Dana-Oliviana Geavlete1, Carmen Beladan1,2, Andreea Călin1, Marian Croitoru1, Ruxandra Jurcuț1,2,
Oana Tăutu1,3, Bogdan A. Popescu1,2, Carmen Ginghină1,2
1 Cardiology Department, “Prof. Dr. C.C Iliescu” Emergency Institute for Cardiovascular Diseases, Bucharest London, London, UK
2 ”Carol Davila” University of Medicine and Pharmacy, Bucharest,
3 University Emergency Hospital Bucharest, Romania
INTRODUCTION
Atherosclerotic renal artery stenosis (RAS) is the main cause of secondary hypertension leading to renal insufficiency, refractory hypertension and considerable cardiovascular events1. RAS is encountered among 7% of adults over 65 years of age, 25% in dialyzed patients for end-stage renal failure and up to 50% in those presenting generalized atherosclerosis (ATS)2,3. The co-existence of RAS and ATS in other vascular beds has been well documented. In patients with coronary artery disease, the prevalence of RAS increases with the extension and severity of coronary involvement. This correlation was emphasized as leading to adverse cardiovascular events such as myocardial infarction (MI), stroke and cardiovascular death 4-6. It also seems that patients with a history of stroke and/or carotid artery stenosis are more likely to have RAS when compared to those without this type of pathology7. As the mortality increases with the higher severity of RAS lesions8, revascularization was expected to improve the outcome in “high-risk” patients presenting with symptomatic chronic heart failure (CHF), angina or flash pulmonary edema9,10. The recent CORAL (Cardiovascular Outcomes in Renal Atherosclerotic Lesions) study failed to demonstrate the benefit of revascularization in addition to medical treatment by comparison to medical therapy alone in RAS patients11. Still, the clinical phenotype should be considered in order to determine the revascularization outcome. Beyond the actual controversies, high-risk patients presenting bilateral significant RAS, episodes of acute pulmonary edema, angina as well as the association between progressive renal deterioration and refractory hypertension may be expected to benefit the most from revascularization12. The bilateral involvement of significant RAS was shown to imply a worse prognosis when compared to cases of unilateral stenosis while referring to the overall mortality and kidney function (38% mortality at 2 years in medically treated bilateral RAS)6,13. Moreover, a higher prevalence of symptomatic CHF, increased left ventricle mass index and left ventricle dilation were observed for this category of patients14. Based on all of the above, it may be stated that the differentiation between bilateral and unilateral RAS holds a certain degree of importance. Screening models for the prediction of high-risk cases may be required in order to obtain the best outcomes regarding renal function preservation and mortality rate subsequent to revascularization. The main objective of the present study was to compare the prevalence of risk factors and the extension of ATS involvement in consecutive patients with significant unilateral versus bilateral RAS. Also, it was aimed to identify the predictors for these specific pathological entities.
MATERIALS & METHODS
A total of 58 patients referred to renal angiography for suspected renovascular hypertension were prospectively enrolled in the study. Significant RAS was defined as angiographic stenosis of at least 60% diameter by visual estimation. The evaluation protocol was based on the following parameters: demographics, cardiac history, cardiovascular risk factors (hypertension, smoking, diabetes, serum lipid levels, dyslipidemia), known duration of hypertension, objective evidence of ATS located at other vascular sites (coronary, carotid and lower extremity arteries), related comorbidities and associated cardiovascular drug therapy.A vascular Doppler scan evaluation of carotid and lower extremity arteries was performed in all patients. Significant carotid artery stenosis was confirmed by carotid angiography subsequent to the initial Doppler scan evaluation. Furthermore, in cases of significant lower extremity peripheral arterial disease (PAD) documented by Doppler scan, peripheral angiography was additionally applied. A complete echocardiographic evaluation and electrocardiogram was performed in all patients in order to select those who underwent a clinically indicated angiography. Coronary artery disease was documented by cardiac catheterization. The renal function was estimated based on the serum creatinine level and glomerular filtration rate (eGFR) using both the Modification of Diet in Renal Disease (MDRD) formula as well as the Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI) equation. The statistical analysis was performed using the IBM Statistical Package for Social Sciences (SPSS) 20.0 statistics software at a significance level of p ≤0.05. The independent sample t test and the Mann-Whitney U test were applied in order to analyze differences between means of 2 independent study subgroups (depending on the normality of continuous data distribution assessed by Kolmogorov-Smirnov test). The Chi-square test was used for analyzing differences between categorical data. Moreover, binary logistic regression was performed in order to assess the association between the presence of bilateral RAS and target variables. Table values were presented as mean ± s.d. (standard deviation) for continuous data and absolute number (percentage) for categorical data.
