Artiom Surev, Grib Andrei, Esanu Andrei, Marcel Abras, Calenici Eugen, Damascan Stefan, Ion Popovici
1 Interventional Cardiology Department, Polivalent Hospital NOVAMED
INTRODUCTION
Coarctation of the Aorta is a congenital heart anomaly involving constriction of an aortic segment , associated with wall thickening of media and the formation of overlapping infolding of neointimal tissue. For the first time it was described by Morgagni in 1760. This pathology represents 5% -7% of all congenital heart diseases, with an incidence of approximately 3 cases to 10 000 neonates1. The typical location of the coarctation of the Aorta is distal to the origin of the left subclavian artery, yet it can be found and at the level of the thoracic or abdominal segment.
Coarctation can be observed in isolation or associ-ated with other congenital heart malformations, such as Aortic bicuspid valve, ventricular septal defect, persistent arterial duct, transpostions of large arteries, defect of atrioventricular duct, obstructive left cardiac defects, including left hipoplastic heart syndrome.
Depending on the balance between the degree of disturbance of the flow and compensatory mechanisms available to overcome it, clinical presentation maybe very variable: from acute heart failure in neonatal up to asimptomatic patient. But the most common symptom for children and adults is arterial hypertension. Untreated Coarctation has a reserved prognosis. Average age of survival is of 38 years; with 75% mortality at the age of 46 years 2 long-term complications are caused by hypertension, including early coronary arterial disease, stroke, endocarditis, aortic dissection and heart failure3.
Coarctation can be diagnosed at any age. As mentioned, in many cases, patients may be asymptomatic until puberty. In other cases, newborns may experience heart failure, acidosis, or cardiogenic shock af-ter closing the arterial duct. In the absence of medical treatment and mechanical correction, the disease progresses rapidly. Prenatal diagnostics can prevent these complications, highlighting the need for malformation correction intervention prior to ductal closure. Prenatal diagnosis presents difficulties due to poor aortic blood flow, caused by persistent arterial duct4.
Patients with less severe coarctation are usually diagnosed later with clinical signs of high blood pressure, decreased or delayed pulse in lower limb arteries, difference in upper and lower limb systolic pressure and uncontrollable tension. Another common sign is systolic murmur, more evident in the thoracic and abdominal levels5.
In compensation, circulation to distal parts is redistributed by collaterals arising from internal thoracic arteries, subclavicular aa., vertebral and anterior spinal arteries. For undiagnosed adults, hypertension is the most common symptom. Others may complain of headache, intermittent claudication of the lower limbs, linked to effort5. However, for patients with blood flow compensated by well-developed collaterals, the pulse on the lower limb arteries and the systolic pressure gradient can be slightly diminished.
Diagnosing the aortic coarctation assumes:
Chest X-ray – The findings vary according to the patient’s clinical picture. The most informative data is provided by the esophageal X-ray with barium – the presence of the classic “E” sign – the dilated left subclavian artery and post-stenotic dilation of the descending aorta6.
Echocardiography – TTE or TEE, is the most commonly used method, being accessible and harmless. The informative value is the quantification of aortic length at its origin from the left ventricle – to the arch; determining ventricular volumes and establishing the systolic pressure gradient in the aortic stenosis area. Echocardiographic parameters evaluated in aortic coarctation using color Doppler: PSV – systolic peak velocity (m/s); EDV – early diastolic velocity (m/s); LDV – late diastolic velocity (M/S); PG – peak gradient of the stenosis area; pulse delay and PI-pulsatile index. Echocar-diography is of a significant diagnostic value in the postoperative or post- stenting period, highligh-ting the effectiveness of malformation correction techniques but also determining in perspective the degree of recurrence of stenosis to several years.7 ECHOCG can also determine the type of CoA: the preductal- when the diameters of the distal aortic arch and of the isthmus are narrowed considerably, the arch and descending aorta do have an iregular stricture; the post-ductal type – the stenotic lesion of the descending aorta, located distal to the left subclavicular artery, echocardiographically presents as a „pumpkin”6.
Fetal echocardiography – is rarely used as a primary diagnostic tool. However, this allows establishing the likelihood of developing CoA by measuring the left and right cavities of the fetal heart, in particular the median-cavitary size(mc). In 2009, Quartermain presented the fetal echocardiography study. He has analyzed 35 fetal ultrasound investigations with left-right ventricular disproportions. Two batches were compared: ventricular disproportion in those who needed surgical correction for CoA and those who did not have coarctation. The results demonstrated that in the group that needed malformation correction, the proportion of mean cavity dimensions (LVmc <RVmc) is lower than in fetus without the need of intervention.
