Letitia-Elena Radu1,2, Ioana Adriana Ghiorghiu2,3, Dan Mihai Dorobantu4, Alina Oprescu5, Carmen Ginghina2,4, Constantin-Virgiliu Arion1,2, Anca Colita1,2, Bogdan A. Popescu2,4
1 Fundeni Clinical Institute, Bucharest, Romania
2 „Carol Davila” University of Medicine and Pharmacy, Bucharest, Romania
3 „Prof. Dr. Victor Gomoiu” Children’s Hospital, Bucharest, Romania
4 „Prof. Dr. C. C. Iliescu” Emergency Institute of Cardiovascular Diseases, Bucharest, Romania
5 Monza Hospital, Bucharest, Romania
Abstract: Purpose – Doxorubicin-based treatment has cardiotoxic effects and may lead to heart failure in cancer treated patients. The aim of the study was to assess the changes in RV function and its correlation to LV systolic function after doxorubicin treatment in pediatric patients. Methods – We included 38 children (median age 5 years) with acute lymphoblastic leukemia undergoing standard chemotherapy, including doxorubicin, with a median cumulative dose of 231 mg/m2. Echocardiograms were performed before treatment initiation (T1), at reinduction end (T2) and at one year after treatment start (T3). Peak systolic tricuspid annular velocity (RV-S), left ventricular ejection fraction (LVEF) and peak septal and lateral systolic mitral annular velocities (SS and SL) were among the measurements performed. Results – RV-S decreased between T1-T2 in 25 (73%), between T2-T3 in 17 (50%) and returned to pre-treatment values in 7 (9%) pati-ents. SS decreased between T1-T2 in 25 (65%), between T2-T3 in 14 (37%) and returned to pre -treatment values in 16 (42%) patients. In patients with a LVEF decrease of at least 5% between T1-T3 (n=10), we found a higher decrease in RV-S from T1-T3 (median -4.1 vs -1.3 cm/s), a lower RV- S value at T3 (median 10.8 vs 13 cm/s, p=0.07) and a lower SS value at T2 (median 6.3 vs 7.5 cm/s, p=0.02) when compared to the rest. Conclusions – We found that RV-S significantly decreases at one year after doxorubicin treatment initiation, more significantly in those patients where the LVEF also decreased. As opposed to SS, RV-S only returns to pretreatment values in less than 10% of children at one year after treatment start. RV function evaluation can prove to be a valuable asset in the follow-up after doxorubicin treatment in children. Keywords: cardio-oncology, doxorubicin toxicity, tissue Doppler echocardiography, right ventricular longitudinal function
INTRODUCTION
Acute lymphoblastic leukemia (ALL) is the most common malignancy in children and the most common cancer to cause death before the age of 20. Regardless, recent years have brought progresses in therapy which allowed for close to 90% survival after treatment1. This has led to an increasing population of chemotherapy survivors, with many regimens including anthracyclines (e.g. doxorubicin), with known cardiotoxic effects, most likely through myocardial fibrosis2,3 . Anthracycline related toxicity is generally classified into acute, occurring in the first week, early, in the first year and late onset, after one year4.
The current guidelines recommend echocardiographic evaluations before, during and after treatment with anthracyclines, with evaluation of left ventricular (LV) function by left ventricular ejection fraction (LVEF), preferably by three-dimensional techniques, and by global longitudinal strain2. Acquiring proper images for advanced echocardiography analyses (e.g. speckle tracking, three-dimensional echo) is often not possible in children. Tissue Doppler imaging (TDI) has long been proposed as a promising technique for evaluating patients undergoing chemotherapy5. Due to its ease of use, this technique is an attractive choice in the pediatric population.
