Diana Alexandra Cherata1,2, Luigi Paolo Badano1, Doina Carstea2, Denisa Muraru1
1 Department of Cardiac, Thoracic and Vascular Sciences, University of Padua, Italy
2 Department of Cardiology, “Filantropia” Municipal Hospital, Craiova, Romania
Abstract: Despite cancer therapeutics-related cardiac dysfunction (CTRCD) can be initially asymptomatic, if not detected and properly managed, it may progress to severe and irreversible heart failure. Therefore, identification of high-risk patients and early detection of subclinical myocardial dysfunction are fundamental tasks for the management of cancer patients undergoing chemo- and/or radiotherapy, involving both cardiologists and oncologists. Although systematic and periodical assessment of left ventricular ejection fraction (LVEF) by two-dimensional echocardiography
(2DE) is conventionally used to monitor LV function during and after chemotherapy, three-dimensional echocardiography (3DE) has been reported to have the best accuracy and reproducibility for LVEF assessment, when compared to cardiac magnetic resonance (CMR). However, LVEF reduction occurs at late and often irreversible stages of CTRCD. Conversely, measurement of LV myocardial deformation by two-dimensional speckle tracking (2DSTE), and particularly measurement of LV global longitudinal strain (GLS) has demonstrated to identify CTRCD at early stages, when LVEF is still normal. Accordingly, baseline and periodical evaluation of GLS have now been introduced by the current recommendations regarding cardiac monitoring of cancer patients.
The purpose of this review is to summarize currently available evidences on the role of the different echocardiographic techniques to monitor LV function in cancer patients treated with potentially cardiotoxic chemotherapeutics with an emphasis on the benefits of novel imaging techniques and on how the latter can be applied in the various clinical settings.
Keywords: cardiotoxicity, cancer treatment-related cardiac dysfunction, three-dimensional echocardiography, three-dimensional speckle tracking, cardio-oncology.
INTRODUCTION
Early detection strategies, improved surgical approaches, as well as new drugs and more aggressive treatment protocols have reshaped the prognosis of cancer patients in the last decades. As a result, mortality rates from cancer have dropped by 23% since 19911. However, increased life expectancy, associated with the aging of the cancer patient population, has resulted in a rise of adverse cardiovascular effects, particularly in patients with history of previous cardiovascular diseases1. Cancer treatment-related cardiac dysfunction (CTRCD) has a poor prognosis, once it becomes clinically manifested, and it is the leading cause of morbidity and mortality in cancer survivors2. Lack of recovery of left ventricular (LV) function after therapy cessation was reported in up to 40–58% of the patients developing CTRCD3, and treatment has been shown to be more effective when initiated early, before irreversible cardiac damage occurs4. Thus, identification of high-risk patients, early detection of CTRCD, and research to identify markers of subclinical myocardial dysfunction are fundamental tasks for the management of cancer patients, involving
both cardiologists and oncologists. CTRCD diagnosis is conventionally based on the calculation of LV ejection fraction (LVEF) using various cardiac imaging modalities. According to current recommendations, LVEF decrease of more than 10% to a value of less than 53%-assessed by two-dimensional echocardiography (2DE) using the biplane Simpson’s method and evidenced by repeated studies defines CTRCD5. Further characterization of CTRCD relates on presence/absence of symptoms and reversibility. Among the various cardiac imaging modalities, 2DE
is the most frequently use in the cardio-oncological field because of its wide availability, easy repeatability, radiation-free nature, cost-effectiveness and clinical value. The addition of recent echocardiographic techniques, such as contrast, three-dimensional (3DE) and 2D/3D speckle-tracking echocardiography (STE), has fueled the use of echocardiography that has become
a cornerstone in the evaluation of patients’ before, during and after cancer therapy5. Although not stated in the defi nition of CTRCD, integrating LVEF assessment with biomarkers, such as
troponin and brain natriuretic peptide (NT-pro BNP) levels, and new parameters obtained with 3DE and STE may increase the detection of CTRCD. Abnormal troponin values next to impaired LV global longitudinal strain (GLS) by STE increases specifi city for the prediction of CTRCD from 73% to 93%6. If both parameters are abnormal a cardiology consultation is required4. Likewise, the high negative predictive value of NT-pro BNP may be useful in the setting of CTRCD as its elevation raises concern for augmented LV filling pressures. The focus of this review is to summarize currently available evidences on the role of the various echocardiographic techniques to diagnose CTRCD, their advantages, limitations and pitfalls, emphasizing on the benefi ts of novel imaging techniques and on how the latter can be applied in predicting subsequent development of CTRCD.
Two-dimensional echocardiography left ventricular ejection fraction: pros and cons. Why do we need more?
Both the American Society of Echocardiography (ASE) and European Association of Cardiovascular Imaging (EACVI) recommend the routine use of bi-plane discs’ summation method for measuring LV volumes and calculate LVEF7. LV volumes are obtained from manual tracing of the interface between the compacted myocardium and the LV cavity in four- and two-chamber apical views using the end-systolic and end-diastolic frames (Figure 1). Despite the fact that it is recommended to maximize the LV areas, to avoid view foreshortening, and to reduce the depth of the scan sector to improve endocardial tracing accuracy, 2DE still shows suboptimal accuracy in measuring LV volumes. Wrong view orientation, geometric assumptions about LV shape, and/or errors during manual tracing of endocardial border may explain the relatively low reproducibility of LVEF calculated by 2DE8. On average, the reported variability of 2DE LVEF is around 9.5%, failing in accurately detect myocardial dysfunction due to limited extent of regional alterations9. The ability to obtain an accurate and reproducible measure of LVEF is of vital importance for patients receiving chemotherapy, since clinical decision relies almost completely on this measurement10. Since, cessation or dose reduction of chemotherapy drugs should be considered if LVEF drops more than 10% to below 53%5, concerns have been raised about erroneously stopping potentially lifesaving therapies due to changes in LVEF that occur only due to the technical variability
of the modality in repeat testing. Indeed, patients at risk for CTRCD require serial evaluation of LVEF at the beginning of cancer therapy, once half of the cumulative dose has been administered, before every subsequent dose and 3, 6 and 12 months after its completion5. Accordingly, in addition to the accuracy, reproducibility and test/re-test repeatability of the imaging technique used to measure LVEF is required to obtain consistent results across the different steps of the therapeutic protocol, and by different observers. Accordingly, ASE/EACVI expert consensus regarding adult cancer patients, recommends to use the best technique available in the echocardiography laboratory (ideally 3DE) for LVEF calculation in patients exposed to potentially cardiotoxic chemotherapy.