Multi-spiral computed tomography (MSCT/ MDCT) – a non-invasive method of diagnosing CoA malformation, using the multiplanar and tridimensional technique, that determines with a 100% specificity morphology of aortic coarctation, in particular by determining the location, length and degree of stenosis. At the same time, it determines the presence of the collateral cir-culation, the ratio of the narrowed zone in comparison with subclavicular artery, and other asso-ciated cardiovascular malformations1.
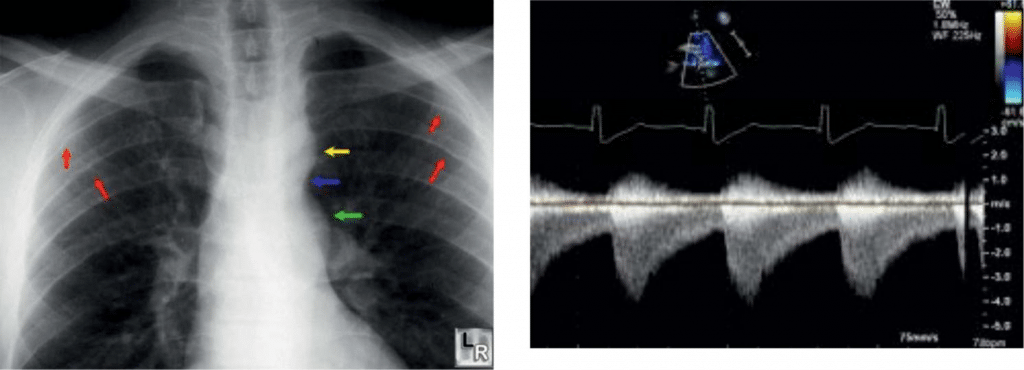
Figure 1. Thoracic radiography in the Aortic Coarctation. Yellow arrow Ao arch; blue – coarctation; red-thinned rib areas – collateral network; green – the post-stenotic area.
Figure 2. Transthoracic echocardiography of aortic coarctation in supra-sternal prosition, Doppler regimen. High velocity systolic jet and slow diastolic jet.
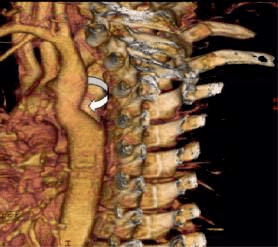
Figure 3. MSCT – aortic narrowing distal to the left a.subclavicular origin.
INDICATIONS FOR TREATMENT
Without surgery, prognosis for patients with aortic coarctation is unfavorable. In 1970, Campbell exami-ned autopsy results and clinical records of 465 patients with coarctation who survived one year after in-tervention. The mean age of death was 34 years, with a mortality of 75% at 43 years. Causes of death include congestive heart failure (26%), aortic rupture (21%), bacterial endocarditis (18%) and intracranial haemor-rhage (12%)8.
Thus, in 2008, the American College of Cardiology and the American Heart Association (ACC/AHA) guidelines developed guidelines for surgical or endovascular surgery in patients with aortic coarctation as follows:
-peak cutoff gradient 20 mmHg; which is the difference between the proximal and distal peak pressure of the stenosed segment.
-coarctation gradient on the peak <20 mmHg, and radiological evidence of secondary collateral flow.
-the rest gradient alone may be an unsafe indica-tor of severity when there is significant collateral circulation.
Infants with “critical” coarctation are at risk for heart failure and death after closing the arterial duct. The identification of these patients is essential and therapeutic measures include the maintenance of duct permeability and continuous intravenous infusion of prostaglandin E8,9.
Correction of aortic coarctation is preferred to be performed at pre-school age, with the goal of preventing the development of chronic systemic arterial hypertension. At the same time, surgical interventi-on in mature is associated with a much higher rate of postoperative complications and even the risk of perioperative death than in children or adolescents8,9. Surgical Treatment Methods:
1. resection of the coaction area and restoration of thoracic aortic continuity through termino-terminal anastomosis
2. intraluminal stenosis diaphragm resection follo-wed by synthetic graft enlargement istmoplasty
3. resection of the coarctation zone and the inter-position of a synthetic material conduit
4. bypass of the stenosis segment by means of a synthetic prosthesis10
The first method of surgical treatment of aortic coarctation described by Crafoord in 1944 consisted in the resection of the coarctation area with termino-terminal anastomosis. Early studies showed a high rate of re-coarctation in over 50% of patients10.
Vosschulte described in 1961 prosthetic patch aortoplasty as an alternative technique for coarctation correction. This technique can be applied to longer coarctation segments, avoiding a circumferential suture line and minimizing aortic mobilization and intercostal artery ligation.
Subclavicular flap aortoplasty was a modified approach reported by Waldhausen and Nahrwold in 1966. This technique avoids a circumferential suture line and the use of prosthetic material that can allow better growth and can be used to repair of a longer coarctation segment11.