The impact of anthracycline treatment on LV function is well known2, but the impact on the right ventricle (RV) is less studied. There is increasing proof that RV dysfunction is a better predictor of outcomes than LV function alone in a number of cardiac conditions6, making monitoring of RV function an attractive tool for cardiac assessment during and after anthracycline treatment. A recent echocardiographic report from Norway has shown that a third of the long term adult survivors of childhood ALL have RV dysfunction at a mean follow-up of 21 years. In this setting, RV dysfunction is three times more frequent in those with impaired LV function6, with 53% of survivors showing subnormal and 27% abnormal RV function by cardiac magnetic resonance7. Additionally, RV diastolic dysfunction was reported in early cardiotoxicity. Data on cardiac function in children after ALL treatment is available, heterogeneous in terms of study design and conclusions, but few of these studies looked at early onset cardiotoxicity or RV function specifically after low dose treatment4,8-13.
The aim of the study was to assess the early effects of low- dose doxorubicin treatment in children with ALL on LV and RV function and the potential value of longitudinal RV systolic dysfunction as an early sign of cardiac toxicity in a prospective study, with repeated measurements over one year from treatment initiation. A prospective protocol including patients receiving low doses of anthracyclines allows for a more accurate determination of the patterns of variability of cardiac function during therapy, in patients treated with modern protocols.
METHODS
Patient selection and hematologic treatment This prospective, non-randomized observational study evaluated acute and early-onset cardiotoxicity after standard chemotherapy treatment for ALL in pediatric patients. The study was approved by Fundeni Clinical Institute Ethics Committee. We enrolled children between 1-18 years newly diagnosed with ALL and treated in our department between February 2015 and August 2017. The treatment was according to the BFM-ALL IC 2002 protocol, to which we ad-ded the monitoring of minimal residual disease at days 15, 33 and 78. We were not able to use BFM-ALL IC 2009 protocol, due to the inability of providing Oncaspar®. Patients are stratified in three treatment groups: standard risk (SR, n=17), intermediate risk (IR, n=15) and high risk (HR, n=6). The cumulative dose (CD) of anthracycline (doxorubicin) was different for each group, with a maximum cumulative doxorubicin dose of 300 mg/m2 in all patients: SR received 6-8 doses of 30 mg/m2 of doxorubicin (2-4 in induction and 4 in reinduction), IR received 8 doses of 30 mg/m2 (4 in induction and 4 in reinduction) and HR patients received 10 doses of 30 mg/m2 (4 in induction, 2 in high risk blocks and 4 in reinduction). In exceptional cases, AC dose was decreased due to low neutrophil count. Treatment protocol is presented in more detail in the Supplemental Figure 1.
Echocardiography
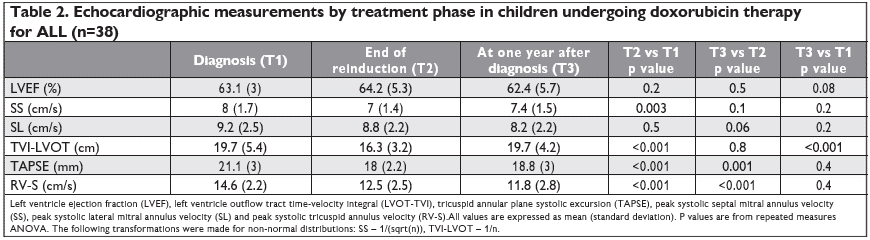
After a signed informed consent, echocardiographic evaluation, including TDI, was performed at 3 diffe-rent time points: at diagnosis before treatment start (T1), at the end of reinduction after all anthracycline doses (T2), and at approximately one year after diagnosis, which was generally at 2-4 months after the last doxorubicin dose administration (T3). Timing of the evaluations in the context of the hematological treatment is presented in Figure 1. The scans were performed and analyzed online on a Vivid E95 (GE Vingmed Imaging, Horten, Norway) by two experienced echocardiographers (IAG and AG). The following measurements were performed: left ventricle ejection fraction (LVEF) with the M mode Teichholz method, LV outflow tract time- velocity integral (LVOT-TVI), tricuspid annular plane systolic excursion (TAPSE), peak systolic septal mitral annulus velocity (SS), peak systolic lateral mitral annulus velocity (SL) and peak systolic tricuspid annulus velocity (RV-S). Additionally, LV diastolic function parameters were measured: early filling transmitral flow velocity (E), late filling transmitral flow velocity (A), E-wave deceleration time (EDT), isovolumic relaxation time (IVRT), and E/A ratio, but due to non-normal distributions and widely dispersed values with poor normalization after various transformations these were not used in the analyses to avoid spurious results. All acquisitions and measurements were performed in concordance with current recommendations14,15. In order to reduce the exam time in this population of children undergoing chemotherapy, we limited it to only the measurements included in the pre -established protocol, and additionally, the Teichholz method doubled by visual assessment was used for estimation of LVEF, as it is quicker to obtain in children compared to the Simpson Biplane method.
