Mariana Radoi1, Emma Weiss2, Elisabeta Badila2,3
1 Faculty of Medicine, Transilvania University, Brasov, Romania
2 „Carol Davila” University of Medicine and Pharmacy, Bucharest, Romania.
3 Emergency Clinical Hospital, Bucharest, Romania
DEFINITION
Orthostatic arterial hypotension (OhTN) is defined as “a progressive and sustained fall in systolic blood pressure (SBP) from baseline value ≥20 mmHg or di-astolic blood pressure (DBP) ≥ 10 mmHg, or a decre-ase in systolic blood pressure to <90 mmHg” when transitioning from the supine to the upright position1. In those with a SBP > 160mmH when supine, OhTN is defined as a fall in SBP of at least 30mmHg2,3.
The current defi nition does not include the 3 mi-nutes time of BP drop as criteria for the diagnosis, in-troduced in 1996 by the OhTN definition Consensus4, but it is currently considered as part of the classical OhTN picture. The reason for excluding this criteria is a consequence of studies which have shown that OhTN during passive orthostatism at the tilt test may develop late, between 3 and 15 minutes in 15% of ca-ses, and even after 16 minutes in 39% of cases5. Clinical studies have shown that OhTN occurs late after ac-tive orthostatism in >50% of patients with neurologic conditions6, and evolves to classical OhTN during the course of the following 10 years in 50% of patients5.
Using devices that measure BP and heart rate simul-taneously in patients with symptoms during the first minute of orthostatism consistent with OhTN, an ini-tial OhTN was described when a drop in SBP of at least 40 mmHg and in DBP of at least 20 mmHg during the first 15 seconds, with a recovery in the following 30 seconds7.
PREVALENCE
Studies providing prevalence data have mainly assessed classical OhTN and show significant variability accor-ding to age, comorbidities, and associated medicati-on. It was reported in <5% of subjects <65 years8, in 20% of those >65 years9, 30% of those >75 years10, in >50% of institutionalized elderly fragile subjects11, and in 64% of elderly admitted patients12. It was frequently associated with medication – vasodilatator drugs and tricyclic antidepressants, and with alcohol consumpti-on13. Orthostatic hypotension was reported in 23-50% of patients with Parkinson’s disease14, and in 20% of patients with type 2 diabetes mellitus15. Symptomatic forms of OhTN are slightly less frequent than asymp-tomatic ones, and are reported in 2% of subjects <65 years9, and 16% of those suffering from Parkinson’s16.
PATHOPHYSIOLOGY
OhTN is a consequence of the inefficiency of compen-satory mechanisms to correct BP when transitioning to an upright position. Gravitation redistributes 500-1000 ml of blood to the subdiaphragmatic vascular bed with the change from clino to orthostatism, which in turn leads to relative hypovolemia followed by a re-duction of ~40% in stroke volume and a decrease in arterial pressure during the first moments of ortho-statism. The decrease in arterial pressure stimulates the sympathetic nervous system and diminishes the in-tervention of the parasympathetic nervous system by stimulating baroreceptors of the carotid sinus and the aortic arch17. Sympathetic reference further stimula-tes autonomous centers in the central nervous system which includes hypothalamic centers, the solitary nu-cleus, medulla oblongata and medullar centers, which further transmit the impulse through afferent fibers to release norepinephrine (NE) in the preganglionic and postganglionic synaptic cleft. This postganglionic NE release leads to systemic and splanchnic vasoconstric-tion, and increased inotropism and heart rate which restores arterial pressure affected by the relative ort-hostatic hypovolemia18. Posture related hemodynamic changes are usually accompanied by increased NE plasma levels.
The release of NE is reduced in the postganglia segments in OhTN associated with central or peripheral nervous system disorders such as multiple system atrophy (MSA), Parkinson’s disease (PD), pure autonomic failure (PAF), and Lewy body dementia (LBD) – all primary neurogenic causes of OhTN. The nerves are damaged by deposits of -synucleoprotein occurring both in the central and peripheral autonomous nervous system. The mechanism underlying synucleinopathy associated OhTA (at the onset of PAF, in PD, in LBD development) is the decrease in postsynaptic NE release, evaluated by the reduction of NE blood levels which has been useful in clinical practice in neurology to guide the OhTN therapy specific for these cases13. Unlike for this patient subsets, in those with MSA, who indeed associate OhTN in 70 – 80% of cases19, the initial autonomic nervous system lesions occur centrally and remain so for a long period during disease progression, while peripheral residual sympathetic tonus and near-normal NE blood levels are preserved20. PAF and MSA are the most frequent etiologies for OhTA of the young, while PD and LBD are more frequent in OhTN of the middle aged and elderly patients.
In the elderly, OhTN secondary to autonomous nervous system dysfunction/injury can be amplified by vascular disease (increased arterial stiffness) or cardi-ac disease (chronotropic incompetence and myocar-dial stiffness) associated with a reduction in vasocon-strictive, chronotropic, and inotropic response to NE.
Postprandial hTN has been reported in 37% of patients with OhTN21, occurring through unclear mechanisms, which may involve sympathetic baroreflex dysfunction, the release of vasodilating intestinal peptides, and the increased postprandial blood flow in the splanchnic vascular bed22.
