Bogdan Enache1,2, Decebal Gabriel Latcu1, Naïma Zarqane1, Nadir Saoudi1
1 Department of Cardiology, Princess Grace Hospital, Monaco
2 Department of Cardiology, „Victor Babes” University of Medicine and Pharmacy Timişoara, Romania
Abstract: Atrial fibrillation (AF) is a widespread, lifelong disease with signifi cant costs for the healthcare systems and the quality of life of AF patients. Besides the commonly encountered rhythm and rate control therapies, one should have in mind that simple measures, most of the time adjunct to medication and catheter ablation, may significantly improve the long-term results. In this paper, we set out to review the most recent research focusing on the management of atrial fi brillation outside of drug and ablation therapies with the aim of extracting practical recommendations for the everyday cardiological practice. Virtually any AF patient should be tested for sleep apnea and treated accordingly. Overweight or obese AF patients should be motivated to lose 10% of their initial body mass. Future developments in gene therapy may provide further innovative therapies for our AF patients.
Keywords: atrial fibrillation, non-pharmacological treatment, non-ablative treatment, obesity, sleep apnea
INTRODUCTION
Atrial fibrillation (AF) is the most frequently encoun-tered arrhythmia in the medical practice, affecting millions of people worldwide. Contemporary estima-tes say that one in four adults in Europe and the USA will develop AF in their lifetime1. The importance of AF and its management is not only due to its prevalen-ce, but also due to the fact that AF is one of the ma-jor causes of stroke, heart failure, and cardiovascular morbidity2.
The 2016 European Society of Cardiology Guidelines for the management of AF recommend the “manage-ment of precipitating factors” and they include “lifes-tyle changes and treatment of underlying cardiovas-cular conditions” alongside the canonical rate and rhythm control management strategies. Furthermore, the guidelines give a top role in improving life expec-tancy to this management of precipitating factors. However, the lion’s share of the guidelines text itself is concentrated rather on the rate and rhythm control strategies either by way of medication and/or catheter (even surgical) ablation.
Catheter ablation of AF has now a strong indication in case of symptomatic AF (class I/IIa after medication failure to control symptoms depending on paroxysmal/ persistent form). This technique (Figure 1) is efficient for rhythm management3, improves quality of life4 and likely has a survival benefi t in heart failure patients5,6.
In this review we wish to focus on the potential benefits of non-pharmacological and non-ablative management of AF, as stand-alone therapies or on top of medication and ablation.
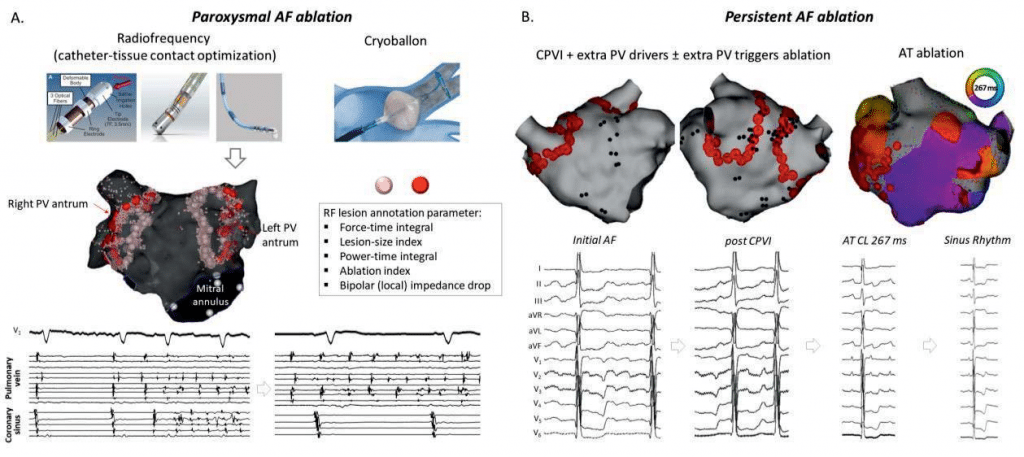
Figure 1. Catheter ablation of AF. A. Paroxysmal AF ablation is currently performed using radiofrequency or cryotherapy. Circumferential point-by-point ablation of the pulmonary vein (PV) antra with operator-tuned targets is mostly used, with lesion quality assessment by integrative parameters including power, time, contact force, generator impedance drop or bipolar (local) impedance drop. The aim of this ablation is complete and persistent bidirectional disconnection of the PV, eventual without dormant conduction (as assessed by adenosine challenge). In the lower part of the panel AF onset triggered by a PV discharge before disconnection is no longer present at the end of the ablation, since recurrent PV discharge is no longer conducted to the atrium which remains in sinus rhythm. B. Persistent AF ablation has a similar initial step with but may also address potential extra PV drivers of AF. AF progressively organizes during the procedure until it eventually stops, sometimes by conversion into atrial tachycardia (AT); a perimitral flutter activation map with a 267 ms cycle length (CL) is shown in the right part of the panel. Further ablation of AT (here by a mitral isthmus line) results in sinus rhythm resumption.