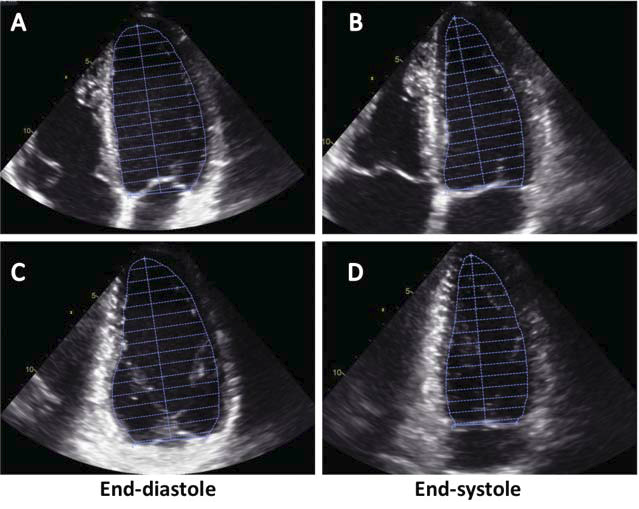
Figure 1. Calculation of left ventricular volumes by two-dimensional echocardiography using the bi-plane discs’ summation method (Simpson method). Left ventricular cavity is divided into 20 discs by manually delineating the endocardium in the apical four- (A and B) and two-chamber views (C and D).
Left ventricular opacification: to visualize the endocardial border
Some of the inaccuracy of 2DE is due to suboptimal image quality and objective difficulties in identifying the
endocardial border to trace. Previous reports have shown that LV opacification using transpulmonary contrast agents (Figure 2) improves endocardial visualization and therefore the accuracy of 2DE LV volume and EF assessment in comparison with computed tomography and cardiac magnetic resonance (CMR)imaging11-13. Even more important, LV opacification has been reported to improve inter-observer reproducibility to a level comparable with CMR14. However, improved accuracy is not restricted to patients with poor baseline image quality12. Therefore, the use of this technique has been suggested to improve accuracy and reproducibility of LVEF measured with 2DE in cancer patients. There is limited data about the use of LV opacification to monitor LVEF during chemotherapy. Thavendiranathan et al.15, in a comparative study among the different echocardiographic techniques, reported that contrast increased the temporal variability of all tested echocardiographic techniques (2DE, 2D triplane and 3DE). Conversely, interobserver variability of contrast 2DE was signifi cantly better than fundamental 2DE and close to that measured
with 3DE.
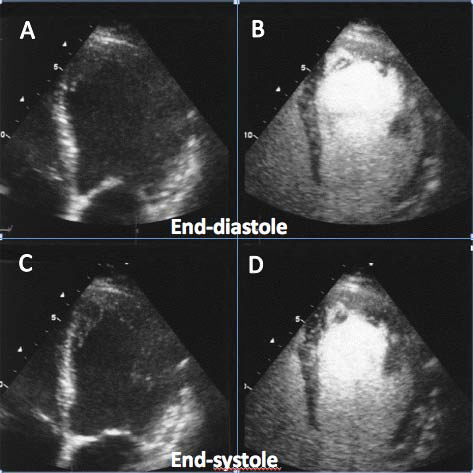
Figure 2. Use of echocardiographic contrast agent (Sonovue, Bracco S.p.A, Italy) to opacify the left ventricular cavity in a patient with sub-optimal endocardial visualization of the lateral wall and the apex in fundamental imaging (panels A and C). With 1 mL of contrast agent the endocardium of the left ventricle is clearly visualized in all segments (panels B and D).
Three-dimensional echocardiography left ventricular ejection fraction: beyond geometrical assumptions
The greatest advantage of 3DE in the evaluation of the LV is that, with this technique, the commonest causes of LV volume underestimation with conventional 2DE (i.e., foreshortening of the LV longitudinal axis, plane position errors, and geometrical assumptions about LV shape) are no longer real issues16,17. With 3DE, only one acquisition of the LV is required to obtain volumes and
ejection fraction (Figure 3). The acquisition is usually performed from the apical approach and requires that the whole LV is included within the 3D data set. LV data set analysis can be performed using computerized automated or semi-automated endocardial surface detection software packages, which do not rely on specific geometric assumptions regarding LV geometry and require only minimal human interaction, therefore improving measurement reproducibility18. Transthoracic 3DE has been extensively validated against CMR and has been demonstrated to be more time-saving, reproducible, and accurate than conventional 2DE for LV volumes and ejection fraction measurement19. In most publications, transthoracic 3DE has been shown to slightly underestimate both LV end-diastolic and endsystolic volumes in comparison with those measured with CMR20. A meta-analysis of 23 studies comparing transthoracic 3DE with CMR volumes and ejection
fraction demonstrated biases of -19 ± 34 mL, -10 ± 30 mL, and -1 ± 12% for LV end-diastolic and end-systolic volumes, and ejection fraction, respectively21. For years, the usefulness of 3DE in everyday practice was limited by the absence of reference values for LV chamber volumes and ejection fraction. Recently, several publications have addressed this gap in the literature20-25.
Walker et al26, in 50 breast cancer patients, showed that LV volumes measured by 3DE were signifi cantly closer to those measured by multiple-gated acquisition and CMR than volumes calculated with 2DE. Armstrong et al27, in survivors of childhood cancer patients, showed that 3DE was more accurate (sensitivity 53%, false-negative rate 47%) than 2DE (sensitivity 25% and
false-negative rate 75%) in detecting LVEF <50% at CMR. In addition, a recent comparative study showed that among 56 cancer patients undergoing chemotherapy with stable LV function, non-contrast 3DE showed significantly lower temporal variability than the other tested echocardiographic techniques (2DE, contrast- 2DE, 2D triplane, contrast 2D triplane, contrast 3DE)15. Non-contrast 3DE measurement of LVEF provided also the desired level of test/re-test variability of 5.6% (95% confidence interval: 5.0-6.2%), whereas test/re-test variability of 2DE was 9.8% (close to 10% variation that will raise the issue of CTCRD). 3DE appears to be the technique of choice for monitoring LV function during and after chemotherapy28.
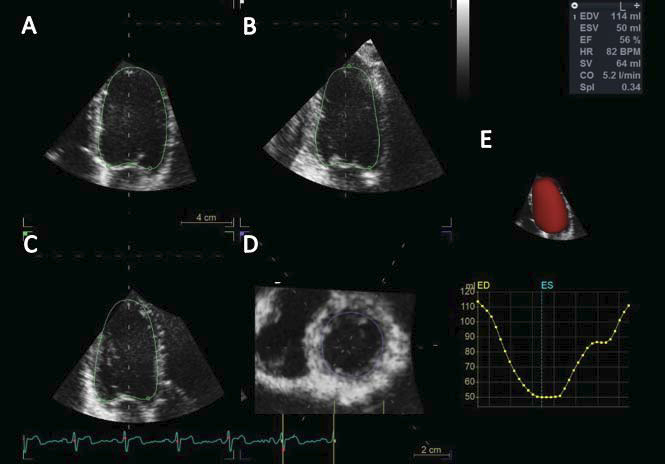
Figure 3. Measurement of left ventricular volumes and ejection fraction using a three-dimensional full-volume data set of the left ventricle. Three longitudinal views and one transversal view obtained by slicing the three-dimensional data set are used to assess the accuracy of the semiautomated endocardial tracking and edited it as needed: apical four chamber view (Panel A), apical two chamber view (Panel B), apical long axis view (Panel C), and short axis view (Panel D). The final beutel of the left ventricle and the curve showing the changes of left ventricular volume during the cardiac cycle are displayed in panel E.