An absolute decrease of more than 5% in LVEF from T1 to T3 was considered significant and was used to define two groups: patients with LVEF de-crease, and patients without. Values at T1/T2/T3 and changes from T1-T2/T2-T3/T1-T3 for measured parameters were compared in these two groups. In 4 patients no measurement of RV-S was done at T2 due to technical issues.
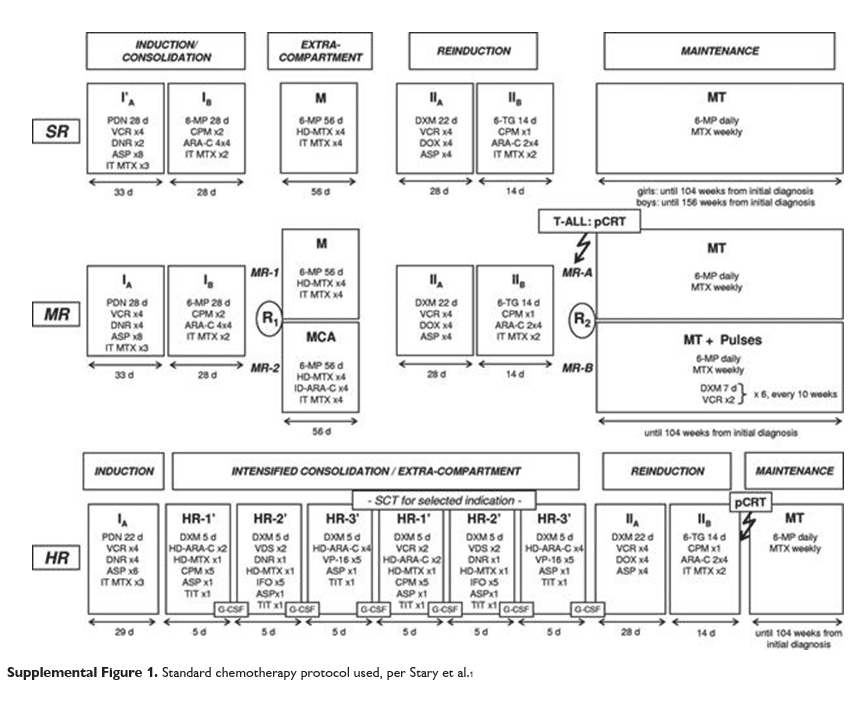
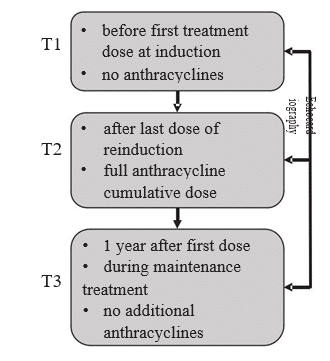
Figure 1. Echocardiography and hematological treatment timing.
Statistical analyses
Continuous numerical values were given as median and interquartile range (IQR) unless otherwise stated. Categorical values are given as proportion from total. Changes between the three measurement moments were evaluated with the repeated ANOVA test and post-hoc pairwise comparison of predictive margins, after transformation of non-normal variables using the method resulting in the lowest V statistic by the Shapiro-Wilk test. Differences between independent groups were evaluated using the Mann-Whitney U test. All analyses were performed using the STATA 12 SE statistical package (StataCorp LP, Texas, USA).