CAUSES
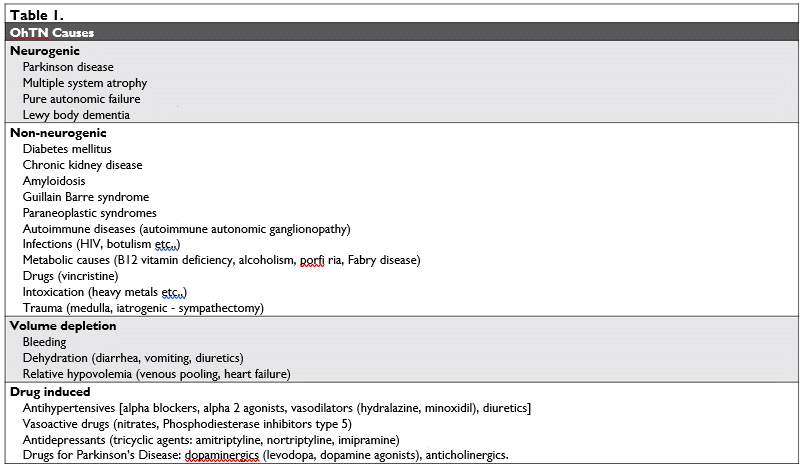
OhTN associated to neurologic conditions affecting the central or peripheral autonomous nervous system (synucleinopathies: PD, PAF, MSA, LBD) is considered a result of primary neurogenic causes. OhTN asso-ciated with peripheral autonomous nervous system dysfunction/injury in the setting of chronic diseases such as diabetes, amyloidosis, neoplasia, or autoimmu-ne diseases (Guillain-Barre syndrome), chronic renal disease, metabolic diseases (porphyria, Fabry disease, vitamin B deficiency, alcoholism), trauma or medullar iatrogenic lesions (sympathectomy)23, infections (HIV, botulism, etc.) is considered a result of secondary ne-urogenic causes1, also denoted non-neurogenic cau-ses24 (Tabel no 1).
In the setting of a normal autonomic function, OhTN can be the consequence of absolute hypovo-lemia (post-hemorrhagic, severe dehydration, adrenal failure, cardiac failure under excessive diuretic thera-py) or of excessive iatrogenic vasodilation (vasodilator drugs for hypertension or myocardial ischemia – ni-trates; psychotropic drugs – antidepressants, neuro-leptics; alpha-blockers for prostate adenomas; SGLT2 inhibitors in diabetes – dapaglifl ozin25; opiates, anti-cholinergics). In such cases it occurs as an acute ma-nifestation or on top of a chronic condition which is aggravated by iatrogenesis.
CLINICAL PRESENTATION
The symptoms of OhTN are polymorphic, have low specifi city and include vertigo, dizziness, blurred vision, thoracic pain, oftentimes cervical pain. They are a result of sudden transition from clino to orthostatism and may be more frequent during the night in patients with nocturnal diuresis or those administering medication before bedtime (antihypertensive drugs, neuroleptics, alpha-blockers). The nocturnal rise in BP levels increases glomerular arterial pressure, diuresis and natriuresis, leading to morning hypovolemia and an increased risk of morning OhTN26.
OhTN is the identified cause of syncope in 15% of cases27. Orthostatic syncope is confi rmed by the si-multaneous drop in BP levels1. The symptoms are in-fluenced mainly by the orthostatic BP levels, and much less by the amount of pressure drop1. OhTN leads to a syncope when SBP drops under 75 mmHg, the cere-bral autoregulation pressure threshold.
The symptoms of OhTN in synucleinopathies are associated to the clinical manifestations of the main neurologic condition – motor dysfunction, autonomic dysfunction (OhTA, postprandial hTA, supine HTN, gastrointestinal dysfunction, urogenital dysfunction) and to cognitive decline18. PAF resulting from the pre-dilect deposition of -synuclein in the postganglia seg-ment can be considered a human model for OhTN, as the motor component seen with other synuclinopathi-es is absent in PAF. In PAF forms exclusively/predomi-nantly associating postganglia lesions, the postganglia release of NE and its plasma levels are decreased and do no increase with orthostatism28. MSA, due to the initial depositions of -synuclein predominantly in the oligodendroglia of the central nervous system, asso-ciates Parkinson-like and cerebellar manifestations. When OhTN is seen at the onset of MSA it is often long-standing6. In PD, OhTN was reported in 20 – 50% of patients, asymptomatic in 18% of cases, frequently associated with long-standing severe PD. It may be aggravated by levodopa or vasodilator drugs29. LBD is characterized by the association between cognitive decline and autonomic dysfunction, including OhTN, which significantly reduces survival30.
Postprandial hTN, occurs 2 hours after a meal, more frequently in the first 30-60 minutes, upon as-suming the upright position, especially after a heavy meal, hot foods, high on carbohydrates, after alcohol consumption, often on top of the use of psychotropes or diuretics22. It is more frequent in patients with dia-betes, PD or Alzheimer’s dementia31.