However, it is important to realize that this technique has several limitations as well. 3DE is not as widely available as 2DE because of cost, and it relies heavily on highquality images and operator expertise to achieve the superior performance mentioned above. Tsang et al.29, demonstrated the need of the formal standardization of the analytical approach among the readers in the echocardiography laboratory to eliminate the systematic bias and improve the agreement among readers in the measurement of LV volumes.
Two-dimensional speckle tracking echocardiography: available evidences, pros and cons.
Regardless of how it is measured, LVEF measurement remains a relatively insensitive parameter to detect early CTRCD. This is because a decrease in LVEF does not occur until a critical amount of myocardial damage has occurred and cardiac compensatory mechanisms are exhausted. Accordingly, to overcome the limitations of using parameters like LVEF which are heavily load dependent, the assessment of myocardial function by measuring its deformation during systole (strain) was introduced. Tissue Doppler Imaging (TDI) was the fi rst echocardiographic technique used for strain analysis, but recently STE has become the preferred technique for clinical practice because it overcomes the technical limitations of TDI30 (Table 1). In the LV, the myofibers geometry changes smoothly from a right-handed helix in the subendocardium to a left-handed helix in the subepicardium, such that the helix angle varies continuously from positive at the endocardium to negative at the epicardium. At the equator of the LV, midwall fibers are oriented circumferentially; contraction of these fibers mainly contributes to a decrease in the minor axis of the ventricle (radial contraction) and is responsible for generation of the largest part of the stroke volume. Longitudinally and slightly oblique oriented fi bers in the subendocardium contribute to the shortening of the LV long axis by 12 to 15 mm, to LV torsion, and also to LV stroke volume. This intricate arrangement of myocardial fi bers results in a complex LV mechanics during the myocardial contraction and relaxation. Myocardial contraction occurs in the longitudinal direction (the base moves towards the apex), the radial direction (walls thickening), and the circumferential direction (cavity size decreases perpendicular to the long axis of the chamber). Since myocardial fibers in the subendocardium are particularly sensitive to chemotherapy damage31, and GLS being the most robust and reproducible among the myocardial strain parameters32, this parameter has raised the interest of the investigators to detect early and subclinical myocardial dysfunction in patients at risk of CTCRD33. GLS has been reported to decrease earlier than LVEF, at cumulative doses of chemotherapeutic agents that traditionally were not involved in CTRCD development34- 36. Moreover, GLS has been shown to predict the subsequent occurrence of CTRCD and heart failure (HF)6,37,38. Abnormal GLS values before, during, or early after
chemotherapy was predictive of long-term all- cause mortality in patients with multiple malignancies39. The extent of GLS change among subsequent studies during chemotherapy that could predicts subsequent CTRCD ranged from 10 to 15.9%33,37, ASE/EACVI expert consensus suggests that in the case of a GLS change of more than 15% from baseline, subclinical CTRCD should be defined and prompt cardiac evaluation along with specific treatment are required5. 2D STE appears to have a pivotal role in one of the most important scenario in clinical oncology: early detection
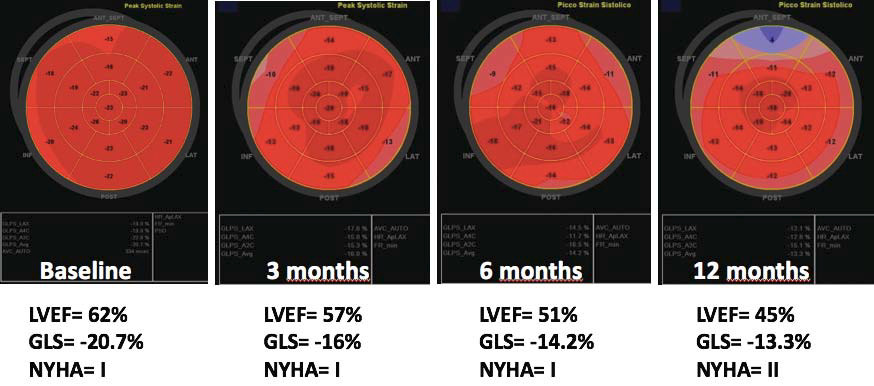
of CTRCD during cancer treatment (Figure 4).
Figure 4. Example showing the role of global longitudinal strain (GLS) in the early detection of cancer treatment-related cardiac dysfunction in a patient with mantle cell lymphoma treated with anthracyclines. At baseline study, both GLS and left ventricular ejection fraction (LVEF) were within normal limits. At 3-month follow-up study: LVEF slightly decreased, though remaining in the normal range. Conversely, GLS was definitely abnormal (-28% compared to baseline values). The patient has no symptoms. At 6-month follow-up study: LVEF was signifi cantly decreased (-18% compared to baseline and below 53%) along with a further reduction of GLS compared to the 3-month study. The patient is still asymptomatic. At 12-month follow-up study a further reduction
of both LVEF and GLS were detected and the patient complained with fatigue and dyspnea on efforts. Abbreviations GLS, global longitudinal strain; LVEF, left ventricular ejection fraction; NYHA, New York Heart Association functional class.
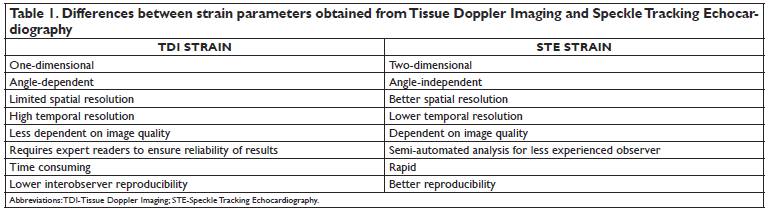
However, the 2DE technique can affect the accuracy of GLS measurement by using foreshortened views or changing the spatial orientation of the apical views from study to study. Moreover, the actual amount of myocardium analyzed using the three 2DE apical views is only a small fraction of total LV myocardial mass. Finally, the myocardium analyzed in the diastolic frame will not necessarily be encompassed in the same systolic frame view due to LV rotation and basal-apical shortening (i.e. limited spatial resolution and the through out-of-plane motion of the tomographic views are known limitations of 2DE GLS)30. The main issues which limited the routine clinical use of 2D STE were the lack of reference values to distinguish between normal and abnormal GLS and the reported significant intervendor variability of strain measurements obtained using different echocardiographic systems40-44. Normal values for the different strain components have been reported in both adults7,45,46 voluand children47. To address the issue of intervendor variability of the strain measurements, the EACVI, the ASE and all the companies manufacturing echocardiographic systems and/or developing software packages to analyze echocardiographic images have joined in a Task Force to standardize myocardial strain imaging48. Application of the standardized criteria set by the EACVI/ ASE-Industry Task Force has proven to be effective in reducing both the intervendor49 and the test/re-test50 variability of GLS measured by STE to clinically acceptable values which were below the variability of LVEF in the same patients.