I. SLEEP APNEA SYNDROME
Sleep apnea syndrome (SAS) has been recently estima-ted to have a prevalence in the general adult popula-tion between 9 – 38%7. SAS is known to be associated with a host of cardiovascular conditions: hypertension (especially some drug-resistant forms)8, heart failure9, coronary artery disease10, and, of course, the focus of our review, AF11. For illustrative purposes, Figure 2 shows a patient with a CPAP machine as well as a polysomnography report with periods of sleep apnea.
In the particular case of AF, sleep apnea is not only a risk factor, but the treatment of SAS (by continuo-us positive airway pressure [CPAP] machine therapy during the night) reduces the number of AF recurren-ces and the total AF burden12. Although not rando-mized, one study13 suggests that in the setting of AF ablation, patients with SAS treated with CPAP therapy have a 50% lower risk of AF recurrence, similar to patients without SAS. Conversely, patients with SAS not treated with CPAP had modest results after abla-tion, with recurrence rates similar to patients who had never had AF ablation but who had had SAS treated by CPAP. A recently published meta-analysis (total n=1217)14 confi rms that CPAP therapy lowers the risk of AF recurrence after catheter ablation and estima-tes that 18% recurrences might be attributed to not receiving CPAP therapy. Evidence is accumulating for the benefits of CPAP on slowing of the progression towards permanent AF15, the reduction of arrhythmic recurrences after electrical cardioversion16 and better rate control.17
II. OBESITY: ON THE IMPORTANCE OF WEIGHT LOSS IN AF PATIENTS
Obesity has been associated with the development and progress of AF. A meta-analysis18 of population cohort studies on 78602 patients with average follow-ups between 4.7 and 25.2 years estimates a 49% incre-ase in the risk of developing AF in obese patients (this effect having a direct correlation with the body mass index). A recent cohort of Romanian patients with AF and heart failure shows that obesity has a prevalence of around 24%19.
Studies of animal models have shown an association between obesity and atrial electrostructural remode-ling. Abed et al.20 studied thirty sheep which were fed with a high-calorie diet and compared to a control group by means of cardiac magnetic resonance ima-ging, hemodynamic studies, electrophysiology study with high-density multisite biatrial epicardial mapping and, finally, direct structural histological study. They found that the obese sheep had increased atrial vo-lumes, increased left atrial pressures, increased atrial interstitial fibrosis, progressive conduction abnorma-lities with slowing of atrial conduction. Overall, the authors found that weight gain was associated with a greater burden of induced and spontaneous AF dis-proportionately to the hemodynamic impact of obe-sity and suggesting a direct pathogenic role.
The Legacy trial21 evaluated the long-term impact of weight loss on rhythm control in obese persons with AF. Patients with AF (n=355) were entered into a weight management program (a structured motiva-tional and goal-directed program using face-to-face counseling) with the primary outcome being freedom from AF as evaluated by periodical 7-day Holter mo-nitoring. With a 6-year follow-up period, the study found that progressive weight loss had a dose-depen-dent effect on long-term freedom from AF. The effect was particularly strong in those patients that achieved a weight-loss of more that 10% of their initial body weight, these patients having a 6-fold greater freedom from AF. Put differently, at 6 years 90% of those that had a weight loss >10% were free from AF, compared to only 40% in those that did not lose weight. Even in a subgroup of patients without the use of rhythm con-trol strategies (including ablation), the cohort that lost >10% of the initial weight had a 45% rate of freedom from AF compared to only 15% of those that did not lose weight.