The resection and replacement with an interposition graft was described by Gross in 1951. This approach is rarely used in present.
In 1977, Amato et al. Described a modification of the Crafoord resection and of the end-to-end anasto-mosis technique in which a longitudinal incision and anastomosis were created in the proximal aorta. Anastomosis extended to an edge largely avoids the use of prosthetic material and allows the resection of coarctation and residual ductal tissue, but the wider incision is less prone to restenosis and allows the extension of the transverse aorta, which is particularly useful in neonates12,13.
The decision to refer for surgical approach for coarctation correction should be considered and seems to be more grounded when during a single thoracotomy other malformations are being approached. In the case of open-heart surgery for the coarctati-on, one should estimate all the risks associated with a general intervention as well as the ones that could appear in the perspective. It should be mentioned that interventions at a higher age are also associated with difficulty of approaching the coarctation area, due to the presence of numerous collateral originating from the subclavicular artery and the left mammary artery.
Complications associated with the surgical resolution of aortic coarctation are divided into several categories:
residuals, especially high blood pressure that may be present for the rest of life, and hence all complications associated with it; other congenital malformations that require resolution in perspective. recurrent complications and those associated with the consequences of surgery and surgical technique – aortic re-coarctation and aneurism, arrhythmias, etc. complications as is, or global – infectious, trauma-tic, etc.
Re-coarctation
Recurrence of re-coarctation occurs in 10-12% cases of classical intervention and in 4.5% cases of endo-vascular correction in young children. The Dodge-Khatami study (2000) established that the main risk factors in the recurrence of coarctation are: termi-no- terminal anastomosis (ETE), SFA anastomosis and aortic arch hypoplasia. The CarlosIbarra-Pérez study (1969) attributes a special role to the re-coarctation caused by graft thrombosis, kinking of the subclavi-cular artery and suture line fibrosis but also residual duct tissue.
– Aneurysms are found after all types of surgical repair of aortic coarctation, but more often after Dacron patch aortoplasty, with reported incidence of up to 90% during a follow-up period of over 20 years. The use of multiple polytetr-afluoroethylene patches instead of Dacron was initially promising, but presents a risk of approximately 7% for aortic aneurysm and a 25% re-coarctation risk.
– Intracranial aneurysms (IAs) have a 5-fold greater incidence in CoA patients compared to the po-pulation lacking this malformation. The incidence of IAs varies between 2.5% and 50% (associated with aortic coarctation). The incidence of intra-cranial aneurysm rupture is 4.8% in CoA patients.
– Residual arterial hypertension – being the most common postoperative complication in CoA patients, occurs most frequently in patients ope-rated at a higher age. Modern surgical approach involves resolving malformation at the stage of diagnosis. J J O’Sullivan et al (2002) established a direct correlation between systolic blood pressure and residual obstruction in the descending aorta, measuring by Doppler echocardiography of pulse wave velocity in 119 children in the post-operative period. The results determined that 34 children (28.3%) had residual arterial hypertension.
– Stroke is directly associated with arterial hyper-tension, involving several mechanisms. Increased intraluminal pressure leads to extensive alteration of endothelium and smooth myocytes in intracerebral arteries. Endothelial damage may in-crease the permeability of the hematoencephalic barrier with the occurrence of local or multifocal brain edema. Endothelial lesions and interactions with modified blood cell endothelium may cause local thrombi formation and ischemic disorders. Fibriloid necrosis can cause infarctions caused by focal stenosis and occlusions. Degenerative changes of smooth muscle and endothelial cells predispose to intracerebral hemorrhage. In addition, hypertension accelerates the arteriosclerotic process with increased peripheral vascular resistance. It is worth mentioning that arterial hypertension itself is a self-sustaining factor in subrachnoid hemorrhage, which at its turn involves a risk of death of about 46%.
– Endocarditis after surgical correction is a frequent common complication, especially in patients with CoA associated cardiac malformations, such as valvulopathies.
Initially, surgical therapy was the only treatment option for aortic coarctation. In 1982, the first case of balloon transluminal angioplasty was reported by Lock. Subsequent studies have shown the effectiveness of endovascular treatment, reporting a reduced rate of recoarction – from 8% to 32%.
First for angioplasty, standard Mansfield Inc. and Meditech Inc. balloons were used. The size of the balloon was selected so that the diameter of the inflated balloon to be 0 to 2 mm greater than the aortic isthmus at the base of the left subclavian artery. The effectiveness of this method is controversial due to the high incidence of forward-looking recurrence with the frequent need for reintervention10. The main complications that arise from balloon angioplasty include: aortic re-coarctation, aortic aneurysms, aortic dissection or rupture.