Three-dimensional speckle tracking echocardiography: a tool with great potentialities to monitor myocardial function!
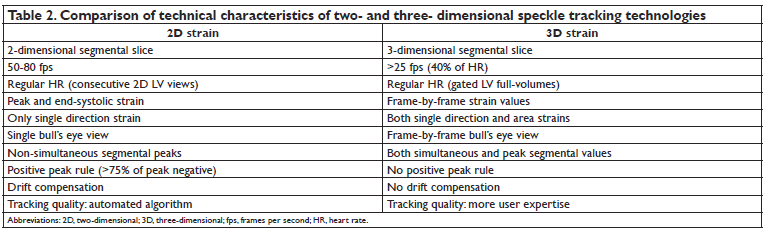
The recently developed 3D STE holds promises to represent a further advance in LV myocardial deformation imaging. Theoretically, it should overcome the main technical limitations of 2DE STE by allowing to analyze the whole LV myocardial mass and to follow myocardial speckle motion in the 3D space51,52. As a result, provided that 3DE data set have reasonable spatial and temporal resolution, 3DSTE has the ability to estimate true 3D myocardial deformation (Table 2). Simultaneous assessment of the various components myocardial wall motion together with LV volumes is one of the advantages of 3DSTE. Global and regional longitudinal, circumferential and radial strains together with LV volumes, EF calculation, LV mass and stroke volume assessment, can be obtained from the same data set reducing both the acquisition and the analysis time53. All these unique characteristics are likely to propel 3DSTE strain parameters as a future diagnostic tool to
assess myocardial mechanics.
However, for an effective clinical and research application, we need the normative values of 3DSTE parameters. Accordingly, reference values for 3DSTE strain components and the effects of demographic, hemodynamic and technical factors on these values, have been recently reported by Muraru et al54. In addition to conventional strain parameters (i.e.
longitudinal, radial and circumferential strain values), 3DSTE provides a composite deformation parameter (e.g. area strain), an area tracking-based parameter, measured at mid myocardial wall, which takes into account both longitudinal and circumferential myocardial deformation. 3DSTE global AS was reported to detect subclinical LV myocardial damage in adults after aortic coarctation repair55, and demonstrated accuracy and reproducibility as a regional wall motion abnormality parameter56. 3DSTE deformation parameters have been reported to be sensitive markers of subclinical LV impairment after surgery for aortic coartaction55 or percutaneous coronary interventions56, in patients with aortic valve disease57 and in hypertensive patients58.
There are few studies which applied 3DSTE to detect myocardial dysfuction in cancer patients who received potentially cardiotoxic chemotherapy. Yu et al.59 demonstrated that childhood cancer survivors had significantly reduced 3DSTE GLS and torsion (p<0.001) in comparison to healthy controls. Another study showed that GLS evaluated with 3DSTE was superior to biomarkers and LVEF in predicting future development of CTRCD60. A recent study compared 2D and 3D echocardiographic measurements of LV function parameters with those measured with CMR in 57 oncological survivors61. They found that LVEF by 3DE below 55%, 3D end-systolic volume indexed larger than 29 mL/ m2 and 3DE GLS higher than -17.5% were the most sensitive echocardiographic parameters to identify subclinical myocardial dysfunction at CMR defined as LVEF lower than 55% and/or abnormal values of peak global longitudinal strain. Finally, 3DSTE area strain was found useful for early detection of cardiac dysfunction associated with the use of anthracycline, and may thus prove to be clinically useful for predicting CTRCD in cancer patients62. However, despite the fact that 3DSTE parameters may have an important role in detecting subclinical myocardial dysfunction and better understanding CTRCDs pathophysiology (Figure 5), studies on oncological patients cardiac function are limited and included small number of patients. Intervendor and intersoftware variability, dependence on the image quality and the need of trained operators, are currently the most important limitations of this promising method54,63.
Clinical and research implications. What do we need to expand our knowledge about CTRCD detection and its management?
It is widely acknowledged that LV myocardial strain assessment now represents an integral part of the evaluation of cardiac function of cancer patients treated with chemotherapy. Despite the significant progresses in reducing the intervendor differences in strain measurements, for an optimal management of cancer patients it is recommended to measure the strain relative change, comparing pretreatment values to measurements obtained during treatment using the same echocardiographic system5. The best approach seems to be the use of individualized baseline strain value, as a reference for further follow up and ideally, each laboratory should produce its own baseline reference values to compare normalcy and pathology in dedicated studies. Moreover, whether or not cardiac intervention or modification to cancer therapy based on strain changes will have a favorable impact on cardiac outcomes in these patients remains to be clarified. EACVI/HFA Cardiac Oncology Toxicity (COT) registry64 is an ongoing multinational, multicenter, randomized controlled study that will specifi cally address this issue. According to the most recent literature, it seems that 3DSTE popularity is increasing and research studies continue to explore its clinical added value over conventional 2DSTE. 3DSTE global strain parameters
seem to have good test-retest measurements reproducibility65, which together with physiological sound measurements and time efficiency, make 3DSTE very attractive for researchers, as well as for clinicians. However, unlike 3DE volume quantification, which is ready for daily clinical use, the current 3DSTE technology is still not developed enough for routine clinical applications30,32. Given its higher feasibility and larger body of evidence, 2DSTE should be used for routine clinical purposes when assessing LV function in patients treated with potentially cardiotoxic regimes5.
3D analysis requires training and experience, which adds the need of specific expertise in acquiring adequate LV 3D data sets, both playing an important role in the reliability of inter-observer reproducibility for 3D measurements, including strain66.