Recently, these researchers took another look at the same group of patients and argue in the REVERSE-AF trial22 that weight loss and management of risk factors may reverse the natural progression of AF di-sease. At 6 years of follow-up, of the patients that had a weight loss of more that 10% of their initial body weight only 3% progressed from paroxysmal to per-sistent AF and 88% reversed from persistent to paro-xysmal or no AF.
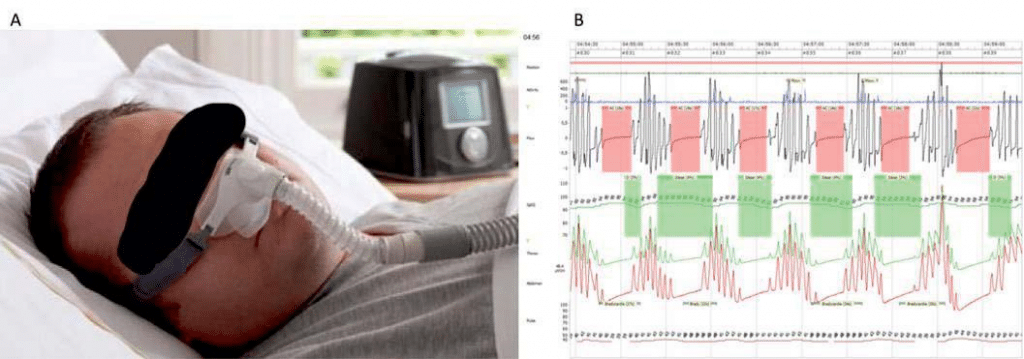
Figure 2. CPAP mask + SAS report. A. Continuous positive airway pressure (CPAP) mask for nocturnal use and CPAP machine in background. B. Poly-somnography report with periods of apnea (red highlighted periods) and subsequent desaturation periods (green highlighted periods) characteristic for central sleep apnea.
Not only does it seem that weight loss and risk fac-tor management programs are very effective, they also seem to be cost-effective23 being cheap to implement and leading to important cost reductions due to less specialist visits, hospitalizations, cardioversions and catheter ablation procedures. Of course, healthcare costs vary between countries and different healthcare systems, but, for example, in the Australian healthcare system the weight loss and risk factor management program lead to an incremental cost-effectiveness ra-tio of $62,653 per quality-adjusted life year gained.
Going further than just the body mass index, resear-ch has been done to understand the role of epicardial fat (as identified by echocardiography, computer to-mography or cardiac magnetic resonance) in arrhyth-mogenesis. Although the mechanisms are from clear and causation is not proven24, still some studies have found a correlation between the amount of epicardial fat and AF recurrence after catheter ablation25,26.
III. PHYSICAL ACTIVITY
The Atherosclerosis Risk in Communities (ARIC) Study27 which, to simplify slightly, was a prospective cohort study of atherosclerotic diseases within four communities in the United States of America started between 1987 and 1989 and comprised of 14219 people aged 45-64 years followed for up to 20 years. Amongst other analyses, the prospective character, the large population and the long follow-up allowed for a study regarding the effects of physical activity on AF28. Phy-sical activity was assessed by questionnaires and con-verted to “poor”, “intermediate” and “ideal” levels. Ideal levels of physical activity conferred an 11% lower risk of developing AF. Even more impactful, an ide-al level of physical activity in men, but not in women (the authors attempted no explanation of this pheno-menon), attenuated the risk of AF in obese patients as follows: obese men with an ideal level of physical activity had a 37% increase risk of AF compared to 156% increased risk in obese men with a poor level of physical activity (after adjusting for blood pressure, diabetes and prior cardiovascular disease).
Things get more complicated as far as physical ac-tivity is concerned because evidence is accumulating in favor of a reversed J-curved effect on AF: athle-tes undergoing intense exercise routines (especially endurance sports) have a higher prevalence of AF29. The most commonly cited explanations include mo-dulators (such as increased vagal tone and gastroe-sophageal refl ux) and substrate modifi ers (pressure and volume overload, atrial stretching, dilatation, and fibrosis). However, there isn’t nearly enough strong evidence to make recommendations against enduran-ce sports30.