RESULTS
General characteristics of the two study groups
Among the 58 patients included in the study group, significant unilateral RAS was found in 31 cases (group 1) and bilateral RAS in 27 cases (group 2).
Table 1. General characteristics of the two study groups
| Unilateral RAS N = 31 |
Bilateral RAS N = 27 |
p | |
| Age (years) | 62.32±14.11 | 64.41±7.06 | NS* |
| Gender | |||
| Females | 11 (35.4 %) | 10 (37.1 %) | NS** |
| Males | 20 (64.5 %) | 17 (62.9 %) | NS** |
* independent sample t test;
** chi-square test;
NS: non-statistically significant differences (p≥0.05);
The severity of renal artery stenosis
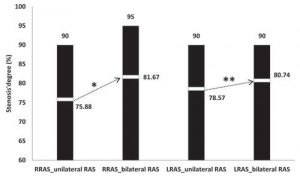
Stenosis of the right renal artery was encountered in 17 patients (54.84%) in the unilateral RAS group with a mean value of stenosis’ degree of 75.88±11.21% (range between 60% and 90%). The left renal artery was affected in the remaining 14 patients (45.16%) characterized by a mean value of 78.57%±11.41% stenosis degree (range 60-90%). The mean value of right artery stenosis in bilateral RAS series was 81.67±10% (range 60-95%), while the degree of left renal artery stenosis displayed a mean value of 78.52±17.03% (range 60-90%). Overall, patients from the bilateral RAS group emphasized a significantly higher degree of both right as well as left renal artery stenosis by comparison to the unilateral RAS study arm (Figure 1).
Blood pressure and renal function in the study groups
The maximum systolic blood pressure recorded in the unilateral RAS group had a mean value of 214.68±27.59 mmHg (range 170-280 mmHg), thus being statistically similar to that recorded in the bilateral RAS series (mean value of 224.33±32. mmHg, range 180-280 mmHg). The renal function determined in bilateral RAS patients was significantly lower when compared to unilateral RAS cases regardless of the used parameter (serum creatinine levels, eGFR estimated by MDRD or by CKD-EPI) (Table 2).
Atherosclerotic risk factors
The presence of all traditional atherosclerotic risk factors (smoking, obesity, dyslipidemia and diabetes mellitus) presented statistically equivalent frequency in both study groups (Table 3). The mean value of total cholesterol levels recorded in the bilateral RAS series (range 106-292 mg/dL), was similar to that determined in the unilateral RAS study arm (197.54±69.21 vs. 174.38±46.47 mg/dL, p=0.249). Additionally, the mean value of total plasma lipids found in the bilateral and unilateral RAS series were statistically similar (627.50±131.3 versus 683.54±180.85 mg/dL, p=0.752).
Figure 1. Severity of renal artery stenosis.
RRAS: right renal artery stenosis LRAS: left renal artery stenosis.
Independent sample t test: *p = 0.024 **p = 0.045.
Table 2. Renal function in study groups
| Unilateral RAS N = 31 |
Bilateral RAS N = 27 |
p | |
| Serum creatinine (mg/dL) | 1.37±0.87 | 1.86±0.56 | 0.013 |
| eGFRMDRD (ml/min/1.73 m2) | 61.64±18.92 | 44.44±15.01 | <0.0001 |
| eGFRCKD-EPI (ml/min/1.73 m2) | 69.78±30.03 | 41.79±14.99 | <0.0001 |
Concomitant atherosclerotic involvement
The majority of cases with significant carotid atherosclerotic stenosis were found in the bilateral RAS group (63.89%). Moreover, the frequency of bilateral carotid stenosis was also significantly higher in bilateral RAS series. There were no statistically significant differences between the bilateral and unilateral RAS groups regarding concomitant coronary artery disease or lower extremity artery disease (Table 4). While analyzing patients with concomitant significant atherosclerotic stenosis in more than one vascular territory, it was noticed that the concomitant atherosclerotic involvement in 2 or more arterial territories was more frequent in the bilateral RAS by comparison to the unilateral RAS study arm (Figure 2). The prevalence of carotid artery stenosis was 62.06% in all RAS patients included in the present analysis.