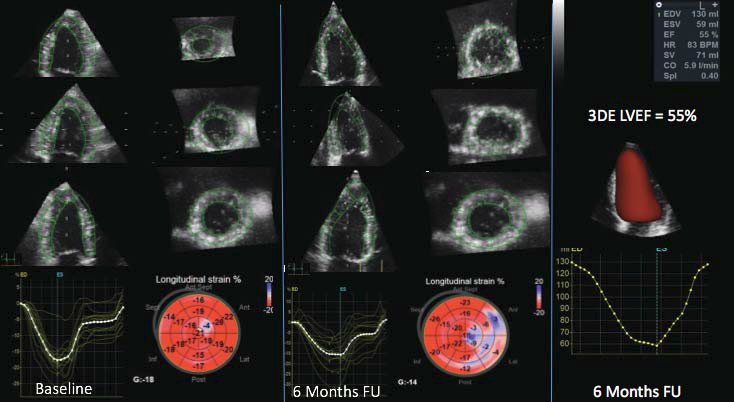
Figure 5. Detection of subclinical cancer treatment-related cardiac dysfunction by three-dimensional global longitudinal strain (GLS) in a patient with non-Hodgkin lymphoma. Compared to the measurements performed at baseline (GLS= -18%, left panel), the study performed at 6 months after anthracycline regimen start showed impairment of GLS (-14%) despite a preserved left ventricular ejection fraction. The patient was asymptomatic. 3DE, threedimensional echocardiography; FU, follow-up; LVEF, left ventricular ejection fraction.
The clinical role of fully automated algorithms to obtain LV volumes and strain parameters from both 2DE67 and 3DE18,68 data sets of the LV remain to be established with properly designed outcome studies. Although various preliminary studies have reported the usefulness of 3DSTE strain parameters, a systematic comparison of 3DE and 2DE strain parameters feasibility, reproducibility and accuracy to detect subclinical CTRCD has never been carried out. A multicenter study, with an adequate sample size to compare
3DSTE to 2DSTE in cancer patients should be designed to provide clinical evidence. The results may impact the clinical use of 3DSTE and may be useful in the design of future chemotherapeutic safety test trials. At present, recommendations concerning cardiac function monitoring of cancer patients are based on expert consensus statements. Prospective controlled outcome studies are urgently required to provide data, which can support recommendations. Finally, including new echocardiographic parameters (e.g. GLS) and echocardiographic techniques (STE and 3DE) into clinical cardio-oncology trials may ultimately prove useful in defining the cardiotoxic profile of new and existing chemotherapeutic agents. Being able to identify subclinical CTRCD may support the identifi cation of patients who can benefit by closer surveillance during and after exposure to potentially cardiotoxic chemotherapy, decreasing the number of cardiac complications and further increase the life expectancy of patients suffering from cancer.
CONCLUSIONS
Although 2DE LVEF is widely used for cardiac function monitoring in clinical practice, it has sown low sensitivity and suboptimal reproducibility in detecting subclinical CTRCD. 3DE has overcome many limitations of 2DE and has significantly improved the accuracy of LV volumes and function measurements to become the method of choice to measure LVEF. The detection of early myocardial impairment (e.g. when LVEF is still preserved) is crucial to start early treatment and improve the patients prognosis. 2DSTE GLS measure the extent of longitudinal myocardial deformation, this myocardial function can be impaired at early stages of CTRCD, when LVEF is still normal. Since it is highly feasible and there is a large body of evidence showing its predictive power for subsequent LV dysfunction and heart failure, 2DSTE should be used together with LVEF to monitor LV function, especially in patients treated with potentially cardiotoxic regimes.
3DSTE strain has potentialities to become the imaging technique of the future to assess LV function. However, to translate the use of 3DSTE from research to clinical routine in the cardio-oncological fi eld, we need more outcome studies to show its added value compared to 2DSTE to predict CTRCD. Finally, current recommendations concerning cardiac function monitoring of cancer patients are largely based on expert consensus statements. Prospective controlled trials are urgently required to provide references to support dedicated guidelines for the early detection, prevention and treatment of CTRCD.
Abbreviations
2DE two-dimensional echocardiography
2DSTE two-dimensional speckle tracking
3DE three-dimensional echocardiography
3DSTE three-dimensional speckle tracking
ASE American Society of Echocardiography
CMR cardiac magnetic resonance
CTRCD cancer-therapeutic related cardiac dysfunction
EACVI European Association of Cardiovascular Imaging
GLS global longitudinal strain
HF heart failure
LV left ventricle
NT-pro BNP brain natriuretic peptide
LVEF left ventricle ejection fraction
STE speckle tracking echocardiography
TDI tissue Doppler imaging
Acknowledgements: Dr. Diana Alexandra Cherata has received a Research Grant from the European Association of Cardiovascular Imaging. Other authors have reported that they have no relationships relevant to the content of this paper to disclose.
References
1. Siegel RL, Miller DK, Jemal A. Cancer statistics 2016. CA Cancer J Clin 2016;66: 7-30
2. Clark RA, Berry NM, Chowdhury MH, Ullah S, Versace VL, Atherthon JJ, Koczawara B, Roder D. Heart failure following cancer treatment characteristics, survival and mortality of a linked health data analysis. Intern Med J 2016; doi: 10.1111/imj.13201 [E-pub ahead of print]
3. Eschenhagen T, Force T, Ewer MS, de Keulenaer GW, Suter TM, Anker SD, Avkiran M, de Azambuja E, Balligand JL, Brutsaert DL, Condorelli G, Hansen A, Heymans S, Hill JA, Hirsch E, Hilfi ker-Kleiner D, Janssens S, de Jong S, Neubauer G, Pieske B, Ponikowski P, Pirmohamed M, Rauchhaus M, Sawyer D, Sugden PH, Wojta J, Zannad F, Shah AM. Cardiovascular side effects of cancer therapies: a position statement from the Heart Failure Association of the European Society of Cardiology. Eur J Heart Fail 2011;13: 1-10
4. Cardinale D, Colombo A, Lamantia G, Colombo N, Civelli M, De Giacomi G, Rubino M, Veglia F, Fiorentini C, Cipolla CM. Anthracyclineinduced cardiomyopathy: Clinical relevance and response to pharmacologic therapy. J Am Coll Cardiol 2010;5: 213–220