DIAGNOSTIC
Active orthostatism with SBP drop of at least 20 mmHg and/or a DBP drop of at least 10 mmHg, by sphygmomanometry, during the first 3 to 10 minutes of orthostatism following 5 minutes of clinostatism is diagnostic for orthostatic hypotension1. Heart rate increases in those non-neurogenic OhTN (>15 beats per minute) and shows little variation in those with neurogenic OhTN (<10 beats per minute)1,6,32.
Ambulatory blood pressure monitoring (ABPM) shows a non-dipper pattern in those with OhTN, es-pecially morning OhTN, a pattern frequently seen in those with neurological pathology and dysautonomia, in patients with diabetes, chronic kidney disease, or the elderly33.
Tilt testing is recommended when: 1) OhTN has a daytime variation which does not allow an offi ce di-agnosis, 2) there is a clinical suspicion of orthostatic syncope in those with motor anomalies that do not allow for „active orthostatism” (i.e. Parkinson’s Disea-se), 3) there is a suspicion for delayed OhTN, 4) eva-luating the risk for symptomatic OhTN in those with neurological pathology and dysautonomia, 5) consi-dering the differential between an orthostatic and a vasovagal syncope, 6) the OhTN syncope cannot be differentiated from a psychogenic pseudosyncope. The procedure is carried out at a room temperature of 20-24°C with the patient lying down on a table to be inclined at 60-80°; at least every 3 minutes BP, heart rate and ECG tracing is carried out. Symptoms or syncope are considered to be a result of OhTN if they occur during testing in active or passive orthostatism, in parallel with a diagnostic drop in BP (at least 20 mmHg drop in SBP and/or 10 mmHg drop in DBP) and if they disappear in clinostatism34. A diagnostic drop in BP in the absence of recurring symptoms/syncope may allow the consideration of symptoms/syncope as being determined by OhTN1. Tilt testing allows the di-fferentiation between a hypotensive versus a vasovagal syncope. In the case of the latter, the BP drop during testing occurs rapidly (within a few minutes from test start) and is associated with relative bradycardia and prodromal symptoms (hot fl ush, diaphoresis, nausea). It is a useful test to select patients who might benefit from physical therapy, but is less relevant in monito-ring therapeutic response in those with OhTN and neurologic pathology and dysautonomia1.
Testing to evaluate for the etiology of OhTN inclu-de: 1) plasma NE levels, in elected cases, in the absen-ce of specific neurologic manifestations, when there is a clinical suspicion for the occurrence of OhTN as a first sign of a synucleinopathy; the level of serum NE is diagnostic for neurogenic OhTN associated with PAF or PD when it does not double after 5-10 minutes of orthostatism13; 2) plasma levels of vitamin B12, as there are data showing that B12 vitamin defi ciency can associate OhTN35; 3) laboratory testing for autoimmu-ne diseases and serum levels of ganglionic acetylcholi-ne receptor autoantibodies, to diagnose paraneoplasic syndromes, when there is a clinical suspicion for other causes of OhTN36.
PROGNOSIS
Chronic OhTN has been associated with a poor pro-gnosis. A meta-analysis on data available up to 2014 has shown that classical OhTN is an independent pre-dictor for all-cause mortality37-39. In the Malmö Pro-ject including 33 346 community patients aged over 45 years, all-cause mortality was 1.6 times higher in those with OhTN40. Classical OhTN was associated with a higher rate of coronary heart disease41, and in the case of the elderly with an increased risk for myocardial infarction42. In the Malmö Project, acute coronary events significantly correlated with a decre-ase in DBP40. Classical OhTN has been associated with a higher risk for atrial fibrillation, hospital admissions for heart failure43, and an increased risk for non-lethal ischemic stroke44 and cognitive decline in those aged over 75 years43,45. In the fragile elderly OhTN is a se-vere marker for deconditioning and presence of co-morbidities carrying a severe prognosis46. Even though OhTN is associated with a higher risk of falls, it does not equate to a similar risk for fractures47. In those with synucleinopathies and delayed OhTN, 10 year mortality was 24%, and 56% after the OhTN associ-ated the features of classical OhTN, with only a 6% mortality in the control group 5.
Initial OhTN, a risk factor for trauma from falls, does not appear to influence the risk of death48.
OhTN secondary to reversible causes (dehydrati-on, hemorrhagic anemia, excessive vasodilating or di-uretic therapy, or the association of vasodilators with diuretics, alpha blockers, SGLT-2 inhibitors) resolves after the identification and correction of the trigger.
MANAGEMENT
The management of OhTN starts from the identificati-on and avoidance/elimination of triggers, to non-phar-macological measures and pharmacological therapy.
Patient education is a major element to insure be-nefit in the management of OhTN. Patients should re-ceive explicit but brief information on the underlying mechanism and general symptoms of OhTN, on the fact that it has no specific therapy that non-pharma-cological measures are key elements in symptom con-trol, and that pharmacotherapy is initiated only when non-pharmacological measures have failed to control symptoms and reduce the drop in BP with orthosta-tism.