RESULTS
Between February 2014 and August 2017, a total of 38 patients finished induction, consolidation and re-induction, and were evaluated by echocardiography in all three visits. Demographic and clinical data are detailed in Table 1. The majority of patients were in the SR and IR groups, receiving treatment with a CD of anthracyclines of less than 240 mg/m2 , with only 5 patients receiving a higher CD (257, 264, 293, and 300 mg/m2 respectively), with a median cumulative dose of 231 mg/m2. We did not find any association between hematological disease characteristics and echocardiographic findings in our study, including morphological, cytogenetic and molecular subtypes.
RV- S decreased during treatment from T1 to T2 (mean 14.6 cm/s vs 12.5 cm/s, p<0.001), and remained decreased after one year from diagnosis at T3 (mean 11.8 cm/s, p=0.4 vs T2, p<0.001 vs T1) – Figure 2. RV-S decreased between T1-T2 in 25 (73%) patients, between T2-T3 in 17 (50%) patients, and returned to pre-treatment values in only 7 patients (9%). The same pattern was observed for TAPSE values, with a decrease from T1 to T2 (mean 21.1 vs 18 mm, p<0.001), which was maintained at T3 (mean 18.8 mm, p=0.4 vs T2, p<0.001 vs T1) – Figure 3. Cumulative dose was not found to be a predictor for RV-S or TAPSE dynamic. SS decreased during treatment from T1 to T2 (mean 8 vs 7 cm/s, p=0.003) and showed a trend toward returning to the initial values at T3 (mean 7.4 cm/s, p=0.1 vs T1, p=0.2 vs T2) – Figure 4. SS decreased between T1-T2 in 25 (65%), between T2-T3 in 14 (37%) and returned to pre-treatment values in 16 (42%) patients. There was a trend for SL values to de-crease during treatment, more significantly between T1 and T3 (mean 9.2 vs 8.2 cm/s, p=0.06). The same pattern was observed in TVI-LVOT measurements, with a significant drop from T1 to T2 (mean 19.7 vs 16. 3 cm, p<0.001) and a return to initial values at T3 (mean 19.7 cm, p=0.8 vs T1, p<0.001 vs T2) – Figure 5. Cumulative dose was found to be a predictor for SS (p=0.04) and SL (p=0.06) dynamic. LVEF did not decrease significantly during treatment overall, but in n=10 patients (26%) a decrease of more than 5% from T1 to T3 was observed. In these patients, we found a higher difference in RV-S from T1-T3 (median -4.1 vs -1.3 cm/s, p<0.001), a lower RV- S value at T3 (median 10.8 vs 13 cm/s, p=0.07) and a lower SS value at T2 (median 6.3 vs 7.5 cm/s, p=0.02) when compared to the rest of the group.
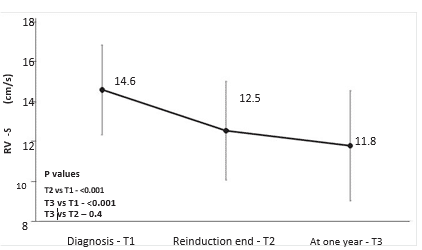
Figure 2. Mean peak tricuspid annular velocity (RV-S) by treatment phase. Vertical lines at each point show standard deviation.
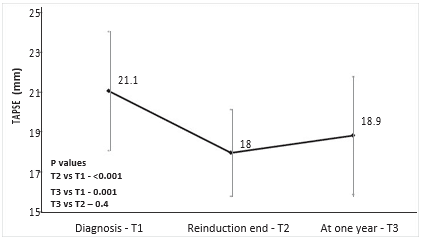
Figure 3. Mean tricuspid annular plane systolic excursion (TAPSE) by treatment phase. Vertical lines at each point show standard deviation.