5. Plana JC, Galderisi M, Barac A, Ewer MS, Ky B, Scherrer-Crosbie M, Ganame J, Sebag IA, Agler DA, Badano LP, Banchs J, Cardinale D, Carver J, Cerqueira M, DeCara JM, Edvardsen T, Flamm SD, Force T, Griffi n BP, Jerusalem G, Liu JE, Magalhães A, Marwick T, Sanchez LY, Sicari R, Villarraga HR, Lancellotti P. Expert consensus for multimodality imaging evaluation of adult patients during and after cancer therapy: a report from the American Society of Echocardiography and the European Association of Cardiovascular Imaging, Eur Heart J Cardiovasc Imaging 2014;15: 1063-1093
6. Sawaya H, Sebag IA, Plana JC, Januzzi JL, Ky B, Tan TC, Cohen V, Banchs J, Carver JR, Wiegers SE, Martin RP, Picard MH, Gerszten RE, Halpern EF, Passeri J, Kuter I, Scherrer-Crosbie M., Assessment of echocardiography and biomarkers for the extended prediction of cardiotoxicity in patients treated with anthracyclines, taxanes, and trastuzumab. Circ Cardiovasc Imaging 2012;5: 596-603
7. Lang R M, Badano LP, Mor-Avi V, Afi lalo J, Armstrong A, Ernande L, Flachskampf FA, Foster E, Goldstein SA, Kuznetsova T, Lancellotti P, Muraru D, Picard MH, Rietzschel ER, Rudski L, Spencer KT, Tsang W, Voigt JU. Recommendations for cardiac chamber quantification by echocardiography in adults: an update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. Eur Heart J Cardiovasc Imaging 2015;16: 233-270
8. Grothues F, Smith GC, Moon JC, Bellenger NG, Collins P, Klein HU, Pennell DJ. Comparison of interstudy reproducibility of cardiovascular magnetic resonance with two-dimensional echocardiography in normal subjects and in patients with heart failure or left ventricular hypertrophy. Am J Cardiol 2002;90: 29-34
9. Jacobs LD, Salgo IS, Goonewardena S, Weinert L, Coon P, Bardo D, Gerard O, Allain P, Zamorano JL, de Isla LP, Mor-Avi V, Lang RM. Rapid online quantification of left ventricular volume from real-time threedimensional echocardiography data. Eur Heart J 2006; 27: 460-468
10. Kongbundansuk S, Hundley WG. Noninvasive imaging of cardiovascular injury related to the treatment of cancer. JACC Cardiovasc Imaging 2014;7: 824–838
11. Hundley WG, Kizilbash AM, Afridi I, Franco F, Peshock RM, Grayburn PA. Administration of an intravenous perfl uorocarbon contrast agent improves echocardiographic determination of left ventricular volumes and ejection fraction: comparison with cine magnetic resonance imaging. J Am Coll Cardiol 1998;32: 1426-1432
12. Malm S, Frigstad S, Sagberg E, Larsson H, Skjaerpe T. Accurate and reproducible measurement of left ventricular volume and ejection fraction by contrast echocardiography: a comparison with magnetic resonance imaging. J Am Coll Cardiol 2004;44: 1030-1035
13. Thomson HL, Basmadjian AJ, Rainbird AJ, Razavi M, Avierinos JF, Pellikka PA, Bailey KR, Breen JF, Enriquez-Sarano M. Contrast echocardiography improves the accuracy and reproducibility of left ventricular remodeling measurements: a prospective, randomly assigned, blinded study. J Am Coll Cardiol 2001;38: 867-875
14. Hoffmann R1, von Bardeleben S, ten Cate F, Borges AC, Kasprzak J, Firschke C, Lafi tte S, Al-Saadi N, Kuntz-Hehner S, Engelhardt M, Becher H,Vanoverschelde JL. Assessment of systolic left ventricular function: a multi-centre comparison of cineventriculography, cardiac magnetic resonance imaging, un enhanced and contrast-enhanced echocardiography. Eur Heart J 2005;26: 607-616
15. Thavendiranathan P, Grant AD, Negishi T, Plana JC, Popović ZB, Marwick TH. Reproducibility of echocardiographic techniques for sequential assessment of left ventricular ejection fraction and volumes: application to patients undergoing cancer chemotherapy. J Am Coll Cardiol 2013;61: 77-84
16. Chukwu EO1, Barasch E, Mihalatos DG, Katz A, Lachmann J, Han J, Reichek N, Gopal AS. Relative importance of errors in left ventricular quantitation by two-dimensional echocardiography: insights from three-dimensional echocardiography and cardiac magnetic resonance imaging. J Am Soc Echocardiogr 2008;21: 990-997
17. Mor-Avi V, Lang RM. The use of real-time three-dimensional echocardiography for the quantification of left ventricular volumes and function. Curr Opin Cardiol 2009;24: 402-409
18. Tsang W, Salgo IS, Medvedofsky D, Takeuchi M, Prater D, Weinert L, Yamat M, Mor-Avi V, Patel AR, Lang RM. Transthoracic 3D echocardiographic left heart chamber quantification using an automated adaptive analytics algorithm. JACC Cardiovasc Imaging 2016;9: 769- 782
19. Badano LP, Boccalini F, Muraru D, Bianco LD, Peluso D, Bellu R, Zoppellaro G, Iliceto S. Current clinical applications of transthoracic three-dimensional echocardiography. J Cardiovasc Ultrasound 2012; 20: 1-22
20. Shimada YJ, Shiota T. A meta-analysis and investigation for the source of bias of left ventricular volumes and function by three-dimensional echocardiography in comparison with magnetic resonance imaging. Am J Cardiol 2011;107: 126-138
21. Fukuda S, Watanabe H, Daimon M, Abe Y, Hirashiki A, Hirata K, Ito H, Iwai-Takano M, Iwakura K, Izumi C, Hidaka T, Yuasa T, Murata K, Nakatani S, Negishi K, Nishigami K, Nishikage T, Ota T, Hayashida A, Sakata K, Tanaka N, Yamada S, Yamamoto K, Yoshikawa J. Normal values of real-time 3-dimensional echocardiographic parameters in a healthy Japanese population: the JAMP-3D Study. Circ J 2012;76: 1177-1181
22. Muraru D, Badano LP, Peluso D, Dal Bianco L, Casablanca S, Kocabay G, Zoppellaro G, Iliceto S. Comprehensive analysis of left ventricular geometry and function by three-dimensional echocardiography in healthy adults. J Am Soc Echocardiogr 2013;26: 618-628
23. Aune E, Baekkevar M, Rodevand O, Otterstad JE. Reference values for left ventricular volumes with real-time 3-dimensional echocardiography. Scand Cardiovasc J 2010;44: 24-30