NON-PHARMACOLOGIC MANAGEMENT
It includes avoiding assuming orthostatism abruptly, avoiding static orthostatism and prolonged bed rest. Particularly in patients with postprandial hTN it is re-commended to avoid heavy meals with high carbohy-drate content and alcohol consumption. One should associate measures to increase physical condition by exercise (counter pressure measures) which should increase isometric muscle tone predominantly in the lower limbs, favoring venous return and reducing symptoms of OhTN. The type of exercise should consider the age and physical status of patients, whi-le the actual exercise should start with education on the topic and medical supervision. Valsalva maneuvers should be avoided as they can precipitate a synco-pe. Whenever possible, physical exercise carried out in pools (avoiding high temperatures) are preferred because they increase orthostatic tolerance as the pressure of the water against the body reduces the effect of the gravity on the sub-diaphragmatic venous vascular bed13. Benefi ts of physical exercise have been reported in those with autonomous dystonia and va-sovagal syncope49,50.
Hypovolemia should be avoided by ensuring a dai-ly oral water intake of 1500-2000 ml. In those with postprandial hTN an average 500 ml of water should be ingested before every meal. The salt intake will be recommended depending on the clinostatism BP level or the presence of heart failure. Administering 500 ml cold water reduces OhTN during the first 10-15 mi-nutes from administration, most probably as a result of increasing NE plasma levels51,52. Sleeping with the head resting on a 30° inclined pillow (similar to tilt testing) sensitizes the carotid and aortic baroreceptors and those of the renin angiotensin aldosterone system and reduces morning OhTN53.
High waist elastic stockings reduce venous pooling by applying an external pressure in the lower limbs and become necessary for those with chronic venous insufficiency; orthostatic inflatable elastic abdominal supports reduce subdiaphragmatic venous pooling and are recommended particularly in patients with neuro-genic OhTN54-56.
Identifying the drugs potentially triggering OhTN should be followed by dose reduction or even their avoidance. The decision should consider the risk be-nefit ratio – the risk for OhTN and their benefits for the condition for which they were indicated (diabetes, arterial hypertension, heart failure, chronic kidney di-sease, cancer, etc.). In patients with OhTN and clinos-tatism hypertension, the lack of BP control leads to cardiovascular events; maintaining a high clinostatism BP also increases glomerular filtration and diuresis, le-ading to the aggravation of OhTN. In this setting BP lowering therapy has to be supervised closely, as re-aching the BP target in clinostatism will also improve OhTN symptom control. Even though there are little data from studies dedicated to the role of BP lowering medication in the development of OhTN, angioten-sin converting enzyme inhibitors, angiotensin recep-tor blockers, and calcium blockers are generally con-sidered to be less associated with OhTN than alpha blockers, diuretics, and beta blockers1.
The correction of vitamin B12 deficiency (<250pg/ mL)57 and the treatment of anemia by subcutaneous recombinant erythropoietin (50U/kg 2-3 times/week) in patients with primary autonomous dysfunction reduce the risk of an orthostatic BP drop, through mechanisms yet unknown58,59.
PHARMACOLOGICAL THERAPY
Pharmacological therapy is recommended when non-pharmacological measures have failed to control symp-toms and reduce the drop in BP with orthostatism36. The drugs used act either by increasing peripheral vascular resistance – midodrine, droxidropa and ato-moxetine, or by increasing intravascular volume – flu-drocortisone.
Drugs traditionally recommended for OhTN are midodrine and fludrocortisone, although their benefit to reduce OhTN is modest and limited by their adver-se effects.
Midodrine acts as an alpha agonist through its meta-bolite desglymidodrine which leads to vasoconstricti-on and the reduction of orthostatic BP drop, but also the increase of BP in clinostatism. Its effects last for up to 3 to 4 hours and should be monitored in pati-ents developing clinostatic hypertension 36 and heart failure. Its efficiency has been mostly studied in pri-mary neurogenic OhTN60,61, to a much less extent in secondary neurogenic OhTN62. Midodrine is the first drug recommended in OhTN36. Its oral administrati-on, titrated from 2.5 mg up to 15 mg, is given 3-4 ho-urs before waking time, to avoid morning OhTN. An earlier administration would be ineffective and contri-bute to clinostatism hypertension. A clinostatism SBP over 180 mmHg is a countraindication for midodrine. Secondary effects include headache, urine retention, piloerection51,61.
Fludrocortisone, a synthetic mineralocorticoid, re-duces OhTN mainly by volume expansion secondary to water and salt retention, but also through an incre-ase of NE and angiotensin II pressor effect, which con-tributes to the acceleration of target organ damage such as left ventricle hypertrophy and chronic kidney disease63. The volume expansion may lead to an incre-ase in clinostatic BP, an effect more pronounced when associated with midodrine. Its addition to midodrine is warranted only by the lack of symptom control with the latter. On the long term, adverse effects of fludro-cortisone include hypokalemia and hypercorticism. It should be avoided in those with heart failure64. Drugs recently tested to correct OhTN dysautono-mia are doxidropa and atomoxetine.