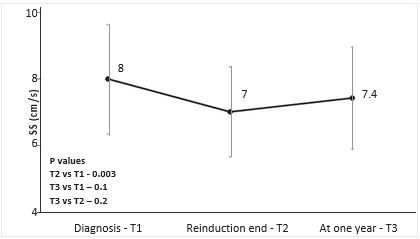
Figure 4. Mean peak septal mitral annular velocity (SS) by treatment phase. Vertical lines at each point show standard deviation.
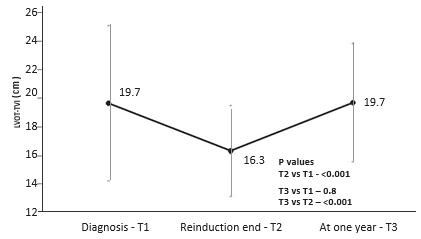
Figure 5. Mean left ventricular outflow tract time-velocity integral (LVOT-TVI) by treatment phase. Vertical lines at each point show standard deviation.
DISCUSSION
This is the first prospective study showing the early cardiotoxic effects of low dose anthracycline therapy in children with ALL on both the LV and RV using TDI methods. Our main finding is that RV longitudinal systolic function decreases in more than two thirds of patients after treatment start, and returns to pre-treatment values in less than 10% at one year. This is in contrast to the LV longitudinal function, which also decreases in two thirds of the patients at treatment start, but returns to pre-treatment values in more than 40% at one year. Additionally, we found that in those patients where the LVEF decreases the most (more than 5%), the decrease in RV-S is more important.
Data on RV systolic function in anthracycline therapy come from studies which use different evaluation protocols, reach contradictory results and as such there is no clear consensus. Agha et al. report a decrease in diastolic function of the RV at the end of induction in a group of heterogeneous malignancies, paradoxically an increased RV TEI index and a slightly reduced TAPSE10. Baysal et al found no differences in RV systolic function but only in the performance index in a group of 20 mixed pediatric malignancies at least 6 months from treatment end13. Kocabas et al. reported lower tricuspid annular velocities by TDI after anthracycline treatment, in a group of mostly ALL, with patients receiving CD of more than 300 mg/m2, but without a comparison between pre- and post- treatment4 . Ganame et al. report similar TAPSE values in control and treated patients, at a median of 5 years8. A report on 19 children receiving high doses of anthracyclines showed decreased diastolic RV velocities compared to controls, but without a cle-ar definition of when during the treatment were the echocardiograms performed9.
In our study we show that RV peak systolic velocities measured at the lateral tricuspid annulus level decrease after reinduction in ALL therapy, and conti-nue to be reduced at one year after treatment start, a novel finding in children. This is found in a homo-genous group of patients, undergoing treatment under the same protocol, with CD of less than 300 mg/ m2 (only a minority had more than 240 mg/m2). This is a significant advantage over previous reports, as it allows for a better understanding of the pattern of changes. Additionally, the prospective nature of the study minimizes confounders inherent to retrospective or cross-sectional protocols.
Maybe the most interesting finding is how the changes in RV and LV function over the course of the treatment differ. The fact that few patients recover to pre-treatment RV longitudinal systolic parameters, while close to half do recover their LV longitudinal systolic function seems to suggest that the RV is more sensitive to the cardiotoxic effects of doxorubicin. In-terestingly, when measuring TAPSE we found that the values tend to return to pre-treatment levels, allowing for speculation that TAPSE is not as sensitive as RV-S. One proposed mechanism for this cardiotoxicity is mitochondrial dysfunction, with oxidative stress16, which could trigger cell hypoxia, apoptosis and fibro-sis, mechanisms described in the pathogenies of heart failure17. We know from studies looking at the effects of cardiopulmonary bypass on cardiac function that the RV is affected more and with longer effects (i.e. more than one year) compared to the LV18,19. Since the exact mechanisms of either of these processes are not entirely understood, it is only speculative that they share similarities. Nevertheless, our observation does raise the debate on how the two ventricles res-pond and adapt to injury.