Histological and intravascular ultrasound studies have demonstrated that frequent angioplasty is associ-ated with rupture of vessel intima and medi. Although some of these may spontaneously heal, disruption of vascular integrity contributes to a relatively increased incidence of aneurysm formation. The CCISC obser-vational study during 2004-2012 has showed that 24% of patients with native coarctation developed aortic aneurysm in echocardiographic monitoring after balloon angioplasty11.
The use of stents in the treatment of aortic coarctation was first proposed in 1992. Unlike balloon angioplasty, in the case of implantation of the stent it does not require the supradilation of the aorta. Thus, the pressure applied to the aortic wall is smaller and more evenly distributed, resulting in a lower rate of complications such as dissection and aneurysm formation.
The stents provide better structural support, thus reducing the incidence of aortic wall lesions and restenosis, often in the case of balloon angioplasty12.
A retrospective analysis after stenting in 17 institutions (565 patients) from 1989 to 2005 have demonstrated a procedural success rate of 97.9% with no significant residual gradient or serious complication in 553 of 565 (97.9%) patients. The overall rate of complications was 14.3%, with aortic wall complications (aneurysm or dissection) of 3.9%. A 5-year follow-up confirmed the efficacy of this method. At the same time, the re- coarctation was confirmed in 20% of patients, and 4% required repeated interventions. Other major aortic wall complications – aneurysm or dissection, have been reported in 1% of cases13.
The stents have been shown to be more effective in preventing vascular recoil, and if this complication occurs, subsequent redilation is relatively simple and safe. The neointimal growth and remodeling of the aortic wall is more controllable and prevents the formation of pseudoaneurism14.
Initially, the stents that were used in aortic coarctation, could be adjusted to the size of the aortic segment, eg biliohepatic, Palmaz XL, etc.16.
Another attempt was to use self-expanding stents. Their advantage is good compliance with the vascular lumen, thus reducing the risk of malposition, and from the drawbacks the permanent trauma of this type of device to the aortic wall can be mentioned.
The use of endovascular stents in small children remains a problem because repeated adjustments due to the child’s growth17.
Another “ideal” stent concept would involve a device that can be adjusted from large diameters to small diameters covered with a synthetic material to prevent mechanical complications (dissection or aortic rupture) with a “delicate” delivery system, and simple to use18,19.
MATERIALS AND METHODS
In this study were analyzed short-term results of the implantation of the graft-stent “BeGraft aortic Bentley”. Being a relatively new device officially announced in 2016, it was designed to solve stenosis both on the aorta and on the peripheral vessels.
Stent-graft structure is made up of a metallic cobalt-chromium (CoCr) alloy; a synthetic polytetraflu-oroethylene (ePTFE) graft with multiple micropores and an expandable semicompliant balloon, with platinum or iridium markers. The advantages of this type of construction is the maximum compliance of the device and the synthetic graft which it is covered with.
The BeGraft Bentley aortic stent is available in 3 base diameters, with a variable diameter of 12 to 24 mm, which can be brought up to 30 mm. There have been reported cases with Bentley aortic stent post-dilation up to 30 mm when using large balloons, inflated to maximum pressures. The recommended applied pressure of the BeGraft aortic balloon ranges from 7 bar for 12-14 mm diameter and 5 bar for postdilation diameter 24-30 mm. The length of the stent varies between 19 and 59 mm. It is compatible with sheath diameter of 9, 11 and 14 F.
Stent-graft involves a modern concept of intra-arterial biomechanics – there are three design variants of the Co-Cr metal casing to precisely adjusting aortic arterial angioaritectonics. This approach to stent assembling allows the elastic recoil phenomenon to be avoided after implantation, and the ratio of the radial forces applied to the vessel walls was also calculated. All these “anatomical” device features, provide excellent flexibility after implantation and ensure optimal expansion.
Indications for the use of BeGraft aortic stent assume the conditions of primary or secondary aortic coarctation in children and adults, but also the conditions necessary for the restoration and improvement of iliac artery permeability.
Own experience of NovaMed clinic
During the period 01.2017 – 01.2018, 8 cases of aortic coarctation were solved by stenting. Patients age was from 6 to 32 years. All cases were approached endo-vascular with the implantation of a “BeGraft Bentley” dedicated graft stent.
Preprocedural characteristics of patients under-going endovascular treatment. The main cause for patients undergoing endovascular intervention- CoA (in all 8 cases), was associated with heart failure in 3 patients. Arterial hypertension, which de facto develops in all patients, has proven to be in all 8 cases, and claudication – an indirect symptom associated with coarctation, has been observed in 7 patients. The post-stenting complications, the most common being the recoarctation was absent in all, and pseudoaneurism-diagnosed in one case.
