Decebal Gabriel Latcu1, Bogdan Enache1, Ahmed Moustafa Wedn1, Sok-Sithikun Bun1, Nadir Saoudi1
1 Centre Hospitalier Princesse Grace, Avenue Pasteur, 98000 Monaco
Robotics has been used for radiofrequency ablation of human arrhythmias for more than 15 years; among 2 widely distributed systems1,2, only Stereotaxis (Saint-Louis, Missouri, USA) is still commercially available. Our experience with the Stereotaxis system goes back more than a decade3,4 and its advantages still make it the system of choice, in our center, for a number of arrhythmias.
THE REMOTE MAGNETIC NAVIGATION (RMN) SYSTEM
RMN uses a steerable magnetic fi eld which allows the remote manipulation inside the heart chambers of a very soft magnetic catheter embedded with an ablation electrode. The RMN system is composed by two giant magnets (Niobe ES, Stereotaxis) positionned each side of the fl uoroscopy table (Axiom Artis, Siemens, Germany), which create a magnetic fi eld of a 0.1 T maximal intensity (Figure 1). The orientation of the magnetic field is remotely controlled by the operator (Figure 2) via a dedicated software (Navigant, Stereotaxis). Additional dedicated systems (V-Drive / V-drive Duo) and disposables (Quick-Cas / V-Cas / VCas Defl ect5, Stereotaxis) connected to the ablation catheter allow the advancement and the retraction of the catheter, of the sheath, as well as defl ection / undeflection / rotation of a remotely controlled fixed curve or steerable sheath. These may be completed by a remotely controlled system for a rigid circular catheter (V-Loop, Stereotaxis). In the following paragraphs some evidence-based data for specific arrhythmias ablation with RMN will be presented.
RMN FOR ABLATION OF ATRIAL FIBRILLATION (AF)
AF ablation with RMN has been performed since 2008 when the fi rst irrigated magnetic catheter became available. Retrospective comparison6 with manual ablation of AF did not show any difference in the ablation result. The longer procedure time for RMN (223 vs 166 min) is compensated by a shorter fluoroscopy time (13 vs 34 min) and possibly inferior complication rate (without any cardiac perforation in the RMN group vs 2.4% in the manual group) but this difference did not reach signifi cance since the study was underpowered. These results were confirmed by another comparative series7; even more, a dedicated prospective study on RMN8 showed comparable results to historical manual ablation data and lack of serious adverse events. A large international multicenter survey9 among RMN users does not report any atrio-esophageal fi stula when using the system, whilst this complication, even rare, is still present while using manual catheters. Persistent AF could represent an elective indication for RMN, as longer procedure times are warranted; also, common left atrial dilation facilitates magnetic navigation. In our initial experience10 on 28 patients having persistent AF ablation with RMN (mean duration of actual AF episode of 10±16 months), with a follow-up 11±6 months after 1.25 procedures/patient, 68% of the cases didn’t have any arrhythmia recurrence. No major complication occurred. The advantage for the operator to perform these lengthy procedures (235±68 min) in a seated position without the lead coat is undeniable. It is worth noting that RMN also renders possible AF ablation by aortic retrograde approach11, which may be useful in case of impossible transeptal approach in congenital abnormalities with inferior vena cava agenesis / interruption. AF ablation with RMN may be further optimized by the use of a remotely controlled steerable sheath (VCas Defl ect); this improves long-term results, allows faster right pulmonary vein isolation and diminishes radiofrequency delivery time and procedure time5. We recently investigated whether lesion creation with magnetic catheters is comparable with the contemporary gold standard manual catheters with contact force assessment. We showed that during radiofrequency delivery, the electrical modifications suggesting transmurality is faster achieved with remote magnetic catheters than with optimal use of contact force catheters12. This may be in relation with a more stable tissue contact while using magnetic technology13.
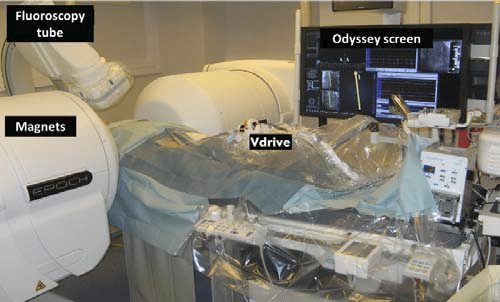
Figure 1. Electrophysiology lab with the RMN: the magnets (Niobe ES), the fluoroscopy tube, the remote catheter control system (Vdrive) and the Odyssey screen.