Table 3. Atherosclerotic risk factors
| ATS risk factor (N, %) |
Unilateral RAS N = 31 |
Bilateral RAS N = 27 |
p |
| Smoking | 21 (51.2) | 20 (48.8) | NS* |
| Obesity | 17 (51.5) | 16 (48.5) | NS* |
| DM | 8 (44.4) | 10 (55.6) | NS* |
| Dyslipidemia | 28 (54.9) | 23 (45.1) | NS* |
| Total serum cholesterol | 187.52 ±67.84 | 168.63±55.58 | NS** |
| Total serum lipids | 659.77 ±175.98 | 646.61±139.54 | NS** |
* chi-square test;
** independent sample t test;
NS: non-statistically significant differences (p≥0.05);
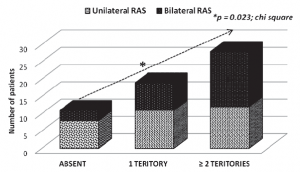
Figure 2. Concomitant signifi cant arterial stenosis
Table 4. Concomitant atherosclerotic involvement
| Unilateral RAS N = 31 |
Bilateral RAS N = 27 |
p* | |
| Significant CAS | |||
| – Absent | 18 (81.8) | 4 (18.2) | 0,002 |
| – Unilateral | 3 (50) | 3 (50) | NS |
| – Bilateral | 10 (33.3) | 20 (66.7) | 0,001 |
| Significant CAD | |||
| – Absent | 17 (54.8) | 14 (45.2) | NS |
| – Uni-vascular | 1 (33.3) | 2 (66.7) | NS |
| – Bi-vascular | 7 (70) | 3 (30) | NS |
| – Tri-vascular | 6 (42.9) | 8 (57.1) | NS |
| Significant lower extremity PAD | |||
| – Absent | 18 (56.2) | 14 (43.8) | NS |
| – Present | 13 (50) | 13 (50) | NS |
| Concomitant significant arterial stenosis | |||
| – Absent | 8 (72.7) | 3 (27.3) | 0.023 |
| – 1 arterial territory | 11 (57.9) | 8 (42.1) | 0.023 |
| – ≥2 arterial territories | 12 (42.8) | 16 (57.2) | 0.023 |
* chi-square test;
CAS: carotid artery stenosis; CAD: coronary artery disease; PAD: peripheral artery disease;
NS: non-statistically significant differences (p≥0.05);
Predictors of bilateral renal artery stenosis The probability of bilateral renal artery stenosis in patients with bilateral significant carotid stenosis from our study group was 9.5 times higher (95% CI of OR:2.41-37.47) than the one of a patient with or without unilateral carotid stenosis (Table 5). For every 1 mL/min/1.73 m2 of decrease in eGFR (assessed by CKD-EPI) below the median value of 49 mL/min/1.73 m2, the probability of bilateral RAS increased with 0.952 (95% of OR: 0.925-0.980). By combining the presence of bilateral significant carotid stenosis with the decrease in eGFR (estimated by CKD-EPI), there was determined an increase in the probability of correctly predicting the presence of bilateral RAS in our study group from 73.1% (by bilateral carotid stenosis alone) and 76.9% (by eGFR CKD-EPI alone) to 78.8% (Figure 3).
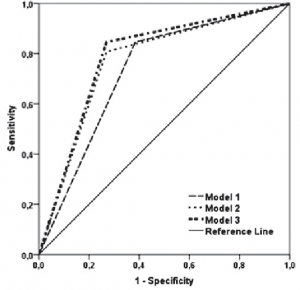
Figure 3. Bilateral renal artery stenosis’ prediction models ROC curves.
ROC curve: receiver operating characteristic (the lines indicate prediction probability);
Model 1: includes only the presence of significant bilateral carotid artery stenosis as independent variable;
Model 2: includes only the values of the eGRF estimated by CKD-EPI equation as independent variable;
Model 3: includes both the presence of significant bilateral carotid artery and eGFR assessed by CDK-EPI equation.