Patient A, 27 years old, normal BMI, with a normostennial constitution, was admitted to the clinic based on persistent high blood pressure level over several years. Coronary artery angiography and aortography were performed, where coarctation of the thoracic segment of the aorta was detected. Presumptive diagnosis – Congenital heart malformation. Aortic coarctation.
For multiple-sided visualization of the stenosis, the patient underwent evaluation by MDCT.
For the purpose of correcting the detected primary congenital malformations, the decision is made to per-form the angioplasty of the deseased aortic segment. The procedure is performed under general anesthesia and cardiac pacing with a good result. A reduction in Ao stenosis is obtained from 95 to 0%. Pre-and post stenotic (pre- and post-stent) invasive pressure measurements revealed – 155/90 mmHg. The transcoarcatation gradient was reduced from 80 mmHg/Hg to 0 mmHg.
Patient B, male, 16 years old, normostenic. From anamnesis – known from 10 years with arterial hyper-tension, with AP values up to 160-180/100 mmHg. Initially detected by ultrasound in the „typical” aortic coarctation spot, subsequently assessed by contrast CT. Treated endovascular with implantation of the stent – graft Bentley.
All patients who has undergone endovascular intervention were monitorized and investigated at 1, 3, 6 months of post-stenting. Recent anamnestic details, clinical and paraclinical like ECG, transthoracic echocardiography data were analyzed. At 6th month, all investigations were repeated, including MDCT assessment of the posterior mediastinum.
Immediately after the intervention, 2 patients accu-sed moderate chest pain with gradual improvement, most likely related to arterial extension syndrome.
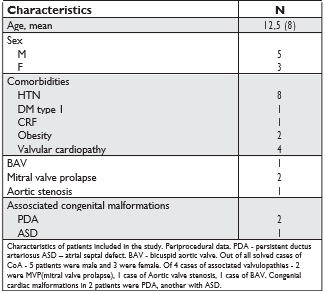
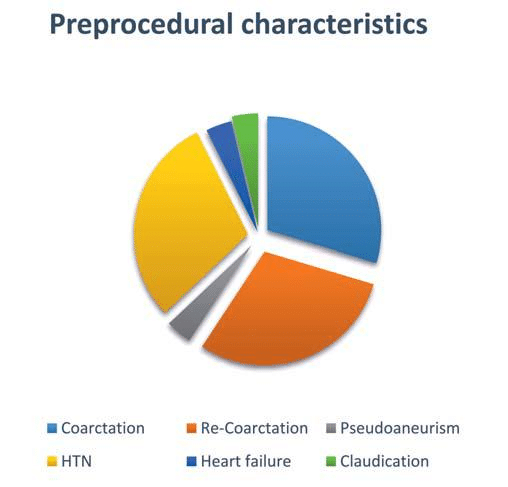
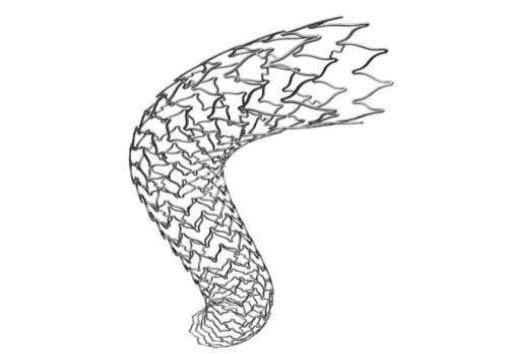
Figure 4. Virtual visualization of an aortic graft-stent Bentley. The reticulated chasiss of the stent can be selected from three designs proposed by the manufacturer.
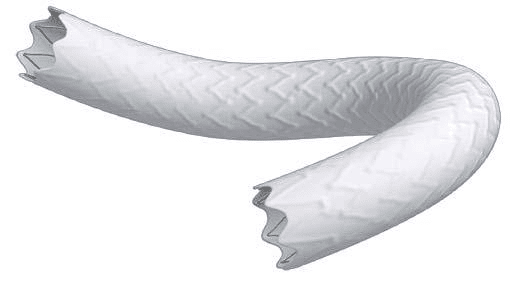
Figure 5. Three-dimensional virtual projection of aortic stent- graft Bent-ley.