The follow-up reported in our series is of one year, and as such we are unable to provide data on later changes in cardiac function. There are numerous stu-dies documenting RV dysfunction in adult survivors of anthracycline treatment, but with a lower prevalence than that reported here6,7. This would suggest that in fact some patients will fully recover their RV function after one year, while others will not. Our cohort will continue to be studied and expanded in the future, in order to allow for a longer follow-up to address this issue.
It is well known and documented that the LV func-tion is affected by anthracycline treatment, with periodic evaluation of the LV being recommended by current guidelines2 . For low doses of anthracyclines a 5 year incidence of LV dysfunction of less than 5% is cited, but pediatric cancers are among the risk factors2. A large registry study in pediatric cancer survivors found a cumulative incidence for heart failure at 40 years of 0.5% (for low risk) and 11.7% (for high risk)20. Additionally, an older study also found that LV function recovers in almost all patients at treatment cessation, albeit with less sensitive echocardiographic methods21. We found that most children have a sub-clinical decrease of LV longitudinal function, but most return to pre-treatment values, when measuring both longitudinal function SS and cardiac output by LVOT-TVI, in concordance to the low incidence of LV dys-function observed in adults. This raises the issue of early identification of children with persisting LV dys-function, as it is recommended that cardiac protecti-on therapy be started as early as possible, to prevent irreversible heart failure2.
Monitoring RV systolic function could play a role in the evaluation of subclinical cardiac dysfunction, and allow for early identifying of specific treatment tar-gets. In our study we found that in those children with an absolute decrease in LVEF of more than 5% there is also a more important decrease in RV longitudinal function and lower RV- S at one year. These results are promising, and further following this cohort will allow us to determine the predictive value of measuring RV systolic function parameters in identifying the patients with clinically significant LVEF decrease.
Limitations
Due to the small number of patients and the follow-up duration of just one year there were no cases of overt heart failure to allow an analysis of prediction power for hard endpoints. On the other hand, measurements performed at precise moments during treatment allow for a more detailed analysis of the pattern of early cardio-toxicity. In order to shorten the duration of the examination (the patients were undergoing chemotherapy and required as little exposure to non-sterile environments as possible) we chose to evaluate the LVEF by the Teichholz method, given that obtaining the views needed for an accurate Simpson Biplane can sometimes be time consuming, or not possible in non-cooperating children. For the same reason we did not opt for speckle tracking imaging techniques, but rather we used the TDI method, as it requires only one simple measurement. The variation in measurements due to the age of the children was limited by the fact that we used mainly comparisons at different time points for the same patient group.
CONCLUSIONS
Cardiotoxicity during and after chemotherapy, especially in children, is an increasingly prevalent issue, with more and more cancer-treated survivors being exposed to the risk of heart failure. Understanding the mechanisms leading to irreversible cardiac dys-function and early identification of targets for cardiac treatment is a priority. We found that the RV has a different pattern of early dysfunction when compared to the LV in children, returning to pre-treatment values in only a minority of children at one year. Further studies are needed to determine if this observation holds clinical or predictive implications.
Conflict of interest: none to declare.
Ethical approval: All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed consent: Informed consent was obtained from all individual participants included in the study.