IV. CONTROL OF THE PARASYMPATHETIC TONUS
The relationship between vagal tone and AF was es-tablished long-ago31. Indeed, beyond pulmonary vein triggers, the modulating factor of atrial vulnerability is to a certain degree the parasympathetic tone, and small variations may precipitate AF (Figure 3). The ne-urogenic theory of AF is more complex32 and has led to new approaches in AF management and prevention.
IVa. Neuromodulation
A technology-based non-invasive intervention is neu-romodulation (more specifically vagus nerve stimulati-on) which has already been used as a therapy in epilep-sy33 as well as in heart failure34. In 2016 Stavrakis et al. published the first-in-human clinical neuromodulation for patients with AF35. They performed a randomized sham (or placebo) controlled trial of transcutaneous electrical stimulation of the auricular branch of the right vagus nerve at the tragus (also called low-level tragus electrical stimulation or LLTS) in patients at the beginning of AF ablation procedures (so during the electrophysiology study, before any ablation was star-ted). The stimulation voltages were well below both the discomfort threshold and the threshold necessary for slowing the sinus rate or the atrio-ventricular con-duction. They induced AF in all patients and calculated the duration and AF cycle length. This was followed by one hour of LLTS in the treatment group. After one hour, they once again induced AF in all patients. What they found was that the patients in the LLTS group had a decreased AF duration and a longer AF cycle length. The authors’ conclusion was that neuromodulation might be a therapeutic option with certain advantages (painless, non-invasive, and pharmacologically inert) in some clinical scenarios.
Another potential target for stimulation is spinal cord stimulation. Although in the early stages (animal experiments), initial research suggests that spinal cord stimulation protects against AF36 and even suppresses AF37.
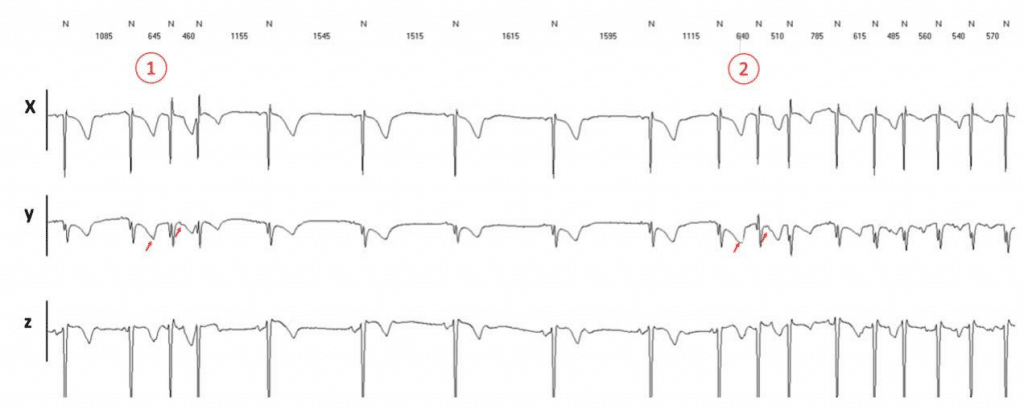
Figure 3. Holter ECG monitoring (3 leads) showing recurrent premature atrial contractions (PAC). The same 2 short-coupled PACs with identical coupling (in 1 and 2, red arrows) induce AF only in the second instance. There is a slight lengthening of the sinus cycle length (normal to normal intervals) just before 2 (from 1515 ms to approx. 1600 ms) suggestive of an increase of the vagal tone, possibly favoring the onset of AF.
IVb. Psychotherapy: less evidence, but what is the risk?
The effects of psychotherapy on the cardiovascular system and on patients with cardiovascular diseases have been less rigorously studied, although isolated research papers date back to the 1960s38. In the last 10 years, however, things have changed dramatically, and psychotherapy trials have become more and more an area of active interest. Mindful-ness is a psychological process which focuses on one’s attention to experiencing the present moment. Ori-ginally based on Buddhist practices, meditation and yoga, it has become popular in a secularized form often named Mindfulness-Based Stress Reduction (MBSR) in the wider fi eld of Behavioral and Cognitive Therapies. There have been over 100 randomized control trials most regarding MBSR and chronic pain, anxiety disor-der, substance abuse disorder and depression39.