DISCUSSION
Renal artery stenosis is the most common cause of secondary hypertension, with atherosclerosis being the substrate in over 90% of cases. The condition leads to refractory hypertension, renal insufficiency and cardiovascular diseases. The real prevalence of RAS was generally reported between 5-10% of the hypertensive population, although much higher rates were described in elderly patients with atherosclerotic profile (concomitant coronary artery disease, carotid artery stenosis and lower extremity peripheral artery disease) 15. Arterial hypertension due to RAS may cause progressive renal damage 16. Unilateral RAS leads to elevated renin and aldosteron levels that promote vasoconstriction and increase peripheral vascular resistance. On the other hand, in bilateral RAS, renin secretion is elevated in both kidneys, diuresis is secondarily affected and hypertension is maintained due to the aldosteron effect on water and sodium retention resulting in volume expansion 17,18. As completely different activation patterns are involved for the renin-angiotensin-aldosteron system (RAAS) depending on whether RAS is unilateral or bilateral, the present study aimed to compare the prevalence of risk factors as well as the extension of the atherosclerotic disease affecting other territories in consecutive patients with unilateral by comparison to bilateral RAS. With regard to the co-existence of cardiovascular risk factors and their influence in developing atherosclerotic RAS, a prospective study reported that 30% of patients referred for coronary angiography were diagnosed with RAS, 15% of which represented cases of significant stenosis (11% unilateral and 4% bilateral RAS). Also, more than 30% of patients with symptomatic coronary artery disease were demonstrated to have RAS, a condition susceptible to worsening the prognostic in these patients 19. Certain important markers for significant RAS were reported: age >60 years, associated coronary artery disease (RAS prevalence increases with extension and severity of the number of affected coronary vessels) and symptomatic congestive heart failure 19,20. It was also emphasized that RAS is present in 20-50% of the patients with peripheral artery disease and, according to a recent study, in more than 31% of patients with carotid artery stenosis 21. Moreover, RAS seemed to increase the incidence of carotid artery stenosis and lower extremity peripheral artery disease by 24% and 37%, respectively 22,23. The previously determined prevalence of carotid artery stenosis in RAS patients in particular was 46% when compared to 12% in non-RAS cases 24. However, there are no reports that differentiate unilateral from bilateral RAS with regard to the perspective of associated ATS lesions. The present study supports a strong association between coronary, lower extremity peripheral and carotid artery disease in RAS patients regardless of the unilateral or bilateral renal artery involvement. The higher rates of concomitant ATS found in both groups by comparison to those previously reported could be the result of different study design as well as the limited number of enrolled patients. Furthermore from this perspective, it may be useful to mention that the present analysis involved symptomatic, hypertensive patients undergoing renal arteriography for high clinical suspicion of reno-vascular disease. Similar studies solely took into consideration patients undergoing coronary angiography and described the frequency of incidentally found RAS in asymptomatic, generalized ATS cases. Further along this line, the study also showed that the majority of patients with significant carotid artery stenosis were recorded in the bilateral RAS group (85.18%) when compared to unilateral RAS (41.93%). Moreover, the frequency of bilateral carotid artery stenosis was significantly higher in the bilateral RAS series (66.7% versus 33.3%). This finding supported a probability of bilateral RAS in a patient with bilateral significant carotid artery stenosis of 9.5 times higher than in one with unilateral significant carotid artery stenosis or none. The overall trend for all included patients was represented by an increasing degree of carotid artery stenosis along with a higher RAS severity. While considering the number of associated significant ATS lesions in different arterial territories (coronary, carotid, lower extremity), it was noticed that concomitant ATS involvement in 2 or 3 territories was recorded more frequently in the bilateral RAS group when compared to the unilateral one. The present data may reflect a larger total body plaque burden in patients with bilateral RAS, thus possibly emphasizing a causal relationship between the specific RAAS activation pattern and an advanced generalized ATS process. As a marker of progressive renal insufficiency induced by renal ischemia, an increased creatinine serum level was found to constitute an independent predictive factor for the presence of RAS 25. Additionally, prior to revascularization, there was documented a correlation between the stenosis severity and eGFR (regardless of the estimation method) 26. While evaluating the serum creatinine levels and the eGFR in the present series, it was found that renal impairment was significantly higher in the bilateral RAS group, regardless of the used parameter. During the course of the present analysis, the predictive performance of the risk model was assessed “through the looking glass” of the ROC curve. Consequently, the presence of bilateral RAS was predicted by the association of bilateral significant carotid artery stenosis with a decreased eGFR of up to 78.8%.