References
1. S.P. Hunger, C.G. Mullighan, Acute Lymphoblastic Leukemia in Children, N. Engl. J. Med. 373 (2015) 1541–1552.
2. J.L. Zamorano, P. Lancellotti, D. Rodriguez Muñoz, V. Aboyans, R. Asteggiano, M. Galderisi, G. Habib, D.J. Lenihan, G.Y.H. Lip, A.R. Lyon, T. Lopez Fernandez, D. Mohty, M.F. Piepoli, J. Tamargo, A. Torbicki, T.M. Suter, S. Achenbach, S. Agewall, L. Badimon, G. Barón-Esquivias, H. Baumgartner, J.J. Bax, H. Bueno, S. Carerj, V. Dean, Ç. Erol, D. Fitzsimons, O. Gaemperli, P. Kirchhof, P. Kolh, P. Nihoyannopoulos, P. Ponikowski, M. Roffi, A. Vaz Carneiro, S. Windecker, G. Minotti, D. Cardinale, G. Curigliano, E. De Azam-buja, S. Dent, C. Ero, M.S. Ewer, D. Farmakis, R. Fietkau, P. Kohl, P. McGale, J. Ringwald, J. Schulz-Menger, J. Stebbing, R.K. Steiner, S. Szmit, 2016 ESC Position Paper on cancer treatments and cardio-vascular toxicity developed under the auspices of the ESC Committee for Practice Guidelines, Eur. Heart J. 37
3. S.E. Lipshultz, M.J. Adams, S.D. Colan, L.S. Constine, E.H. Her-man, D.T. Hsu, M.M. Hudson, L.C. Kremer, D.C. Landy, T.L. Mill-er, K.C. Oeffinger, D.N. Rosenthal, C.A. Sable, S.E. Sallan, G.K. Singh, J. Steinberger, T.R. Cochran, J.D. Wilkinson, Long-term cardiovascular toxicity in children, adolescents, and young adults who receive cancer therapy: Pathophysiology, course, monitoring, management, prevention, and research directions: A scientific statement from the American Heart Association, Circulation. 128 (2013) 1927–1955.
4. A. Kocabaş, F. Kardelen, H. Ertuğ, B. Aldemir-Kocabaş, Ö. To-sun, A. Yeşilipek, V. Hazar, G. Akçurin, Assessment of early-onset chronic progressive anthracycline cardiotoxicity in children: Dif-ferent response patterns of right and left ventricles, Pediatr. Car-diol. 35 (2014) 82–88.
5. M. Lotrionte, G. Palazzoni, R. Natali, G. Comerci, A. Abbate, S. Di Persio, G.G.L. Biondi-Zoccai, Appraising cardiotoxicity associated with liposomal doxorubicin by means of tissue Doppler echocar-diography end-points. Rationale and design of the LITE (Liposomal doxorubicin-Investigational chemotherapy-Tissue Doppler imaging Evaluation) randomized pilo, Int. J. Cardiol. 135 (2009) 72–77.
6. J.R. Christiansen, R. Massey, H. Dalen, A. Kanellopoulos, H. Hamre, E. Ruud, C.E. Kiserud, S.D. Fosså, S. Aakhus, Right ventricular function in long-term adult survivors of childhood lymphoma and acute lymphoblastic leukaemia, Eur. Heart J. Cardiovasc. Imaging. 17 (2016) 735–741.
7. K. Ylänen, T. Poutanen, P. Savikurki-Heikkilä, I. Rinta-Kiikka, A. Eerola, K. Vettenranta, Cardiac magnetic resonance imaging in the evaluation of the late effects of anthracyclines among long-term survivors of childhood cancer, J. Am. Coll. Cardiol. 61 (2013) 1539–1547.
8. J. Ganame, P. Claus, A. Uyttebroeck, M. Renard, J. D’hooge, B. Bijnens, G.R. Sutherland, B. Eyskens, L. Mertens, Myocardial Dys-function Late After Low-Dose Anthracycline Treatment in As-ymptomatic Pediatric Patients, J. Am. Soc. Echocardiogr. 20 (2007) 1351–1358.
9. B. Yağci-Küpeli, A. Varan, H. Yorgun, B. Kaya, M. Büyükpamukçu, Tissue Doppler and myocardial deformation imaging to detect myocardial dysfunction in pediatric cancer patients treated with high doses of anthracyclines, Asia. Pac. J. Clin. Oncol. 8 (2012) 368–374.
10. H. Agha, L. Shalaby, W. Attia, G. Abdelmohsen, O.A. Aziz, M.Y.A. Rahman, Early Ventricular Dysfunction After Anthracycline Chemotherapy in Children, Pediatr. Cardiol. 37 (2016) 537–544.