Droxidropa is a synthetic aminoacid converted in NE by the enzymatic intervention of an aminoacid de-carboxylase available in the nervous tissue and other tissues. Its mechanism of action appears to be rela-ted to the increase in NE synthesis and release in the sympathetic nervous fibers from the neuro-vascular space, but also to the increase in NE synthesis in the extraneural space51. Droxidropa was proved effective in reducing neurogenic OhTN65, and was approved by the FDA for treatment of OhTN in PD, MSA, PAF and non-diabetic peripheral autonomous neuropathies, as a result of clinical studies and a meta-analysis published in 201666. The 2017 ACC/AHA/HRS Guidelines for the management of syncope recommends doxidropa with a IIa level of indication to treat orthostatic synco-pe67. Its administration is recommended depending on the 24h profile of OhTN, and is titrated from 100 mg to 600 mg, 3 times daily13. In 30% of patients there is no clinical improvement68. In those with neurogenic OhTN, the low NE level in clinostatism (<220 pg/mL) predicts the clinical response to doxidropa69. The ESC 2018 Guidelines on syncope comment on the lack of data to supports its efficacy on long term use which would require more clinical trials1.
Atomoxetine acts by blocking NE reuptake in the postganglionic sympathetic synapses as a result of its binding to the NE cotransporter. The mechanism of action explains the pressor effect of atomoxetine in patients with MSA, where peripheral neurons are unaffected, its limited effects in patients with PAF, where these neurons are primarily affected, or in tho-se with PD and LBD, where the autonomous periphe-ral system is affected during the course of disease20. The NE plasma levels predict the response to atomo-xetine70. It may be associated with droxidropa, with careful monitoring and attention to the potential ar-rhythmogenic effects of their co-administration. The association between atomoxetine and pyridostigmine, which increases nicotinic neurotransmission in the sympathetic ganglia by inhibiting acetylcholinesterase, has additive effects and improves orthostatic toleran-ce in those with severe sympathetic autonomous dys-function71. In specific situations, OhTN therapy may include: Desmopressin indicated in patients with morning OhTN after excessive nocturnal diuresis, howe-ver with little clinical benefit in studies1.
Acarbose and octreotide are indicated in those with postprandial hTN. Oral acarbose reduces glucose absorption and postprandial insulin le-vels, infl uencing postprandial hTN through its va-sodilating effect72. Octreotide reduces postpran-dial hTN by reducing the release of vasodilating intestinal peptides, however with limited indicati-ons due to the subcutaneous administration and adverse effects (abdominal pain and diarrhea)72.
CONCLUSIONS
Orthostatic hypotension is a cardiovascular anomaly frequently found in clinical conditions that either pri-marily or secondarily affect the central and/or periphe-ral autonomous nervous system. It is associated with poor outcomes. Further research is mandated to con-firm the relationship between OhTN and cardiovascu-lar morbidity and mortality, and to assess whether it is indeed a marker of cardiovascular disease severity or a risk factor for the development of major cardiovas-cular events. The efficacy of currently available drug therapy is limited, having a modest effect on quality of life, at the expense of lack of evidence of prognostic significance.
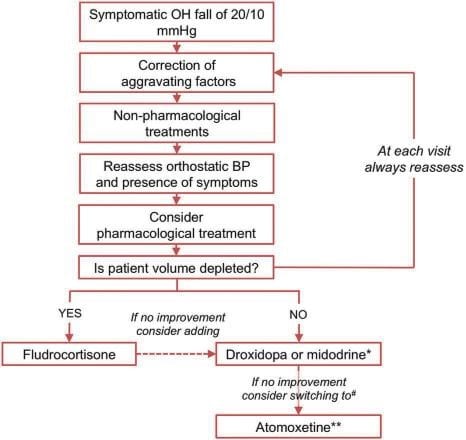
Figure 1. Therapeutic algorithm.
Conflict of interest: none declared.
References
1. 2018 ESC Guidelines for the diagnosis and management of syncope. Eur Heart J 2018; 39: 1883–1948.
2. Wieling W and Schatz IJ. The consensus statement on the defini-tion of orthostatic hypotension: a revisit after 13 years. J Hypertens 2009; 27:935-93.
3. Freeman R, Wieling W, Axelrod FB et al. Consensus statement on the definition of orthostatic hypotension, neutrally mediated synco-pe and the postural tachycardia syndrome. Clin Auton Res 2011; 21: 69-72.
4. Anonymous. Consensus statement on the definition of orthostatic hypotension, pure autonomic failure, and multiple system atrophy. Neurology. 1996;46:1470.
5. Gibbons CH, Freeman R. Clinical implications of delayed orthostatic hypotension: a 10-year follow-up study. Neurology 2015;85:1362–7.
6. Pavy-Le Traon A, Piedvache A, Perez-Lloret S, et al., for the Euro-pean MSA Study Group. New insights into orthostatic hypotension in multiple system atrophy: a European multicentre cohort study. J Neurol Neurosurg Psychiatry 2016;87:554–61.
7. Finucane C, O’Connell MD, Fan CW, et al. Agerelated normative changes in phasic orthostatic blood pressure in a large population study: findings from The Irish Longitudinal Study on Ageing (TILDA). Circulation 2014;130:1780–9.
8. Juraschek SP, Daya N, Appel LJ, et al. Orthostatic hypotension in middle-age and risk of falls. Am J Hypertens 2017;30:188–95.
