during exercise must be considered, such as volume overload, changes in preload and in peripheral resistance52.
Unlike mitral regurgitation, for aortic regurgitation there are no data to support the role of echocardiographic assessment of aortic regurgitant orifice area, regurgitant volume or PASP during exercise.
To summarize, although not used on a large scale, stress testing can provide important data regarding functional capacity and clinical status53 (symptomatic or asymptomatic) in patients with valvular heart disease. Imaging stress testing can also assess hemodynamic changes during exercise (transvalvular gradient, regurgitation severity, LV function, and pulmonary pressure). These data can be used for optimal timing of surgical correction or for their predictive value in risk stratification and, in certain subsets of patients, for diagnosis. However, we must also take into account that research in this area is still evolving, that most recommendations are based on expert consensus and not on large clinical trials and thus validation is still needed.
Conflict of interests: none declared.
References
1. Iung B, Baron G, Butchart EG et al. A prospective survey of patients with valvular heart disease in Europe: TheEuro Heart Survey on Valvular Heart Disease. European Heart Journal 2003;24: 1231-1243.
2. Vahanian A, Alfieri O, et al, Guidelines on the management of valvular heart disease European Heart Journal 2012; 33:2451-2496.
3. Nkomo V, Pellikka P, Enriquez-Sarano M, McCully R. When to use stress echocardiography in the evaluation of patients with valvular heart disease SAHeart 2010; 7:94-105.
4. Bonow RO et al. Braunwald‘s Heart Disease: A textbook of Cardiovascular Medicine 9th edition Elsevier 2012; 1490-1499.
5. Ginghină C, Mic tratat de cardiologie, Editura Academiei Române 2010; 401-468.
6. Gorlin R, Sawyer CG, Haynes FW, Goodale WT, Dexter L Effects of exercise on circulatory dynamics in mitral stenosis American Heart Journal 1951; 41:192-203.
7. Krause F, Rudolph W. Symptoms, exercise capacity and exercise hemodynamics: interrelationships and their role in quantification of the valvular lesion, Herz 1984; 9:187-199.
8. Sagar KB, Wann S, Paulson WJH, Lewis S. Role of exercise Doppler echocardiography in isolated mitral stenosis, Chest 1987; 92:27-30.
9. Leavitt JI, Coats MH, Falk RH. Effects of exercise on transmitral gradient and pulmonary artery pressure in patients with mitral stenosis or a prosthetic mitral valve: A doppler echocardiographic study. J Am Coll Cardiol. 1991; 17(7):1520-1526.
10. Bonow RO, Carabello BA, Chatterjee K, et al. ACC/AHA 2006 guidelines for the management of patients with valvular heart disease: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Writing Committee to Revise the 1998 Guidelines for the Management of Patients With Valvular Heart Disease). J Am Coll Cardiol 2006; 48:e1-148.
11. Song JK, Kang DH, Lee CW, Lee SG, Cheong SS, Hong MK, Kim JJ, Park SW, Park SJ, Lee SJ. Factors determining the exercise capacity in mitral stenosis. Am J Cardiol 1996; 78:1060-2.
12. Kim HK, Kim YJ, Hwang SJ, Park JS, Chang HJ, Sohn DW, Oh BH,
Park YB Hemodynamic and Prognostic Implications of Net Atrioventricular Compliance in Patients with Mitral Stenosis. J Am Soc Echocardiogr 2007.
13. Brochet E, Detaint D, Fondard O, Early Hemodynamic Changes Versus Peak Values: What Is More Useful to Predict Occurrence of Dyspnea During Stress Echocardiography in Patients with Asymptomatic Mitral Stenosis? Journal of the American Society of Echocardiography 2011; 24(4):392-398
14. Dahan M, Paillole C, Martin D, Gourgon R. Determinants of stroke volume response to exercise in patients with mitral stenosis: a Doppler echocardiographic study. J Am Coll Cardiol 1993;21:384-9.
15. Maréchaux S, Bellouinc A, Polgea AS, Clinical value of exercise Doppler echocardiography in patients with cardiac-valvular disease Archives of Cardiovascular Disease 2008; 101:351-360.
16. Monin JL. Stress haemodynamics for asymptomatic mitral regurgitation: how much does it help? Heart 2005; 91:1383-1384.
17. Leung DY, Griffin BP, Stewart WJ, Cosgrove DM 3rd, Thomas JD, Marwick TH. Left ventricular function after valve repair for chronic mitral regurgitation: predictive value of preoperative assessment of contractile reserve by exercise echocardiography. J Am Coll Cardiol 1996; 28:1198-205.