One of the only randomized control trials to look at the cardiovascular effects of MBSR40 found that pa-tients undergoing meditation showed an increase in respiratory sinus arrhythmia and a decrease in cardi-ac preejection period without a significant change in heart rate. To date, there are no randomized control trials on psychotherapy and AF burden nor AF recur-rence. However, there are studies on the effects of yoga on AF41. The only randomized trial of yoga and AF42 found modest (but statistically signifi cant) impro-vements in quality of life, lower heart rate and lower blood pressure in the group of paroxysmal AF patients undergoing yoga classes.
A significant sinus node variability (as objectively measured by the standard deviation of the normal to normal intervals –SDNN- on Holter ECG recor-dings) is known to be a marker of good cardiovascular health43. On the opposite, low SDNN has been cor-related to a higher mortality in heart failure44, after myocardial infarction45 and diabetes46. If direct, con-scious, control of the heart rate and its variability is not possible, the respiratory-cardiac interaction can be used in order to improve the heart rate variability through cardiac coherence47. Easy to use smartphone apps (some of them free, as RespiRelax, HeartRate+ Coherence) are available (Figure 4). Whether this may directly diminish the arrhythmia burden in AF patients still needs to be proven.
IV. Gene therapy
Gene therapy as a treatment for AF is still far from cli-nical validation. However, numerous preclinical trials48 already confirm several targets as potentially viable for clinical use: fibroblast proliferation, interstitial fibro-sis, ion channels with a role in electrical remodeling, gap junctions underlying cardiomyocyte coupling, and autonomic modulation. The targeting of transcription factors affecting pathways involved in AF susceptibility has already proven a valid concept in animal models49.
V. SMOKING AND DRINKING
The „traditional” cardiovascular risk factors of ciga-rette smoking and alcohol drinking have a noticeable impact on the prevalence of AF. The aforementio-ned ARIC trial has also looked at smoking and AF50. The authors concluded that current smokers have a two-fold risk of AF and more interestingly smoking cessation does not signifi cantly lower this risk. A 2017 meta-analysis of alcohol consumption and the inciden-ce of AF found that high alcohol consumption (more than two standard drinks per day) was associated with a 33% increased risk of AF, moderate consumption (one or two standard drinks per day) with an 11% increased risk and lower alcohol consumption with no increase in the risk of AF51.
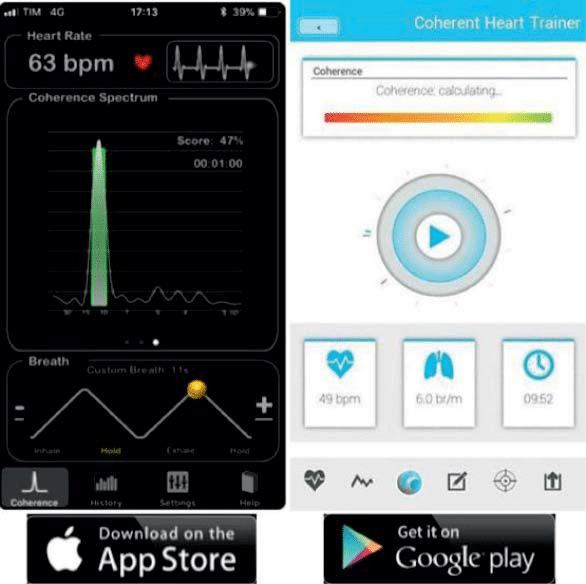
Figure 4. Cardiac coherence apps. Examples of free smartphone apps (HeartRate+ Coherence [left] for iPhone and Coherence Heart Trainer [right] for Android phones) which have breathing exercises while monitor-ing the heart rate through the smartphone’s sensors and camera in order to estimate the level of cardiac coherence.
VI. CONCLUSION: A FEW, BUT IMPORTANT „COMMANDMENTS”
AF is a widespread, lifelong disease with significant costs for the healthcare systems and the quality of life of AF patients. While implementing drug-based rhythm and rate control therapies, one should have in mind that simple measures, most of the time adjunct to medication and catheter ablation, may significantly improve results. We should perfectly treat hyperten-sion and valvular heart disease, as early as possible, to limit the mechano-electrical feedback promoting AF52,53. Test virtually any AF patients for sleep apnea and treat accordingly. Motivate overweight AF pati-ents to lose 10% of their body mass in order to keep them on the higher arrhythmia-free survival curve. Encourage psychotherapeutic measures, in the form of hobbies or oriented stress reduction and cardiac coherence exercises, since they do no harm and may provide a real benefit. Future developments in gene therapy may provide further innovative therapies for our AF patients.