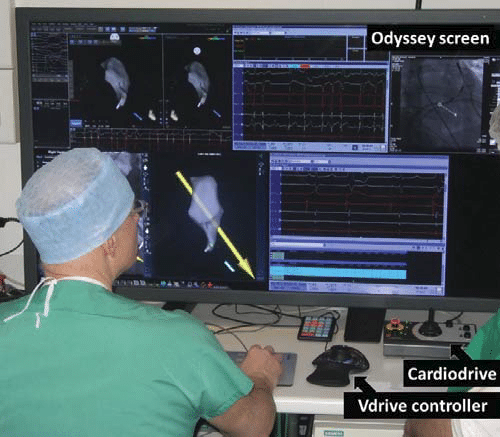
Figure 2. The control room with the Odyssey screen, the Cardiodrive and the Vdrive controller.
RMN FOR ABLATION OF OTHER SUPRAVENTRICULAR ARRHYTHMIAS.
Post AF ablation atrial tachycardia (AT) was until recently another elective indication for RMN technology. Indeed, without having to continuously hold the catheter, the operator could concentrate on annotation and activation mapping, since it’s accuracy determines the procedural success. We compared14 our initial experience of 25 AT patients (RMN) with a control group of manual ablations (32 AT patients). There was no difference in what concerns acute or long-term success (80% vs 78%, p=ns) between the 2 techniques. Nevertheless, if no serious adverse event occurred in the RMN group, in the control group and transitory ischemic attack and a cardiac perforation requiring drainage were reported. The difference in procedure duration between the 2 techniques did not reach signifi cance (RMN 236±67 min, control group 201±72 min). Atrio-ventricular nodal reentrant tachycardia ablation has been feasible with RMN technology from the beginning, since no irrigation is necessary. Finely tuned mapping of the atrio-ventricular node extensions, especially of the rightward inferior extension (commonly the slow pathway), with 1 mm step advancement/ retraction movements of the ablation catheter with direction changes in 1 degree steps, may be fully exploited in this indication. We reported15 a 100% success rate for these procedures, without serious adverse events and with a number of junctional beats inferior to manual technique, favoring a better tissue contact with the magnetic catheters. Typical flutter is a challenge for RMN, possibly because of insuffi cient catheter pressure on the cavo-tricuspid isthmus. Magnetic non-irrigated catheters were proven inferior to manual technique16 but irrigation improved results and seems mandatory in case of anatomical complexity17. For cost-effectiveness reasons RMN might be an alternative to manual catheters for cavo-tricuspid isthmus ablation only in case of concomitant AF ablation or in case of superior approach18. Procedural success of CTI ablation may be warranted with the RMN technology if concomitant use of a steerable sheath. AT in case of congenital heart disease is diffi cult for complex anatomies with limited catheter access. Direct robotic manipulation of the distal tip of a soft catheter, specific for RMN, is a great advantage in comparison to rigid, manually driven catheters, in case of twisting path from the puncture site to the ablation target19. Fluoroscopy exposure is thus signifi cantly reduced20,21. Retrograde transaortic approach for AT ablation in Mustard-Senning or cavo-pulmonary derivation patients seems particularly successful with RMN22,23,24. Accessory pathways, incisional flutters and sinus node reentry have all been reported with the successful use of the RMN.
RMN FOR VENTRICULAR TACHYCARDIA (VT) ABLATION
Feasibility and safety of catheter ablation with the RMN have been reported for right ventricular outfl ow tract VT25, fascicular VT26, ischemic scar-related VT27,28, including epicardial VT29, as well as in other heart disease- related VT30. An increasing amount of data31,32 seem to suggest even superior results for VT ablation with RMN in comparison to manual technique. A randomized study is currently including VT patients and will assess whether substrate-based ablation of VT with RMN has clinical advantages over manual catheter manipulation33.