9. Rutan GH, Hermanson B, Bild DE, Kittner SJ, LaBaw F, Tell GS. Orthostatic hypotension in older adults. The Cardiovascular Health Study. CHS Collaborative Research Group. Hypertension. 1992 Jun; 19(6 Pt 1):508-19.
10. Low PA. Prevalence of orthostatic hypotension. Clin Auton Res 2008;18(S1):8–13.
11. Gupta V, MD, Lewis A. Lipsitz. Orthostatic Hypotension in the El-derly: Diagnosis and Treatment The American Journal of Medicine. 2007;120:841-84.
12. Feldstein C, Weder AB. Orthostatic hypotension: a common, seri-ous and under recognized problem in hospitalized patients. J Am Soc Hypertens 2012;6:27–39.
13. Jose-Alberto Palma, PhD, Horacio Kaufmann. Epidemiology, Diag-nosis, and Management of Neurogenic Orthostatic Hypotension. Movement disorders Clinical practice 2017;298-308.
14. Velseboer DC, RJ Haan, W Wieling, DS Goldstein, RM Bie Preva-lence of orthostatic hypotension in Parkinson’s disease: a systematic review and meta-analysis. Parkinsonism Relat Disord, 2011.
15. Gaspar L, Kruzliak P, Komornikova A, et al. Orthostatic hypotension in diabetic patients—10-year follow-up study. J Diabetes Complica-tions 2016;30:67–71.
16. Palma JA, Gomez-Esteban JC, Norcliffe-Kaufmann L, et al. Ortho-static hypotension in Parkinson disease: how much you fall or how low you go? Mov Disord 2015;30:639–645.
17. Naschitz E J. and Rosner I. Orthostatic hypotension: framework of
the syndrome. Postgrad Med J. 2007 Sep; 83(983): 568–574.
18. Roy Freeman, Ahmad R. Abuzinadah, Christopher Gibbons, Pearl Jones, Mitchell G. Miglis, Dong In Sinn. Orthostatic Hypotension, State-of-the-Art Review. JACC 2018;72:1294–309.
19. Low PA, Reich SG, Jankovic J, et al. Natural history of multiple sys-tem atrophy in the USA: a prospective cohort study. Lancet Neurol 2015;14:710–719.
20. Jordan J, Shibao C, Biaggioni I. Multiple system atrophy: using clini-cal pharmacology to reveal pathophysiology. Clin Auton Res 2015; 25:53–59.
21. Vloet L. C., Pel-Little R. E., Jansen P. A., Jansen R. W. (2005). High prevalence of postprandial and orthostatic hypotension among geri-atric patients admitted to Dutch hospitals. J. Gerontol. A Biol. Sci. Med. Sci. 60, 1271–1277.
22. Trahair LG , Horowitz M, Jones KL. Postprandial hypotension: a sys-tematic review. J Am Med Dir Assoc. 2014 Jun;15(6):394-409.
23. Claydon V.E., Krassioukov A. V. (2006). Orthostatic hypotension and autonomic pathways after spinal cord injury. J. Neurotrauma 23, 1713–1725. 10.1089/neu.2006.23.1713.
24. Vinik AI, Nevoret M, Casellini C, Parson H. Neurovascular function and sudorimetry in health and disease. Curr Diab Rep. 2013;13(4): 517-32.
25. Chao E. C., Henry R. R. (2010). SGLT2 inhibition — a novel strategy for diabetes treatment. Nat. Rev. Drug Discov. 9, 551–559.
26. Fanciulli A, Jordan J, Biaggioni I, et al. Consensus statement on the definition of neurogenic supine hypertension in cardiovascular auto-nomic failure by the American Autonomic Society (AAS) and the Eu-ropean Federation of Autonomic Societies (EFAS): Endorsed by the European Academy of Neurology (EAN) and the European Society of Hypertension (ESH). Clin Auton Res. 2018; 28(4):355-362.
27. Sutton Richard. Clinical Classification of Syncope. Progress in Car-diovascular Diseases 2013;55(4):339-344.
28. Kaufmann H, Norcliffe-Kaufmann L, Palma JA, et al., for the Auto-nomic Disorders Consortium. Natural history of pure autonomic failure: a United States prospective cohort. Ann Neurol 2017;81: 287–97.
29. Ha AD, Brown CH, York MK, Jankovic J. The prevalence of symp-tomatic orthostatic hypotension in patients with Parkinson’s disease and atypical parkinsonism. Parkinsonism Relat Disord 2011;17:625– 628.
30. Stubendorff K, Aarsland D, Minthon L, Londos E. The impact of autonomic dysfunction on survival in patients with dementia with Lewy bodies and Parkinson’s disease with dementia. PloS One 2012;7:e45451.
31. Luciano G. L., Brennan M. J., Rothberg M. B. (2010). Postprandial hy-potension. Am. J. Med. 123, 281–286.
32. Jamnadas-Khoda J, Koshy S, Mathias CJ, Muthane UB, Ragothaman M, Dodaballapur SK. Are current recommendations to diagnose or-thostatic hypotension in Parkinson’s disease satisfactory Mov Disord 2009;24(12):1747-51.