Conflict of interest: none declared.
References
1. Mou L, Norby FL, Chen LY, O’Neal WT, Lewis TT, Loehr LR, Soli-man EZ, Alonso A. Lifetime Risk of Atrial Fibrillation by Race and Socioeconomic Status: ARIC Study (Atherosclerosis Risk in Com-munities). Circ Arrhythm Electrophysiol. 2018;11(7):e006350–15. doi:10.1161/CIRCEP.118.006350.
2. Kirchhof P, Benussi S, Kotecha D, Ahlsson A, Atar D, Casadei B, Castella M, Diener H-C, Heidbuchel H, Hendriks J, Hindricks G, Manolis AS, Oldgren J, Popescu BA, Schotten U, Van Putte B, Vardas P, Agewall S, Camm J, Baron Esquivias G, Budts W, Carerj S, Cas-selman F, Coca A, De Caterina R, Deftereos S, Dobrev D, Ferro JM, Filippatos G, Fitzsimons D, Gorenek B, Guenoun M, Hohnloser SH, Kolh P, Lip GYH, Manolis A, McMurray J, Ponikowski P, Rosen-hek R, Ruschitzka F, Savelieva I, Sharma S, Suwalski P, Tamargo JL, Taylor CJ, Van Gelder IC, Voors AA, Windecker S, Zamorano JL, Zeppenfeld K. 2016 ESC Guidelines for the management of atrial fi brillation developed in collaboration with EACTS. Euro Heart J. 2016;37(38):2893-2962. doi:10.1093/eurheartj/ehw210.
3. Taghji P, Haddad El M, Phlips T, Wolf M, Knecht S, Vandekerck-hove Y, Tavernier R, Nakagawa H, Duytschaever M. Evaluation of a Strategy Aiming to Enclose the Pulmonary Veins With Contiguous and Optimized Radiofrequency Lesions in Paroxysmal Atrial Fibril-lation: A Pilot Study. JACC Clin Electrophysiol. 2018;4(1):99-108. doi:10.1016/j.jacep.2017.06.023.
4. Gonzalez J., Macle L., Deyell M. W., Bennett M. T., Dubuc M., Dyrda K.,Thibaut B, Andrade J. G. (2014). Effect Of Catheter Ablation On Quality Of Life In Atrial Fibrillation. J Atr Fibrillation, 6(6), 1063. http://doi.org/10.4022/jafib.1063
5. Liang JJ, Callans DJ. Ablation for Atrial Fibrillation in Heart Failure with Reduced Ejection Fraction. Card Fail Rev. 2018;4(1):33-37. doi:10.15420/cfr.2018:3:1.
6. Marrouche NF, Brachmann J, Andresen D, Siebels J, Boersma L, Jor-daens L, Merkely B, Pokushalov E, Sanders P, Proff J, Schunkert H, Christ H, Vogt J, Bänsch D. Catheter Ablation for Atrial Fibrillation with Heart Failure. N Engl J Med. 2018;378(5):417-427. doi:10.1056/ NEJMoa1707855.
7. Senaratna CV, Perret JL, Lodge CJ, Lowe AJ, Campbell BE, Matheson MC, Hamilton GS, Dharmage SC. Prevalence of obstructive sleep ap-nea in the general population: A systematic review. Sleep Med Rev. 2017;34:70-81. doi:10.1016/j.smrv.2016.07.002.
8. Mehra R, Benjamin EJ, Shahar E, Gottlieb DJ, Nawabit R, Kirch-ner HL, Sahadevan J, Redline S. Association of Nocturnal Arrhyth-mias with Sleep-disordered Breathing. Am J Respir Crit Care Med. 2006;173(8):910-916. doi:10.1164/rccm.200509-1442OC.
9. Bradley TD, Floras JS. Sleep apnea and heart failure: Part I: obstructive sleep apnea. Circulation. 2003;107(12):1671-1678. doi:10.1161/01. CIR.0000061757.12581.15.