24. Kaku K, Takeuchi M, Otani K, Sugeng L, Nakai H, Haruki N, Yoshitani H, Watanabe N, Yoshida K, Otsuji Y, Mor-Avi V, Lang RM. Age- and gender-dependency of left ventricular geometry assessed with real- time three-dimensional transthoracic echocardiography. J Am Soc Echocardiogr 2011;24: 541-547
25. Chahal NS, Lim TK, Jain P, Chambers JC, Kooner JS, Senior R. Population- based reference values for 3D echocardiographic LV volumes and ejection fraction. JACC Cardiovasc Imaging 2012;5: 1191-1197
26. Walker J, Bhullar N, Fallah-Rad N, Lytwyn M, Golian M, Fang T, Summers AR, Singal PK, Barac I, Kirkpatrick ID, Jassal DS. Role of three-dimensional echocardiography in breast cancer: comparison with two-dimensional echocardiography, multiple- 18 gated acquisition scans, and cardiac magnetic resonance imaging. J Clin Oncol 2010;28:3429–3436
27. Armstrong GT1, Plana JC, Zhang N, Srivastava D, Green DM, Ness KK, Daniel Donovan F, Metzger ML, Arevalo A, Durand JB, Joshi V, Hudson MM, Robison LL, Flamm SD. Screening adult survivors of childhood cancer for cardiomyopathy: comparison of echocardiography and cardiac magnetic resonance imaging. J Clin Oncol. 2012;30: 2876-2884
28. Mor-Avi V, Lang RM. Is echocardiography reliable for monitoring the adverse cardiac effects of chemotherapy? J Am Coll Cardiol 2013;61: 85-87
29. Tsang W, Kenny C, Adhya S, Kapetanakis S, Weinert L, Lang RM, Monaghan M. Interinstitutional measurements of left ventricular volumes, speckle-tracking strain, and dyssynchrony using three-dimensional echocardiography. J Am Soc Echocardiogr 2013;26: 1253-1257
30. Mor-Avi V, Lang RM, Badano LP, Belohlavek M, Cardim NM, Derumeaux G, Galderisi M, Marwick T, Nagueh SF, Sengupta PP, Sicari R, Smiseth OA, Smulevitz B, Takeuchi M, Thomas JD, Vannan M, Voigt JU, Zamorano JL. Current and evolving echocardiographic techniques for the quantitative evaluation of cardiac mechanics: ASE/EAE consensus statement on methodology and indications: endorsed by the Japanese Society of Echocardiography. J Am Soc Echocardiogr 2012; 24: 277-313
31. Llesuy SF1, Milei J, Gonzalez Flecha BS, Boveris A. Myocardial damage induced by doxorubicins: hydroperoxide-initiated chemiluminescence and morphology. Free Radic Biol Med 1990;8: 259-264
32. Voigt JU, Pedrizzetti G, Lysyansky P, Marwick TH, Houle H, Baumann R, Pedri S, Ito Y, Abe Y, Metz S, Song JH, Hamilton J, Sengupta PP, Kolias TJ, d›Hooge J, Aurigemma GP, Thomas JD, Badano LP. Definitions for a common standard for 2D speckle tracking echocardiography: consensus document of the EACVI/ASE/Industry Task Force to standardize deformation imaging. Eur Heart J Cardiovasc Imaging 2015;16: 1-11
33. Thavendiranathan P, Poulin F, Lim KD, Plana JC, Woo A, Marwick TH. Use of Myocardial Strain Imaging by Echocardiography for the Early Detection of Cardiotoxicity in Patients During and After Cancer Chemotherapy: A Systematic Review. J Am Coll Cardiol 2014;63: 2751-2768
34. Motoki H, Koyama J, Nakazawa H, Aizawa K, Kasai H, Izawa A, Tomita T, Miyashita Y, Kumazaki S, Takahashi M, Ikeda U.Torsion analysis in the early detection of anthracycline-mediated cardiomyopathy. Eur Heart J Cardiovasc Imaging 2012;13: 95–103
35. Stoodley PW, Richards DA, Boyd A, Hui R, Harnett PR, Meikle SR, Byth K, Stuart K, Clarke JL, Thomas L. Left ventricular systolic function in HER2/neu negative breast cancer patients treated with anthracycline chemotherapy: a comparative analysis of left ventricular ejection fraction and myocardial strain imaging over 12 months. Eur J Cancer 2013;49: 3396-3403
36. Stoodley PW, Richards DA, Boyd A, Hui R, Harnett PR, Meikle SR, Clarke JL, Thomas L. Altered left ventricular longitudinal diastolic function correlates with reduced systolic function immediately after anthracycline chemotherapy. Eur Heart J Cardiovasc Imaging 2013; 14: 228-234
37. Kang Y, Xu X, Cheng L, Li L, Sun M, Chen H, Pan C, Shu X. Twodimensional speckle tracking echocardiography combined with high- sensitive cardiac troponin T in early detection and prediction of cardiotoxicity during epirubicine-based chemotherapy. Eur J Heart Fail 2014;16: 300–308
38. Negishi K, Negishi T, Hare JL, Haluska BA, Plana JC, Marwick TH. Independent and incremental value of deformation indices for prediction of trastuzumab-induced cardiotoxicity. J Am Soc Echocardiogr 2013;26:493-498
39. Rhea IB, Uppuluri S, Sawada S, Schneider BP, Feigenbaum H3. Incremental prognostic value of echocardiographic strain and its association with mortality in cancer patients. J Am Soc Echocardiogr 2015;28: 667-673
40. Manovel A, Dawson D, Smith B, Nihoyannopoulos P. Assessment of left ventricular function by different speckle-tracking software. Eur J Echocardiogr 2010;11: 417
41. Takigiku K, Takeuchi M, Izumi C, Yuda S, Sakata K, Ohte N, Tanabe K, Nakatani S; JUSTICE investigators. Normal range of left ventricular 2-dimensional strain: Japanese Ultrasound Speckle Tracking of the Left Ventricle (JUSTICE) study. Circ J 2012;76: 2623-32
42. Nelson MR, Hurst RT, Raslan SF, Cha S, Wilansky S, Lester SJ. Echocardiographic measures of myocardial deformation by speckletracking technologies: the need for standardization? J Am Soc Echocardiogr 2012;25: 1189-94
43. Bansal M, Cho GY, Chan J, Leano R, Haluska BA, Marwick TH. Feasibility and accuracy of different techniques of two-dimensional speckle based strain and validation with harmonic phase magnetic resonance imaging. J Am Soc Echocardiogr 2008;21: 1318-25
44. Negishi K, Lucas S, Negishi T, Hamilton J, Marwick TH. What is the primary source of discordance in strain measurement between vendors: imaging or analysis? Ultrasound Med Biol 2013;39: 714-20.