18. Lee R, Marwick TH. Assessment of subclinical left ventricular dysfunction in asymptomatic mitral regurgitation Eur J Echocardiography 2007; 8:175-184.
19. Magne J, Lancellotti P, Piérard Luc A. Exercise-Induced Changes in Degenerative Mitral Regurgitation J Am Coll Cardiol. 2010; 56(4):300-309.
20. Stoddard MF, Prince CR, Dillon S, Longaker RA, Morris GT, Liddell NE. Exercise-induced mitral regurgitation is a predictor of morbid events in subjects with mitral valve prolapse. J Am Coll Cardiol 1995; 25:693-699.
21. Tischler MD, Battle RW, Saha M, Niggel J, LeWinter MM. Observations suggesting a high incidence of exercise-induced severe mitral regurgitation in patients with mild rheumatic mitral valve disease at rest. J Am Coll Cardiol 1995; 25:128-33.
22. Magne J, Lancellotti P, Piérard LA. Exercise Pulmonary Hypertension in Asymptomatic Degenerative Mitral Regurgitation Circulation. 2010; 122:33-41.
23. He S, Fontaine AA, Schwammenthal E, Yoganathan AP, Levine RA. Integrated mechanism for functional mitral regurgitation: leaflet restriction versus coapting force: in vitro studies. Circulation 1997; 96: 1826-34.
24. Trichon BH, Felker GM, Shaw LK, Cabell CH, O’Connor CM. Relation of frequency and severity of mitral regurgitation to survival among patients with left ventricular systolic dysfunction and heart failure. Am J Cardiol 2003;91:538-43.
25. Yamano T, Nakatani S, Kanzaki H, Toh N, Amaki M, Tanaka J, Abe H, Hasegawa T, Sawada T, Matsubara H, Kitakaze M. Exercise-induced changes of functional mitral regurgitation in asymptomatic or mildly symptomatic patients with idiopathic dilated cardiomyopathy. Am J Cardiol 2008; 102:481-5.
26. Lebrun F, Lancellotti P, Piérard LA. Quantitation of functional mitral regurgitation during bicycle exercise in patients with heart failure. J Am Coll Cardiol. 2001 Nov 15;38(6):1685-92.
27. Supino PG, Borer JS, Schuleri K, Gupta A, Hochreiter C, Kligfield P, Herrold EM, Preibisz J. Prognostic value of exercise tolerance testing in asymptomatic chronic nonischemic mitral regurgitation. Am J Cardiol 2007; 100:1274-81.
28. Messika-Zeitoun D, Johnson BD, Nkomo V, Avierinos JF, Allison TG, Scott C, Tajik AJ, Enriquez-Sarano M. Cardiopulmonary Exercise Testing Determination of Functional Capacity in Mitral Regurgitation Journal of the American College of Cardiology 2006; 47(12):2521-2527.
29. Morise AP. Exercise Testing in Nonatherosclerotic Heart Disease: Hypertrophic Cardiomyopathy, Valvular Heart Disease, and Arrhythmias. Circulation. 2011; 123:216-225.
30. Bonow RO, Cheitlin M, Crawford M, Douglas PS, et al. 36th Bethesda Conference: recommendations for determining eligibility for competition in athletes with cardiovascular abnormalties. Task Force 3: Valvular Heart Disease. J Am Coll Cardiol 2005; 14:1334-40.
31. Rafique AM, Biner S, Ray I, Forrester JS, Tolstrup K, Siegel RJ. Metaanalysis of prognostic value of stress testing in patients with asymptomatic severe aortic stenosis. Am J Cardiol. 2009; 104:972-977.
32. O’Connor K, Lancellotti P, Donalb E, Piérard LA. Exercise echocardiography in severe asymptomatic aortic stenosis .Archives of Cardiovascular Disease 2010; 103:262-269.
33. Amato MC, Moffa PJ, Werner KE, Ramires JA. Treatment decision in asymptomatic aortic valve stenosis: role of exercise testing. Heart 2001; 86:381-6.
34. Roşca M, Lancellotti P, Magne J, Piérard LA. Stress testing in valvular heart disease: clinical benefit of echocardiographic imaging. Expert Rev Cardiovasc Ther 2011; 9:81-92.
35. Lancellotti P, Lebois F, Simon M, Tombeux C, Chauvel C, Pierard LA. Prognostic importance of quantitative exercise Doppler echocardiography in asymptomatic valvular aortic stenosis. Circulation 2005; 112:I377-382.
36. Maréchaux S, Hachicha Z, Bellouin A, Dumesnil JG, Meimoun P, Pasquet A, Bergeron S, Arsenault M, Le Tourneau T, Ennezat PV,Pibarot P. Usefulness of exercise stress echocardiography for risk stratification of true asymptomatic patients with aortic valve stenosis. Eur Heart J 2010; 31:1390-1397.