Limitations
The number of cases included in the present study was restricted by the inclusion criteria of hypertensive patients presenting a high clinical suspicion of RAS. Patients who had asymptomatic RAS and incidentally discovered RAS (<50% stenosis) were not considered for this particular study. The contribution of concomitant ATS in other vascular areas as well as the presence of cardiovascular risk factors (dyslipidemia, hypertension, diabetes and smoking) could at some point interfere with the indication for renal arteriography in certain patients, thus potentially influencing the study results.
Clinical significance
The present study emphasized the high prevalence of atherosclerotic risk factors and the generalized nature of the ATS process in RAS patients (both unilateral and bilateral). While aiming to assess the probability of significant bilateral RAS, the results of this analysis suggest that a thorough evaluation of the renal function and carotid artery disease in hypertensive patients presenting a high clinical suspicion of RAS should be considered. A carotid artery Doppler scan in hypertensive patients associating high serum creatinine levels and decreased eGRF could be susceptible to helping clinicians in the screening process for high-risk bilateral RAS (which is the main correctable cause of secondary hypertension with tremendous prognostic implications if left untreated).
CONCLUSIONS
In light of the above mentioned findings, it may be stated that significant bilateral RAS is more frequently associated with significant ATS involvement of other vascular territories than unilateral RAS. Moreover, in our study significant bilateral carotid artery stenosis was more prevalent in the group of patients with bilateral RAS. The presence of a significant bilateral RAS condition results in a more severe renal function impairment, regardless of the assessment method (serum creatinine, eGFR by MDRD or by CKD-EPI formula). The screening for bilateral significant carotid artery stenosis by Doppler ultrasound and for renal impairment by eGFR (according to CKD-EPI) could identify hypertensive patients at risk for bilateral RAS, thus making them proper candidates for angiographic evaluation. Acknowledgement: This paper is supported by the Sectorial Operational Programme Human Resources Development (SOP HRD), financed from the European Social Fund and by the Romanian Government under the contract number POSDRU/159/1.5/S/137390.”
References
1. Kalra PA, Guo H, Kausz AT, et al.: Atherosclerotic renovascular disease in United States patients aged 67 years or older: risk factors,
revascularization, and prognosis. Kidney Int 2005, 68:293–301.
2. Scoble JE: Atherosclerotic nephropathy. Kidney Int 1999, 71 (Suppl): S106–S109.
3. Uzu T, Takeji M, Yamada N, et al: Prevalence and outcome of renal artery stenosis in atherosclerotic patients with renal dysfunction. Hypertens Res 2002; 25:537-542.
4. Garovic V, Textor SC. Renovascular hypertension: current concepts. Semin Nephrol 2005; 25:261-71.
5. Garovic VD, Textor SC. Renovascular hypertension and ischemic nephropathy. Circulation 2005; 112:1362-74.
6. Conlon PJ, Little MA, Pieper K, Mark DB. Severity of renal vascular disease predicts mortality in patients undergoing coronary angiography. Kidney Int 2001; 60:1490-7.
7. Kuroda S, Nishida N, Uzu T et al. (2000) Prevalence of renal artery stenosis in autopsy patients with stroke. Stroke, 31, 61–5.
8. Conlon PJ, Athirakul K, Kovalik E, Schwab SJ, Crowley J, Stack R, McCants CB Jr, Mark DB, Bashore TM, Albers F: Survival in renal vascular disease. J Am Soc Nephrol 9: 252–256, 1998.
9. Ducloux D, Jamali M, Chalopin JM: Chronic congestive heart failure associated with bilateral renal artery stenosis. Clin Nephrol 48: 54–55,
1997.
10. Bloch MJ, Trost DW, Pickering TG, Sos TA, August P: Prevention of recurrent pulmonary edema in patients with bilateral renovascular disease through renal artery stent placement. Am J Hypertens 12: 1–7, 1999.
11. Cooper CJ, Murphy TP, Cutlip DE, Jamerson K, Henrich W, Reid DM, Cohen DJ, Matsumoto AH, Steff es M, Jaff MR, Prince MR, Lewis EF, Tuttle KR, Shapiro JI, Rundback JH, Massaro JM, D’Agostino RB Sr, Dworkin LD; CORAL Investigators. Stenting and medical therapy for atherosclerotic renal-artery stenosis. N Engl J Med. 2014 Jan 2;370(1): 13-22.