45. Kocabay G, Muraru D, Peluso D, Cucchini U, Mihaila S, Padayattil-Jose S, Gentian D, Iliceto S, Vinereanu D, Badano LP. Normal left ventricular mechanics by two-dimensional speckle-tracking echocardiography. Reference values in healthy adults. Rev Esp Cardiol (Engl Ed) 2014;67: 651-8
46. Menting ME, McGhie JS, Koopman LP, Vletter WB, Helbing WA, van den Bosch AE, Roos-Hesselink JW. Normal myocardialstrain values using 2D speckle tracking echocardiography in healthy adults aged 20 to 72 years. Echocardiography. 2016. doi: 10.1111/echo.13323. [Epub ahead of print]
47. Levy PT, Sanchez Mejia AA, Machefsky A, Fowler S, Holland MR, Singh GK. Normal ranges of right ventricular systolic and diastolic strain measures in children: a systematic review and meta-analysis. J Am Soc Echocardiogr 2014;27: 549-60
48. Thomas JD, Badano LP. EACVI-ASE-Industry initiative to standardize deformation imaging. A brief update from the co-chairs. Eur Heart J Cardiovasc Imaging 2014;14: 1039-40
49. Farsalinos KE, Daraban AM, Ünlü S, Thomas JD, Badano LP, Voigt JU. Head-to-head comparison of global longitudinal strain measurements among nine different vendors: the EACVI/ASE inter-vendor com parison study. J Am Soc Echocardiogr 2015;28: 1171-81
50. Yang H, Marwick TH, Fukuda N, Oe H, Saito M, Thomas JD, Negishi K. Improvement in Strain Concordance between two major vendors afterthe strain standardization initiative. J Am Soc Echocardiogr 2015;28:642-8
51. Nesser HJ, Mor-Avi V, Gorissen W, Weinert L, Steringer-Mascherbauer R, Niel J, Sugeng L, Lang RM. Quantifi cation of left ventricular volumes using three-dimensional echocardiographic speckle tracking: comparison with MRI, Eur Heart J 2009;30: 1565–1573
52. Jasaityte R, Heyde B, D’Hooge J. Current state of three-dimensional myocardial strain estimation using echocardiography. J Am Soc Echocardiogr 2013;26: 15-28
53. Pérez de Isla L, Balcones DV, Fernández-Golfín C, Marcos-Alberca P, Almería C, Rodrigo JL, Macaya C, Zamorano J. Three-dimensionalwall motion tracking: a new and faster tool for myocardial strain assessment: comparison with two-dimensional-wall motion tracking. J Am Soc Echocardiogr 2009;22: 325-330
54. Muraru D, Cucchini U, Mihăilă S, Miglioranza MH, Aruta P, Cavalli G, Cecchetto A, Padayattil-Josè S, Peluso D, Iliceto S, Badano LP. Left ventricular myocardial strain by three-dimensional speckle-tracking echocardiography in healthy subjects: reference values and analysis of their physiologic and technical determinants. J Am Soc Echocardiogr 2014;27: 858-871
55. Kowalik E, Kowalski M, Klisiewicz A, Hoffman P. Global area strain is a sensitive marker of subendocardial damage in adults after optimal repair of aortic coarctation: threedimensional speckletracking echocardiography data. Heart Vessels 2016; [E-pub ahead of print]
56. Ternacle J, Gallet R, Champagne S, Teiger E, Gellen B, Dubois Randé JL, Gueret P, Lim P. Changes in three-dimensional speckle-tracking-derived myocardial strain during percutaneous coronary intervention. J Am Soc Echocardiogr 2013;26: 1444-1449
57. Li CM, Li C, Bai WJ, Zhang XL, Tang H, Qing Z, Li R. Value of threedimensional speckle-tracking in detecting left ventricular dysfunction in patients with aortic valvular diseases. J Am Soc Echocardiogr 2013;26: 1245-1252
58. Galderisi M, Esposito R, Schiano-Lomoriello V, Santoro A, Ippolito R, Schiattarella P, Strazzullo P, de Simone G. Correlates of global area strain in native hypertensive patients: a three-dimensional speckletracking echocardiography study. Eur Heart J Cardiovasc Imaging 2012;13: 730-738
59. Yu HK, Cheuk DK, Wong SJ, Chan GC, Cheung YF. New three dimensional speckle tracking echocardiography identifi es global impairment of left ventricular mechanics with a high sensitivity in childhood cancer survivors. J Am Soc Echocardiogr 2013;26: 846-852
60. Mornoş C, Manolis AJ, Cozma D, Kouremenos N, Zacharopoulou I, Ionac A. The value of left ventricular global longitudinal strain assessed by three-dimensional strain imaging in the early detection of anthracycline- mediated cardiotoxicity. Hellenic J Cardiol 2014;55: 235-244
61. Toro-Salazar OH, Ferranti J, Lorenzoni R, Walling S, Mazur W, Raman SV, Davey BT, Gillan E, O’Loughlin M, Klas B, Hor KN. Feasibility of echocardiographic techniques to detect subclinical cancer therapeutics–related cardiac dysfunction among high-dose patients when compared with cardiac magnetic resonance, J Am Soc Echocardiogr. 2016;29: 119-131
62. Tatsuya M, Hidekazu T, Akihiro K. Left ventricular endocardial dysfunction in patients with preserved ejection Ffaction after receiving anthracycline. Echocardiography 2014; 31: 848–857
63. Badano LP, Cucchini U, Muraru D, Al Nono O, Sarais C, Iliceto S. Use of three-dimensional speckle tracking to assess left ventricular myocardial mechanics: inter-vendor consistency and reproducibility of strain measurements. Eur Heart J Cardiovasc Imaging 2013; 14: 285-293
64. Lancellotti P, Anker SD, Donal E, Edvardsen T, Popescu BA, Farmakis D, Filippatos G, Habib G, Maggioni AP, Jerusalem G, Galderisi M. EACVI/ HFA Cardiac Oncology Toxicity Registry in breast cancer patients: rationale, study design, and methodology (EACVI/HFA COT Registry)— EURObservational Research Program of the European Society of Cardiology, Eur Heart J Cardiovasc Imaging 2015;16: 466-470
65. Gayat E, Ahmad H, Weinert L, Lang RM, Mor-Avi V. Reproducibility and inter-vendor variability of left ventricular deformation measurements by three-dimensional speckle-tracking echocardiography. J Am Soc Echocardiogr 2011;24: 878-885
66. Mor-Avi V, Jenkins C, Kühl HP, Nesser HJ, Marwick T, Franke A, Ebner C, Freed BH, Steringer-Mascherbauer R, Pollard H, Weinert L, Niel J, Sugeng L, Lang RM. Real-time 3-dimensional echocardiographic quantification of left ventricular volumes: multicenter study for validation with magnetic resonance imaging and investigation of sources of error. JACC Cardiovasc Imaging 2008;1: 413-4123
67. Knackstedt C, Bekkers SC, Schummers G, Schreckenberg M, Muraru D, Badano LP, Franke A, Bavishi C, Omar AM, Sengupta PP. Fully Automated Versus Standard Tracking of Left Ventricular Ejection Fraction and Longitudinal Strain: The FAST-EFs Multicenter Study. J Am Coll Cardiol 2015;66: 1456-1466
68. Muraru D, Badano LP, Piccoli G, Gianfagna P, Del Mestre L, Ermacora D, Proclemer A. Validation of a novel automated border-detection algorithm for rapid and accurate quantitation of left ventricular volumes based on three-dimensional echocardiography. Eur J Echocardiogr 2010;11: 359-368.
 This work is licensed under a
This work is licensed under a