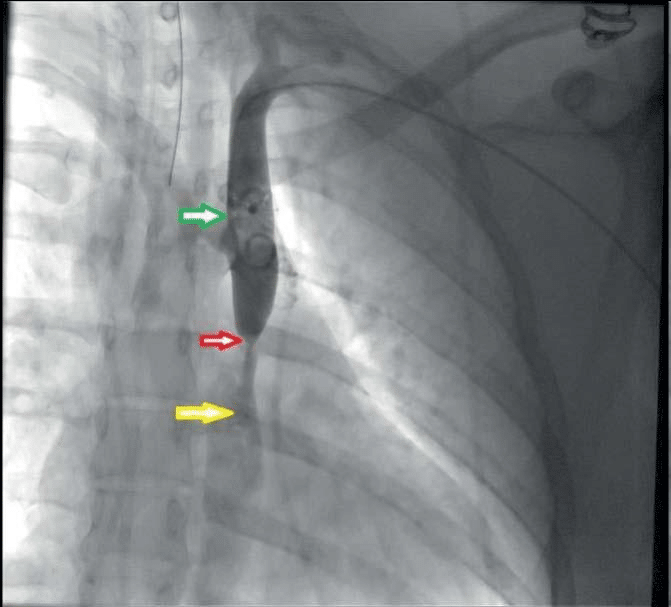
Figure 6. Pre-procedural angiography of patient A. Green arrow – ade-quate arterial fl ow in the aorta arch and descending Ao, Red arrow – severe stenosis 90-95% in the thoracic Ao, distal to the emergence of the left a. subclavia. Yellow arrow – post stenotic dilatation, severe contrastation deficit.
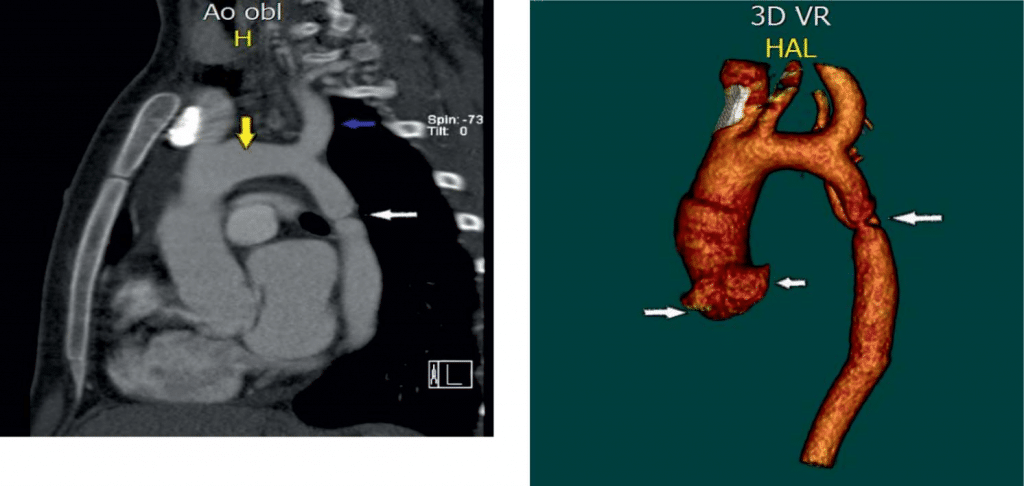
Figure 7. Multi-plane MDCT of the descending aorta, the thoracic seg-ment. Severe stenosis distal to the aortic arch.
Figure 9. Pre-stenting MDCT of Pacient A. 3D VR (Volume Rendering) Mode. The level of aortic coarctation is determined; coronary sinus and aortic arch.
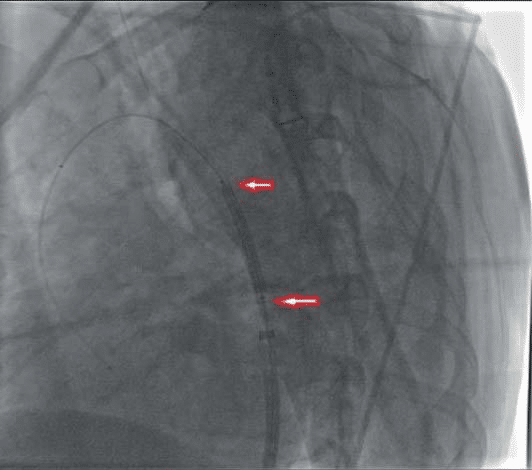
Figure 8. Pre-stenting MDCT of pacient A. Sagittal projection of the aortic arch and descending Ao. The white arrow – descending thoracic Ao stenosis. The yellow arrow – the aorta arch. The blue arrow – Left subclavian artery.