37. Ennezat P, Maréchaux S, Iung B, Chauvel C, LeJemtel TH, Pibarot P. Exercise testing and exercise stress echocardiography in asymptomatic aortic valve stenosis Heart 2009; 95:877-884.
38. Clyne CA, Arrighi JA, Maron BJ, Dilsizian V, Bonow RO, Cannon RO 3rd. Systemic and left ventricular responses to exercise stress in asymptomatic patients with valvular aortic stenosis. Am J Cardiol 1991; 68:1469-76.
39. Marechaux S, Ennezat PV, Lejemtel TH, Polge AS, de Groote P, Asseman P, Nevière R, Le Tourneau T, Deklunder G. Left ventricular response to exercise in aortic stenosis: an exercise echocardiographic study. Echocardiography 2007; 24:955-9.
40. Lancellotti P, Karsera D, Tumminello G, Lebois F, Piérard LA. Determinants of an abnormal response to exercise in patients with asymptomatic valvular aortic stenosis. Eur J Echocardiogr 2008;9:338-43.
41. Lancellotti P, Moonen M, Garweg C, Piérard LA. Image. Afterload mismatch revealed by an exercise biphasic response in aortic stenosis. Arch Cardiovasc Dis 2009;102:593-4.
42. Lafitte S, Perlant M, Reant P. Impact of impaired myocardial deformations on exercise tolerance and prognosis in patients with asymptomatic aortic stenosis European Journal of Echocardiography 2009; 10:414-41.
43. Archer SL, Mike DK, Hetland MB, Kostamo KL, Shafer RB, Chesler E. Usefulness of mean aortic valve gradient and left ventricular diastolic filling pattern for distinguishing symptomatic from asymptomatic patients. Am J Cardiol 1994; 73:275-81.
44. Bruch C, Stypmann J, Grude M, Gradaus R, Breithardt G, Wichter T. Tissue Doppler imaging in patients with moderate to severe aortic valve stenosis: clinical usefulness and diagnostic accuracy, Am Heart J 2004; 148:696-702.
45. Dalsgaard M, Kjaergaard J, Pecini R, Iversen KK, Køber L, Moller JE, Grande P, Clemmensen P, Hassager C. Left ventricular filling pressure estimation at rest and during exercise in patients with severe aortic valve stenosis: comparison of echocardiographic and invasive measurements. J Am Soc Echocardiogr 2009; 22:343-9.
46. Supino PG, Borer JS. Epidemiology of valvular heart diseases: a growing public health problem Heart Fail Clin 2006;2:379-393.
47. Grossman W, Jones D, McLaurin LP. Wall stress and patterns of hypertrophy in the human left ventricle. J Clin Invest 1975; 56:56-64.
48. Tamas E, Broqvist M, Olsson E, Franzén S, Nylander E. Exercise radionuclide ventriculography for predicting post-operative left ventricular function in chronic aortic regurgitation. J Am Coll Cardiol Img 2009; 2:48-55.
49. Borer JS, Bacharach SL, Green MV, Kent KM, Henry WL, Rosing DR, Seides SF, Johnston GS, Epstein SE. Exercise-induced left ventricular dysfunction in symptomatic and asymptomatic patients with aortic regurgitation: assessment by radionuclide cineangiography Am J Cardiol 1979; 42:351-357.
50. Wahi S, Haluska B, Pasquet A, Case C, Rimmerman CM, Marwick T. Exercise echocardiography predicts development of left ventricular dysfunction in medically and surgically treated patients with asymptomatic severe aortic regurgitation Heart 2000; 84:606-614.
51. Hesse B, Lindhardt TB, Acamp W. Anagnostopoulos C, Ballinger J, Bax JJ, Edenbrandt L, Flotats A, Germano G, Stopar TG, Franken P, Kelion A, Kjaer A, Le Guludec D, Ljungberg M, Maenhout AF, Marcassa C, Marving J, McKiddie F, Schaefer WM, Stegger L, Underwood R. EANM/ESC guidelines for radionuclide imaging of cardiac function Eur J Nucl Med Mol Imaging DOI 10.1007/s00259-007-0694-9.
52. Picano E, Pibarot P, Lanclotti P, Monin JL, Bonow RO. The Emerging Role of Exercise Testing and Stress Echocardiography in Valvular Heart Disease J Am Coll Cardiol 2009; 54: 2251-2260.
53. Zdrenghea D, Popa D. Testarea de efort în practica medicala, Editura CLUSIUM, 2009.
 This work is licensed under a
This work is licensed under a