33. Cuspidi C, Sala C, Tadic M, Gherbesi E, Antonio De Giorgi, Guido Grassi, Giuseppe Mancia, Clinical and prognostic signifi cance of a re-verse dipping pattern on ambulatory monitoring: An updated review. J Clin Hypertens. 2017;19:713–721.
34. Lanier BJ, Matthew BM and Clay CM. Evaluation and Management of Orthostatic Hypotension. American Family Physician 2011;84( 5):527-536.
35. Shibao C, Lipsitz LA, Biaggioni I. ASH position paper: evaluation and treatment of orthostatic hypotension. J Clin Hypertens (Green-wich). 2013;15(3):147-153.
36. Magkas N, Tsioufis C, Thomopoulos C, Dilaveris P, Georgiopoulos G, Sanidas E, Papademetriou V, Tousoulis D. Orthostatic hypoten-sion: From pathophysiology to clinical applications and therapeutic considerations. J Clin Hypertens. 2019;21:546–554.
37. Masaki KH, Schatz IJ, Burchfiel CM, Sharp DS, Chiu D, Foley D, Curb JD. Orthostatic hypotension predicts mortality in elderly men: the Honolulu Heart Program. Circulation. 1998;98:2290 –2295.
38. Angelousi A, N Girerd, A Benetos, Frimat L, Gautier S, Weryha G, Boivin JM. Association between orthostatic hypotension and car-diovascular risk, cerebrovascular risk, cognitive decline and falls as well as overall mortality: a systematic review and meta-analysis. J Hypertens 2014;32(8):1562-71.
39. Xin W, Z Lin, S Mi. Orthostatic hypotension and mortality risk: a meta-analysis of cohort studies. Heart 2014;100(5):406-13.
40. Fedorowski A, Stavenow L, Hedblad B, Berglund G, Nilsson PM, Me-lander O. Orthostatic hypotension predicts all-cause mortality and coronary events in middle-aged individuals (The Malmo Preventive Project . Eur Heart J 2010;31:85-91.
41. Rose KM, HA Tyroler, CJ Nardo, Arnett DK, Light KC, Rosamond W, Sharrett AR, Szklo M. Orthostatic hypotension and the incidence of coronary heart disease: the Atherosclerosis Risk in Communities study. Am J Hypertens 2000;13(6 Pt 1):571-578.
42. Luukinen H, K Koski, P Laippala, KE Airaksinen. Orthostatic hypo-tension and the risk of myocardial infarction in the home-dwelling elderly. J Intern Med 2004;255(4):486-93.
43. Jerome L. Fleg, Gregory W. Evans, Karen L. Margolis, Joshua Bar-zilay, Jan Basile, J. Thomas Bigger, Jeffrey A. Cutler,1 Richard Grimm, Carolyn Pedley, Kevin Peterson, Rodica Pop-Busui, JoAnn Sperl-Hillen, and William C. Cushman. Orthostatic Hypotension in the ACCORD Blood Pressure Trial: Prevalence, Incidence, and Prognostic Significance. Hypertension. 2016; 68(4):888–895.
44. Kario K, Eguchi K, Hoshide S, Hoshide Y, Umeda Y, Mitsuhashi T, Shimada K. U-curve relationship between orthostatic blood pres-sure change and silent cerebrovascular disease in elderly hyperten-sives: orthostatic hypertension as a new cardiovascular risk factor. J Am Coll Cardiol. 2002 Jul 3;40(1):133-41.
45. Matsubayashi K, Okumiya K, Wada T, Osaki Y, Fujisawa M, Doi Y, Ozawa T. Postural dysregulation in systolic blood pressure is as-sociated with worsened scoring on neurobehavioral function tests and leukoaraiosis in the older elderly living in a community. Stroke. 1997 28(11):2169-73.
46. Crow RS, Lohman MC, Titus AJ, et al. Mortality risk along the frailty spectrum: data from the National Health and Nutrition Examination Survey 1999 to 2004. J Am Geriatr Soc. 2018;66(3):496-502.
47. Anupama Gangavati, Ihab Hajjar, Lien Quach. Hypertension, Ortho-static Hypotension, and the Risk of Falls in a Community-Dwelling Elderly Population: The Maintenance of Balance, Independent Liv-ing, Intellect, and Zest in the Elderly of Boston Study. Journal of the American Geriatrics Society 2011; 59(3):383-9.
48. Finucane C, O’Connell MD, Donoghue O, Richardson K, Savva GM, Kenny RA. Impaired orthostatic blood pressure recovery is associated with unexplained and injurious falls. J Am Geriatr Soc 2017;65:474–82.
49. Krediet CT, van Lieshout JJ, Bogert LW, Immink RV, Kim YS, Wiel-ingW. Leg crossing improves orthostatic tolerance in healthy sub-jects: a placebo-controlled crossover study. Am J Physiol Heart Circ Physiol 2006;291:H1768–H1772.
50. van Dijk N, Quartieri F, Blanc JJ, et al. Effectiveness of physical coun-terpressure maneuvers in preventing vasovagal syncope: the Physi-cal Counterpressure Manoeuvres Trial (PC-Trial) J Am Coll Car-diol. 2006;48:1652–1657.