12. Ritchie J, Green D, Chrysochou C, Chalmers N, Foley RN, Kalra PA, et al. High-risk clinical presentations in atherosclerotic renovascular
disease: prognosis and response to renal artery revascularization. Am J Kidney Dis; 2013. pii : S0272-6386 (13) 0112-8.
13. Chabova V, Schirger A, Stanson AW, et al. Outcomes of atherosclerotic renal artery stenosis managed without revascularization. Mayo Clin Proc 2000; 75:437–44.
14. Wright JR, Shurrab AE, Cooper A, et al.: Left ventricular morphology and function in patients with atherosclerotic renovascular disease. J
Am Soc Nephrol 2005, 16:2746–2753.
15. Baboolal K, Evans C, Moore RH. Incidence of end-stage renal disease in medically treated patients with severe bilateral atherosclerotic renovascular disease. Am J Kidney Dis 1998;31:971–7.
16. Goldblatt H, Lynch J, Hanzal RF, Summerfille WW. Studies on experimental hypertension, I: the production of persistent elevation of
systolic blood pressure by means of renal ischemia. J Exp Med 1934; 59: 347–379.
17. Liard JF, Cowley AW Jr, McCaa RE, McCaa CS, Guyton AC. Renin, aldosterone, body fluid volumes, and the baroreceptor reflex in the
development and reversal of Goldblatt hypertension in conscious dogs. Circ Res 1974;34:549-60.
18. Bianchi G, Campolo L, Vegeto A, Pietra V, Piazza U. Th e value of plasma renin concentration per se and in relation to plasma and extracellular
fluid volume in diagnosis and prognosis of human renovascular hypertension. Clin Sci 1970; 39:559-76.
19. Harding MB, Smith LR, Himmelstein SI et al. Renal artery stenosis: prevalence and associated risk factors in patients undergoing routine cardiac catherization. J Am Soc Nephrol. 1992 May;2(11):1608-16.
20. Dzielinska Z, Januszewicz A, Demkow M, Makowiecka-Ciesla M, Prejbisz A, et al. Cardiovascular risk factors in hypertensive patients with coronary artery disease and coexisting renal artery stenosis. J Hypertens. 2007 Mar;25(3):663-70.
21. Chrysochou C, Kalra PA. Epidemiology and natural history of atherosclerotic renovascular disease. Prog Cardiovasc Dis 2009; 52: 184-195.
22. Hirsch AT, Haskal ZJ, Hertzer NR, et al. ACC/AHA Guidelines for the Management of patients with Peripheral Arterial Disease (Lower Extremity,
Renal, Mesenteric and Abdominal Aortic): A Collaborative Report from the American Association for Vascular Surgery/Society for Vascular Surgery, Society for Cardiovascular Angiography and Interventions, Society of Interventional Radiology, Society for Vascular Medicine and Biology and the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Writing Committee to Develop Guidelines for the Management of Patients with Peripheral Arterial Disease). Circulation 2006; 113; e463-e465.
23. Louie J, Isaacson JA, Zierler RE et al. Prevalence of carotid and lower extremity arterial disease in patients with renal artery stenosis. Am J
Hypertens. 1994 May;7(5):436-9.
24. Imori Y, Akasaka T, Ochiai T, Oyama K, Tobita K, Shishido K, Nomura Y, Yamanaka F, Sugitatsu K, Okamura N, Mizuno S, Arima K, Suenaga H, Murakami M, Tanaka Y, Matsumi J, Takahashi S, Tanaka S, Takeshita S, Saito S. Co-existence of carotid artery disease, renal artery stenosis, and lower extremity peripheral arterial disease in patients with coronary artery disease. Am J Cardiol. 2014 Jan 1;113(1):30-5.
25. Przewłocki T, Kabłak-Ziembicka A, Tracz W, Kozanecki A, Kopeć G, Rubiś P, Kostkiewicz M, Rosławiecka A, Rzeźnik D, Stompór T. Renal artery stenosis in patients with coronary artery disease. Kardiol Pol. 2008 Aug;66(8):856-62;
26. Yu H, Zhang D, Haller S, Kanjwal K, Colyer W, Brewster P, Steffes M, Shapiro JI, Cooper CJ. Determinants of renal function in patients with renal artery stenosis. Vasc Med. 2011 Oct; 16(5):331-8.
 This work is licensed under a
This work is licensed under a