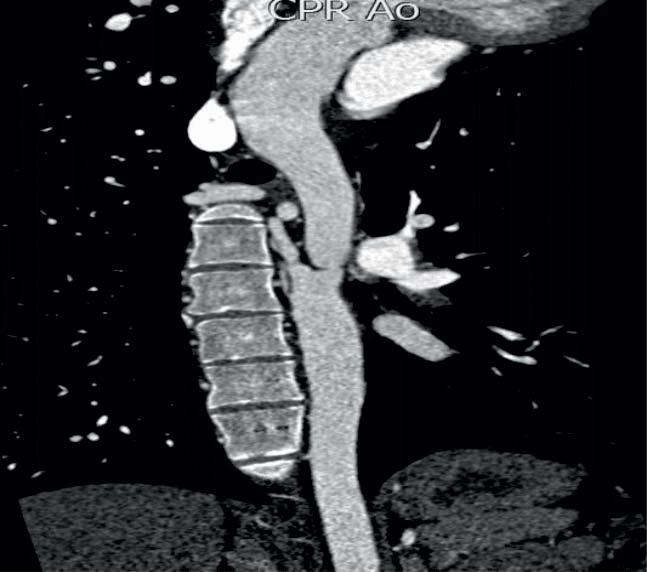
Figure 10. Patient A. Endovascular correction of Aortic Coarctation. On a „AMPLATZ-SUPER-STIFF” guide, the stent-graft „BENTLEY” is 16-38 mm.
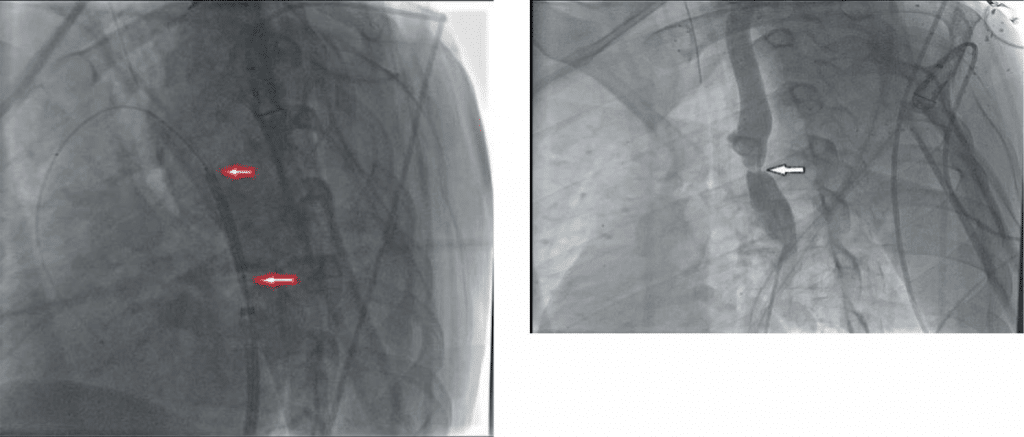
Figure 11. Adjustment of the Bentley aortic stent graft after implantation. Balloon inflated to 10 atm – brought to 18 mm.
Figure 13. Aortic angiography in patient B. Aortic coarctation next to bifurcation with left s. bclavicular artery.
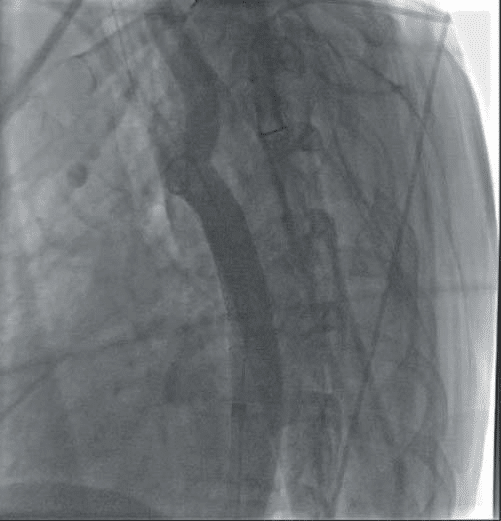
Figure 12. Patient A. Post stenting with good final result. Achieved reduc-tion of Ao stenosis from 95 to 0%.
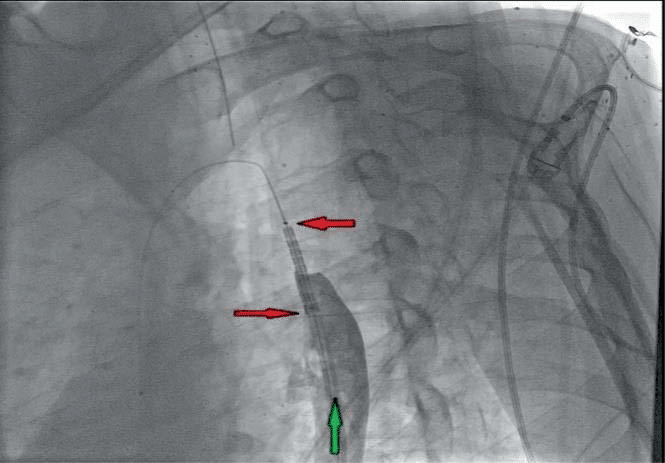
Figure 14. Patient B. Affected aortic segment angioplasty. with the stent-graft „BENTLEY” of 12-29 mm.