CONCLUSION: ACHIEVEMENTS AND CHALLENGES
Ablation using RMN has similar effi cacy compared to the manual technique in a wide range of arrhythmias. RMN has the advantages of improved safety and an undeniable increased comfort for the operator. Complex procedures became feasible with RMN for a stand-alone operator, manipulating both the ablation catheter and the mapping system. Congenital heart disease arrhythmias are an elective indication for RMN; RMN might be superior to manual technique also for VT ablation. In our center, AVNRT and AF ablation are other procedure types for which RMN is systematically considered. Nevertheless, RMN is facing today several challenges. First, the irrigated magnetic-tip catheter, available for almost one decade, has not been upgraded. Several technological improvements have been embedded into manual catheters (contact force measurement, more effi cient cooling with less irrigation fl ow) but are still lacking for magnetic catheters. Shortening the rigid part of the distal tip of the magnetic catheters and approaching the three magnets towards the distal end might improve navigation, catheter stability and electrode- tissue contact. A contact assessment module (“eContactTM”) will be shortly available from Stereotaxis; added to the current catheters it may overcome some of these limitations. Second, for several years, electrophysiology entered the era of multielectrode mapping (MEM) with automatic annotation. More recently, ultra-high-density mapping became the gold-standard for mapping of complex arrhythmias34. Except for the use of the V-loop disposable allowing the use of the circular catheter (LassoTM) for MEM, but with the magnets in the stowed position and less reliably than multielectrode catheters like the PentaRayTM or OrionTM, RMN allows only “point-by-point” mapping. Moreover, also RMN has been used in junction with other mapping systems like Rhythmia35 and Navex36, integration is currently available only with CartoTM (Biosense-Webster, Inc.), which might also be considered a limitation.
Acknowledgements: Dr. Laţcu and Dr. Bun received in the past year consulting fees and speaking honoraria from Boston Scientifi c and Pfizer. Dr. Laţcu is also a consultant for Stereotaxis.
References
1. Faddis MN, Chen J, Osborn J, et al. Magnetic guidance system for cardiac electrophysiology: a prospective trial of safety and effi cacy in humans. J Am Coll Cardiol 2003;42:1952-8.
2. Saliba W, Reddy VY, Wazni O, et al. Atrial fi brillation ablation using a robotic catheter remote control system initial human experience and long-term follow-up results. J Am Coll Cardiol. 2008;51(25):2407-11.
3. Saoudi N, Laţcu DG, Rinaldi JP, Ricard P. La robotique dans le diagnostic et le traitement des troubles du rythme cardiaque. Bull Acad. Natle Med 2008;192(5):1029-41.
4. Laţcu DG, Ricard P, Zarqane N, et al. Robotic magnetic navigation for ablation of human arrhythmias: initial experience. Arch Cardiovasc Dis. 2009;102(5):419-25.
5. Errahmouni A, Laţcu DG, Bun SS, Rijo N, Dugourd C, Saoudi N. Remotely controlled steerable sheath improves result and procedural parameters of atrial fi brillation ablation with magnetic navigation. Europace. 2015 Jul;17(7):1045-50. doi: 10.1093/europace/euu388. Epub 2015 Feb 5.
6. Arya A, Zaker-Shahrak R, Sommer Pet al. Catheter ablation of atrial fi brillation using remote magnetic catheter navigation: a case-control study. Europace. 2011;13(1):45-50.
7. L. Vollmann D, Seegers J, Dorenkamp M et al. Remote magnetic versus manual catheter navigation for circumferential pulmonary vein ablation in patients with atrial fi brillation. Lüthje Clin Res Cardiol. 2011; 100(11):1003-11.
8. Pappone C, Vicedomini G, Frigoli E, et al. Irrigated-tip magnetic catheter ablation of AF: a long-term prospective study in 130 patients. Heart Rhythm 2011:8(1):8-15.
9. Danon A, Shurrab M, Nair KM, Laţcu DG, Arruda MS, Chen X, Szili- Torok T, Rossvol O, Wissner EE, Lashevsky I, Crystal E. Atrial fi brillation ablation using remote magnetic navigation and the risk of atrial- esophageal fi stula: international multicenter experience. J Interv Card Electrophysiol. 2015 Aug;43(2):169-74. doi: 10.1007/s10840- 015-0003-7. Epub 2015 May 3.
10. Arnoult M, Laţcu DG, Ricard P, Saoudi N. Remote magnetic navigation in catheter ablation of persistent atrial fi brillation. Arch Cardiovasc Dis Suppl 2012;4(1):70.