51. Jones KP, Brett Shaw B and Raj RS. Orthostatic hypotension: manag-ing a diffi cult problem. Expert Rev Cardiovasc Ther. 2015 Novem-ber;13(11): 1263–1276.
52. Jordan J, Shannon JR, Black BK, Ali Y, Farley M, Costa F, Diedrich A, Robertson MR, Biaggioni I, Robertson D. The pressor response to water drinking in humans: a sympathetic reflex? Circulation 2000;101:504–509.
53. Anne Pavy-Le Traon. How to manage a patient with autonomic dys-function. How to manage a patient with orthostatic hypotension. Teaching Course 13. 3rd Congress of the European Academy of Neurology Amsterdam, The Netherlands, June 24 – 27, 2017.
54. Smit AA, Wieling W, Fujimura J, et al. Use of lower abdominal com-pression to combat orthostatic hypotension in patients with auto-nomic dysfunction. Clin Auton Res 2004;14:167–175.
55. Fanciulli A, Goebel G, Metzler B, et al. Elastic abdominal binders at-tenuate orthostatic hypotension in Parkinson’s disease. Mov Disord Clin Pract 2016;3:156–160.
56. Okamoto LE, Diedrich A, Baudenbacher FJ, et al. Efficacy of servo-controlled splanchnic venous compression in the treatment of or-thostatic hypotension: a randomized comparison with midodrine. Hypertension 2016;68:418–426.
57. Toru S, Yokota T, Inaba A, et al. Autonomic dysfunction and ortho-static hypotention caused by vitamin B12 deficiency. J Neurol Neu-rosurg Psychiatry 1999;66:804–805.
58. Figueroa JJ, Basford RJ, Phillip A. Preventing and treating orthostatic hypotension: As easy as A, B, C. Cleveland Clinic Journal of Medicine 2010;77(5):298-306.
59. Perera R, Isola L, Kaufmann H. Effect of recombinant erythropoietin on anemia and orthostatic hypotension in primary autonomic failure. Clin Auton Res 1995;5:211–213.
60. Low PA, Gilden JL, Freeman R, et al. Effi cacy of midodrine vs place-bo in neurogenic orthostatic hypotension randomized, double-blind multicenter study. Midodrine Study Group. JAMA. 1997;277:1046-1051.
61. Wright RA, Kaufmann HC, Perera R, et al. A double-blind, dose-response study of midodrine in neurogenic orthostatic hypotension. Neurology. 1998;51:120-124.
62. Smith W, Wan H, Much D, Robinson AG, Martin P. Clinical bene-fi t of midodrine hydrochloride in symptomatic orthostatic hypotension: a phase 4, double-blind, placebo-controlled, randomized, tilt-table study. Clin Auton Res 2016;26:269–277.
63. Norcliffe-Kaufmann L, Axelrod FB, Kaufmann H. Developmental ab-normalities, blood pressure variability and renal disease in Riley Day syndrome. J Hum Hypertens 2013;27:51–55.
64. Ricci F, De Caterina R, Fedorowski A. Orthostatic hypotension: epi-demiology, prognosis, and treatment. J Am Coll Cardiol. 2015; 66(7): 848-860.
65. Kaufmann H, Freeman R, Biaggioni I, et al. Droxidopa for neurogenic orthostatic hypotension: a randomized, placebo-controlled, phase 3 trial. Neurology 2014;83:328–335.
66. Elgebaly A, Abdelazeim B, Mattar O, Gadelkarim M, Salah R, Negida A. Meta-analysis of the safety and efficacy of droxidopa for neuro-genic orthostatic hypotension. Clin Auton Res 2016;26:171–180.
67. Shen WK, Sheldon RS, Benditt DG, et al. 2017 ACC/AHA/HRS guideline for the evaluation and management of patients with syn-cope: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Heart Rhythm Society. Circulation. 2017;136(5):e60-e122.
68. Kaufmann H, Freeman R, Biaggioni I, et al. Droxidopa for neurogenic orthostatic hypotension: a randomized, placebo-controlled, phase 3 trial. Neurology 2014;83:328–335.
69. Palma J-A, Norcliffe-Kaufmann L, Martinez J and Kaufmann H. Su-pine plasma NE predicts the pressor response to droxidopa in nOH. Neurology® 2018;00:e1-e6.
70. Shibao C, Norcliffe-Kaufmann L, Kaufmann H, Biaggioni I. Baseline supine norepinephrine levels predict the improvement in orthostatic symptoms after atomoxetine in patients with neurogenic orthostatic hypotension. Clin Aut Res 2016;26:347.
71. Okamoto EL, Shibao AC, Gamboa A, Diedrich A, Raj RS, Black KB, Robertson D, Biaggioni I. Synergistic Pressor Effect of Atomoxetine and Pyridostigmine in Patients With Neurogenic Orthostatic Hypo-tension. Hypertension. 2019;73:235-241.
72. Shibao C, Gamboa A, Diedrich A, Dossett C, Choi L, Farley G, Biag-gioni I. Acarbose, an alpha-glucosidase inhibitor, attenuates postpran-dial hypotension in autonomic failure. Hypertension. 2007;50(1):54-61.
 This work is licensed under a
This work is licensed under a