10. Javaheri S, Barbe F, Campos-Rodriguez F, Dempsey JA, Khayat R, Javaheri S, Malhotra A, Martinez-Garcia MA, Mehra R, Pack AI, Polotsky VY, Redline S, Somers VK. Sleep Apnea: Types, Mecha-nisms, and Clinical Cardiovascular Consequences, J Am Coll Cardiol. 2017;69(7):841-858. doi:10.1016/j.jacc.2016.11.069.
11. Gami AS, Hodge DO, Herges RM, Olson EJ, Nykodym J, Kara T, Somers VK. Obstructive Sleep Apnea, Obesity, and the Risk of In-cident Atrial Fibrillation. J Am Coll Cardiol. 2007;49(5):565-571. doi:10.1016/j.jacc.2006.08.060.
12. Neilan TG, Farhad H, Dodson JA, Shah RV, Abbasi SA, Bakker JP, Michaud GF, van der Geest R, Blankstein R, Steigner M, John RM, Jerosch-Herold M, Malhotra A, Kwong RY. Effect of sleep apnea and continuous positive airway pressure on cardiac structure and recur-rence of atrial fibrillation. J Am Heart Assoc. 2013;2(6):e000421-e000421. doi:10.1161/JAHA.113.000421.
13. Fein AS, Shvilkin A, Shah D, Haffajee CI, Das S, Kumar K, Kramer DB, Zimetbaum PJ, Buxton AE, Josephson ME, Anter E. Treatment of obstructive sleep apnea reduces the risk of atrial fibrillation re-currence after catheter ablation. J Am Coll Cardiol. 2013;62(4):300-305. doi:10.1016/j.jacc.2013.03.052.
14. Deng F, Raza A, Guo J. Treating obstructive sleep apnea with contin-uous positive airway pressure reduces risk of recurrent atrial fibril-lation after catheter ablation: a meta-analysis. Sleep Med. 2018;46:1-27. doi:10.1016/j.sleep.2018.02.013.
15. Holmqvist F, Guan N, Zhu Z, Kowey PR, Allen LA, Fonarow GC, Hylek EM, Mahaffey KW, Freeman JV, Chang P, Holmes DN, Pe-terson ED, Piccini JP, Gersh BJ, Impact of obstructive sleep apnea and continuous positive airway pressure therapy on outcomes in patients with atrial fibrillation—Results from the Outcomes Regis-try for Better Informed Treatment of Atrial Fibrillation (ORBIT-AF). Am Heart J. 2015;169(5):647–654.e2. doi:10.1016/j.ahj.2014.12.024.
16. Kanagala R, Murali NS, Friedman PA, Ammash NM, Gersh BJ, Ballman KV, Shamsuzzaman ASM, Somers VK. Obstructive sleep apnea and the recurrence of atrial fibrillation. Circulation. 2003;107(20):2589-2594.
17. Dediu GN, Dumitrache-Rujinski S, Lungu R, Frunza S, Diaconu C, Bartos D, Bogdan MA. Positive pressure therapy in patients with cardiac arrhythmias and obstructive sleep apnea. Pneumologia. 2015; 64(1):18-22.
18. Wanahita N, Messerli FH, Bangalore S, Gami AS, Somers VK, Stein-berg JS. Atrial fibrillation and obesity—results of a meta-analysis. Am Heart J. 2008;155(2):310-315. doi:10.1016/j.ahj.2007.10.004.
19. Tica OA, Tica O, Tor R, Hatos A, Cote I, Brendea MN, Rosan L, Mihele L, Moisi M, Popescu MI, Clinical profile and management in non-valvular atrial fibrillation and heart failure patients. Rom J Card 2018;28(1):6-14.
20. Abed HS, Samuel CS, Lau DH, Kelly DJ, Royce SG, Alasady M, Ma-hajan R, KUKLIK P, Zhang Y, Brooks AG, Nelson AJ, Worthley SG, Abhayaratna WP, Kalman JM, Wittert GA, Sanders P. Obesity re-sults in progressive atrial structural and electrical remodeling: Im-plications for atrial fibrillation. Heart Rhythm. 2013;10(1):90-100. doi:10.1016/j.hrthm.2012.08.043.
 This work is licensed under a
This work is licensed under a