11. Miyazaki S, Nault I, Haïssaguerre M, Hocini M. Atrial fi brillation ablation by aortic retrograde approach using a magnetic navigation system. J Cardiovasc Electrophysiol. 2010;21(4):455-7.
12. Bun SS, Ayari A, Laţcu DG, Errahmouni A, Saoudi N. Radiofrequency catheter ablation of atrial fi brillation: Electrical modifi cation suggesting transmurality is faster achieved with remote magnetic catheter in comparison with contact force use. J Cardiovasc Electrophysiol. 2017 Jul;28(7):745-753. doi: 10.1111/jce.13222. Epub 2017 May 24.
13. Bhaskaran A, Barry MA, Al Raisi SI, Chik W, Nguyen DT, Pouliopoulos J, Nalliah C, Hendricks R, Thomas S, McEwan AL, Kovoor P, Thiagalingam A. Magnetic guidance versus manual control: comparison of radiofrequency lesion dimensions and evaluation of the effect of heart wall motion in a myocardial phantom. J Interv Card Electrophysiol. 2015 Oct;44(1):1-8. doi: 10.1007/s10840-015-0023-3. Epub 2015 Jun 30.
14. Laţcu DG, Massaad Y, Mahjoub M, Squara F, Saoudi N. Left atrial fl utter occuring after atrial fi brillation ablation: ablation using remote magnetic navigation versus manual technique. Archives of Cardiovascular Diseases Supplements 2013: 5(1) :69.
15. Ricard P, Laţcu DG, Yaïci K, Zarqane N, Saoudi N. Slow pathway radiofrequency ablation in patients with AVNRT: junctional rhythm is less frequent during magnetic navigation ablation than with the conventional technique. Pacing Clin Electrophysiol. 2010;33(1):11-5.
16. Steven D, Rostock T, Servatius H et al. Robotic versus conventional ablation for common-type atrial fl utter: a prospective randomized trial to evaluate the effectiveness of remote catheter navigation. Heart Rhythm 2008;5:1556-60.
17. Koektuerk B, Chun JK, Wissner E et al. Cavotricuspid Isthmus Anatomy Determines The Success Of Remote Controlled Magnetic Bidirectional Block: A Comparision Between Magnetic 8-mm Solid Tip
And 3.5-mm Magnetic Irrigated Tip Catheter. Indian Pacing Electrophysiol J. 2011;11(4):103-14.
18. Laţcu DG, Bun SS, Ricard P, Saoudi N. Hepatico-Tricuspid Isthmus Ablation for Typical-Like Atrial Flutter by Femoral Approach in Absence of the Inferior Vena Cava: Use of Magnetic Navigation and Three-Dimensional Mapping with Image Integration. Pacing Clin Electrophysiol. 2011 Mar 16. doi: 10.1111/j.1540-8159.2011.03051.x.
19. Ueda A, Suman-Horduna I, Mantziari L, Gujic M, Marchese P, Ho SY, Babu-Narayan SV, Ernst S. Contemporary outcomes of supraventricular tachycardia ablation in congenital heart disease: a single-center experience in 116 patients. Circ Arrhythm Electrophysiol. 2013 Jun;6(3):606-13. doi: 10.1161/CIRCEP.113.000415. Epub 2013 May 17.
20. Schwagten B, Witsenburg M, De Groot NM et al. Effect of magnetic navigation system on procedure times and radiation risk in children undergoing catheter ablation. Am J Cardiol. 2010;106(1):69-72.
21. Wu J, Pfl aumer A, Deisenhofer I et al. Mapping of atrial tachycardia by remote magnetic navigation in postoperative patients with congenital heart disease. J Cardiovasc Electrophysiol. 2010:21(7):751-9.
22. Wu J, Pfl aumer A, Deisenhofer I et al. Mapping of intraatrial reentrant tachycardias by remote magnetic navigation in patients with d-transposition of the great arteries after mustard or senning procedure. J Cardiovasc Electrophysiol. 2008;19(11):1153-9.
23. Schwagten B, Jordaens L, Witsenburg M et al. Initial experience with catheter ablation using remote magnetic navigation in adults with complex congenital heart disease and in small children. Pacing Clin Electrophysiol. 2009;32 Suppl 1:S198-201.