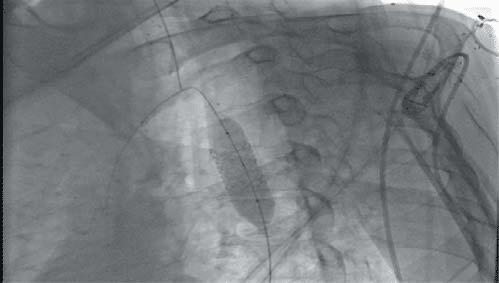
Figure 15. Bentley stent-graft alignment. Inflation at 14 atm – brought to 16-17 mm.
CONCLUSIONS
1. The endovascular approach of aortic coarctation is currently the method of choice for treatment due to the low rate of complications, reduced hospitalization time, relative safety, and not of less importance for patient preference.
2. Bentley aortic stents appear to be very promising, being easily adjustable, with maximum safety and protection compared to older generations or the classic method – by balloon dilation.
3. The great advantage of the Bentley graft stents is the possibility of simultaneous treatment of the aortic coarctation and persistent arterial duct (if present).
The authors have no conflict of interest to declare.
References
1. Agarwala BN, Bacha E. Clinical manifestations and diagnosis of coarctation of the aorta. 2009
2. Warnes CA, Williams RG, Bashore TM, Child JS, Connolly HM, Dea-rani JA. ACC/AHA 2008 Guidelines for the Management of Adults with Congenital Heart Disease: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (writing committee to develop guidelines on the management of adults with congenital heart disease) Circulation. 2008
3. Jenkins NP, Ward C. Coarctation of the aorta: natural history and outcome after surgical treatment. QJM: monthly journal of the Association of Physicians. 1999
4. Ward KE, Pryor RW, Matson JR, Razook JD, Thompson WM, Elkins RC. Delayed detection of coarctation in infancy: implication for tim-ing of newborn follow-up. Pediatrics1990
5. Gatzoulis MA, Swan L, Therriern J. Adult congenital heart disease : A practical guide. Blackwell Publishing; 2006.
6. Brickner ME, Hillis LD, Lange RA. Congenital heart disease in adults; 2000
7. Tanous D, Benson LN, Horlick EM. Coarctation of the aorta: evaluation and management. Current opinion in cardiology. 2009
8. Campbell M. Natural history of coarctation of the aorta. Br Heart J. 1970
9. Seirafi PA, Warner KG, Geggel RL, Payne DD, Cleveland RJ. Repair of coarctation of the aorta during infancy minimizes the risk of late hypertension. The Annals of thoracic surgery. 1998
10. Rao PS, Galal O, Smith PA, Wilson A. Five to nine year follow-up results of balloon angioplasty of native coarctation in infants and children. J Am Coll Cardiol. 1996
11. Gatzoulis MA, Swan L, Therriern J. Adult congenital heart disease : A practical guide. Blackwell Publishing; 2006
12. Huggon IC, Qureshi SA, Baker EJ, Tynan M. Effect of introducing balloon dilation of native coarctation on overall outcome in infants and children. Am J Cardiol
13. Sundt TM III, Orszulak TA, Cook DJ, et al. Improving results of open arch replacement. Ann Thorac Surg. 2008
14. Trimarchi S, Nienaber CA, Rampoldi V, et al. Contemporary results of surgery in acute type A aortic dissection: The International Registry of Acute Aortic Dissection experience. J Thorac Cardiovasc Surg. 2005
15. Lock JE, Keane JF, Fellows KE. The use of catheter intervention procedures for congenital heart disease. J Am Coll Cardiol. 1986
16. Campbell M. Natural history of coarctation of the aorta. Br Heart J. 1970
17. Thanopoulos BV, Eleftherakis N, Tzanos K, Skoularigis I, Triposki-adis F. Stent implantation for adult aortic coarctation. J Am Coll Cardiol. 2008
18. Aortic aneurysm after patch aortoplasty repair of coarctation: a pro-spective analysis of prevalence, screening tests and risks. J Am Coll Cardiol 14
19. Rao PS. Balloon Angioplasty for aortic recoarctation following previous surgery, 1993.
20. Kenny D, Hijazi ZM. Coarctation of the aorta: from fetal life to adulthood. Cardiol J. 2011
21. Sreeram I, Sreeram N, Bennink G. Palliative stent implantation for coarctation in neonates and young infants. Ann Pediatr Card. 2012.
 This work is licensed under a
This work is licensed under a