11. J.T. Poterucha, S. Kutty, R.K. Lindquist, L. Li, B.W. Eidem, Chang-es in left ventricular longitudinal strain with anthracycline chemo-therapy in adolescents precede subsequent decreased left ventricular ejection fraction, J. Am. Soc. Echocardiogr. 25 (2012) 733– 740.
12. G.E. Stapleton, S.L. Stapleton, A. Martinez, N.A. Ayres, J.P. Kov-alchin, L.I. Bezold, R. Pignatelli, B.W. Eidem, Evaluation of Longitu-dinal Ventricular Function with Tissue Doppler Echocardiography in Children Treated with Anthracyclines, J. Am. Soc. Echocardiogr. 20 (2007) 492–497.
13. T. Baysal, Y. Koksal, B. Oran, M. Sen, E. Unal, D. Cimen, Car-diac functions evaluated with tissue doppler imaging in childhood cancers treated with anthracyclines, Pediatr. Hematol. Oncol. 27 (2010) 13–23.
14. L. Lopez, S.D. Colan, P.C. Frommelt, G.J. Ensing, K. Kendall, A.K. Younoszai, W.W. Lai, T. Geva, Recommendations for Quantification Methods During the Performance of a Pediatric Echocardiogram: A Report From the Pediatric Measurements Writing Group of the American Society of Echocardiography Pediatric and Con-genital Heart Disease Council, J. Am. Soc. Echocardiogr. 23 (2010) 465–495.
15. L.G. Rudski, W.W. Lai, J. Afilalo, L. Hua, M.D. Handschumacher, K. Chandrasekaran, S.D. Solomon, E.K. Louie, N.B. Schiller, Guide-lines for the Echocardiographic Assessment of the Right Heart in Adults: A Report from the American Society of Echocardiography. Endorsed by the European Association of Echocardiography, a reg-istered branch of the European Society of Cardiology, and , J. Am. Soc. Echocardiogr. 23 (2010) 685–713.
16. J. V McGowan, R. Chung, A. Maulik, I. Piotrowska, J.M. Walker, D.M. Yellon, Anthracycline Chemotherapy and Cardiotoxicity, Cardiovasc. Drugs Ther. 31 (2017) 63–75.
17. M.G. Rosca, C.L. Hoppel, Mitochondrial dysfunction in heart fail-ure, in: Heart Fail. Rev., NIH Public Access, 2013: pp. 607–622. doi:10.1007/s10741-012-9340-0.
18. M.J. Schuuring, P.P.M. Bolmers, B.J.M. Mulder, R.A.C.M. de Bruin-Bon, D.R. Koolbergen, M.G. Hazekamp, W.K. Lagrand, S.G. De Hert, E.M.F.H. de Beaumont, B.J. Bouma, Right ventricular function declines after cardiac surgery in adult patients with congenital heart disease., Int. J. Cardiovasc. Imaging. 28 (2012) 755–62.
19. F. Roshanali, M.A. Yousefnia, M.H. Mandegar, H. Rayatzadeh, S. Alinejad, Decreased right ventricular function after coronary ar-tery bypass grafting, Tex Hear. Inst J. 35 (2008) 250–255.
20. E.J. Chow, Y. Chen, L.C. Kremer, N.E. Breslow, M.M. Hudson, G.T. Armstrong, W.L. Border, E.A.M. Feijen, D.M. Green, L.R. Meacham, K.A. Meeske, D.A. Mulrooney, K.K. Ness, K.C. Oeffin-ger, C.A. Sklar, M. Stovall, H.J. Van Der Pal, R.E. Weathers, L.L. Robison, A.Y. Yasui, Individual prediction of heart failure among childhood cancer survivors, J. Clin. Oncol. 33 (2015) 394–402.
21. A.B. Lewis, V.L. Crouse, W. Evans, M. Takahashi, S.E. Siegel, Re-covery of left ventricular function following discontinuation of anthracycline chemotherapy in children., Pediatrics. 68 (1981) 67–72.
 This work is licensed under a
This work is licensed under a