24. Ernst S, Babu-Narayan SV, Keegan J et al. Remote Controlled Magnetic Navigation and Ablation with 3D Image Integration as an Alternative Approach in Patients with Intra-Atrial Baffl e Anatomy. Circ Arrhythm Electrophysiol. 2011 Nov 7. [Epub ahead of print]
25. Konstantinidou M, Koektuerk B, Wissner E et al. Catheter ablation of right ventricular outfl ow tract tachycardia: a simplifi ed remotecontrolled approach. Europace. 2011 ;13(5):696-700.
26. Thornton AS, Res J, Mekel JM, Jordaens LJ. Use of advanced mapping and remote magnetic navigation to ablate left ventricular fascicular tachycardia. Pacing Clin Electrophysiol. 2006;29(6):685-8.
27. Arya A, Eitel C, Bollmann A et al. Catheter ablation of scar-related ventricular tachycardia in patients with electrical storm using remote magnetic catheter navigation. Pacing Clin Electrophysiol. 2010; 33(11):1312-8. doi: 10.1111/j.1540-8159.2010.02818.x.
28. Skoda J, Arya A, Garcia F, Gerstenfeld E, Marchlinski F, Hindricks G, Miller J, Petru J, Sediva L, Sha Q, Janotka M, Chovanec M, Waldauf P, Neuzil P, Reddy VY. Catheter Ablation of Ischemic Ventricular Tachycardia With Remote Magnetic Navigation: STOP-VT Multicenter Trial. J Cardiovasc Electrophysiol. 2016 Mar;27 Suppl 1:S29-37. doi: 10.1111/jce.12910.
29. Di Biase L, Santangeli P, Astudillo V et al. Endo-epicardial ablation of ventricular arrhythmias in the left ventricle with the Remote Magnetic Navigation System and the 3.5-mm open irrigated magnetic catheter: results from a large single-center case-control series. Heart Rhythm. 2010;7(8):1029-35.
30. Aryana A, d’Avila A, Heist EK et al. Remote magnetic navigation to guide endocardial and epicardial catheter mapping of scar-related ventricular tachycardia. Circulation. 2007;115(10):1191-200.
31. Bauernfeind T, Akca F, Schwagten B et al. The magnetic navigation system allows safety and high effi cacy for ablation of arrhythmias. Europace. 2011;13(7):1015-21.
32. Turagam MK, Atkins D, Tung R, Mansour M, Ruskin J, Cheng J, Di Biase L, Natale A, Lakkireddy D. A meta-analysis of manual versus remote magnetic navigation for ventricular tachycardia ablation. J Interv Card Electrophysiol. 2017 Jun 17. doi: 10.1007/s10840-017-0257-3. [Epub ahead of print]
33. Di Biase L, Tung R, Szili-Torok T, Burkhardt JD, Weiss P, Tavernier R, Berman AE, Wissner E, Spear W, Chen X, Neužil P, Skoda J, Lakkireddy D, Schwagten B, Lock K, Natale A. MAGNETIC VT study: a prospective, multicenter, post-market randomized controlled trial comparing VT ablation outcomes using remote magnetic navigation-guided substrate mapping and ablation versus manual approach in a low LVEF population. J Interv Card Electrophysiol. 2017 Apr;48(3):237-245. doi: 10.1007/s10840-016-0217-3. Epub 2017 Jan 7.
34. Laţcu DG, Bun SS, Viera F, Delassi T, El Jamili M, Al Amoura A, Saoudi N. Selection of Critical Isthmus in Scar-Related Atrial Tachycardia Using a New Automated Ultrahigh Resolution Mapping System. Circ Arrhythm Electrophysiol. 2017 Jan;10(1). pii: e004510. doi: 10.1161/ CIRCEP.116.004510.
35. Laţcu DG, Bun SS, Saoudi N. Combined remote magnetic navigation and ultra-high-density mapping (Rhythmia™) in slow pathway ablation. Europace. 2016 Jun;18(6):814. doi: 10.1093/europace/euv459. Epub 2016 Feb 5.
36. Nölker G, Gutleben KJ, Muntean B, Vogt J, Horstkotte D, Dabiri Abkenari L, Akca F, Szili-Torok T. Novel robotic catheter manipulation system integrated with remote magnetic navigation for fully remote ablation of atrial tachyarrhythmias: a two-centre evaluation. Europace. 2012 Dec;14(12):1715-8. doi: 10.1093/europace/eus169. Epub 2012 Jun 20.
 This work is licensed under a
This work is licensed under a