Maria Magdalena Gurzun1, Monica Rosca1,2, Andreea Calin1,2, Marinela Serban2, Andreea C. Popescu1,3, Carmen Ginghina1,2, Bogdan A. Popescu1,2
1 University of Medicine and Pharmacy “Carol Davila”, Bucharest, Romania
2 Emergency Institute of Cardiovascular Diseases “Prof. Dr. C. C. Iliescu”, Bucharest, Romania
3 Elias Emergency Hospital, Bucharest, Romania
Abstract: Echocardiography is the main method for mitral valve (MV) anatomy assessment. To establish the relative importance of different imaging modalities, we compared four echocardiographic methods for MV prolapse diagnosis: standard 2D transesophageal echocardiography (TEE), complete 2D-TEE (including additional views to properly visualise all scallops), 3D-TEE, and 3D colour coded TEE modelling. Methods and results – We analysed MV morphology in 137 patients (57,11+/-11 year, 57% male) by these four methods: 38 normal subjects and 99 patients with mitral regurgitation. The complete 2D-TEE reclassified as prolapsing 34 scallops considered normal by standard 2D-TEE (mainly A1 and A3 scallops). By 3D-TEE 15 segments classifi ed as normal by complete 2D-TEE were reclassified as prolapsing, allowing a better diagnosis of A1, P3, and commissural prolapse. Based on 3D-model data 8 segments have been reclassified compared to 3D-TEE. The accuracy of the other methods compared with the gold-standard 3D-TEE was 98% for complete 2D-TEE, 93% for standard 2D-TEE, and 98% for the 3D modelling. Conclusion – The MV cannot be properly analysed by 2D examination without a full, careful, and experienced evaluation (A1, A3, P3, the commissures are more challenging). Therefore, 3D-echocardiography becomes an essential tool for the precise diagnosis of MV prolapse in everyday clinical practice.
Keywords: mitral valve, prolapse, myxomatous mitral valve disease, transesophageal echocardiography, three-dimensional echocardiography
INTRODUCTION
Mitral valve repair is the surgical method of choice for patients with severe mitral regurgitation due to myxomatous mitral valve disease whenever the valve is deemed reparable by detailed imaging assessment and the required surgical expertise is available. Therefore, precise knowledge of mitral valve morpho logy and function is mandatory for proper patient management. Echocardiography is the main method for the diagnosis and detailed mitral valve anatomy description in patients with mitral regurgitation. Morphologically, myxomatous mitral valve disease is characterized by fibromyxomatous changes, mucopolysaccharide accumulation and collagen alternation1. However, the echocardiographic defi nition of this pathology is still debatable and has changed in parallel with different technical developments (from M-mode echocardiography to bi-dimensional echocardiography and 3D echocardiography). In general, it is characterized by systolic displacement of abnormally thickened mitral leafl ets2 into the left atrium3. However, looking at the same patient by different echocardiographic modalities may sometimes lead to different conclusions regarding the mitral valve: prolapsing vs non-prolapsing. Therefore, the purpose of this study was to compare four echocardiographic methods for the diagnosis of mitral valve prolapse: standard 2D transesophageal echocardiography (TEE) (including just the four chamber, two chamber, long axis, and bi-commisural views), complete 2D TEE (including additional and modified views for the proper visualisation of all scallops and the commissures), 3D TEE, and 3D colour coded TEE modelling.
METHODS
Study population
All patients with an established diagnosis of significant mitral regurgitation (MR) due to myxomatous disease referred to our department for transesophageal echocardiography (TEE) were included. A control group – composed of patients who were sent to our echocardiography laboratory for clinically indicated TEE, but in whom no significant cardiovascular pathology was
identified – were also analysed. The exclusion criteria were: ischemic heart disease, history of rheumatic fever or rheumatic valve disease, significant calcification of the mitral annulus, significant concomitant aortic or tricuspid valve disease (>mild), left ventricular systolic dysfunction (left ventricular ejection fraction <60%), non-sinus rhythm. The following clinical data were collected for all patients: age, gender, history of hypertension, symptoms (e.g. dyspnoea, chest pain, palpitations, syncope). The clinical status was defined according to the New York Heart Association (NYHA) classification. Physical examination and 12-lead electrocardiography were performed in all patients.
Transesophageal 2D and 3D echocardiography
All patients had complete echocardiographic examinations (EAE protocol) followed by 3D acquisitions for the mitral valve (3D and colour 3D). Electrocardiographically gated full-volume loops were acquired over 4 cardiac cycles at a frame rate of 15 to 45 frames/s. The volume for mitral valve was a multi-beat acquisition with at least 15 frames per second. The mitral valve morphology was analysed off-line in standard 2D TEE views following a complete TEE protocol4. The standard TEE exam included the four basic views for mitral valve analysis: four chamber, bicommisural, two chamber and long axis views. The complete 2D TEE exam included all views described in Figure 1, allowing the visualisation of each scallop in at least two separate views. We used the Carpentier classification to describe the components of each leaflet. The posterior mitral leaflet is formed by three scallops: P1 (anterolateral), P2 (middle), and P3 (posteromedial), while the corresponding segments of the anterior mitral leaflet are labelled A1, A2, and A35. Every scallop of the mitral valve was characterized as being normal, prolapsing, or fl ail. The prolapse was defined as supra-annular displacement of the leaflet body, with leaflet tips pointing towards the left ventricle, while fl ail was defined the orientation of free margin of the mitral leaflet towards the left atrium, with the leaflet tip pointing towards the left atrium during systole. The visualisation of ruptured chordae was noted. Using 2D images we measured the following parameters of mitral annulus (MA): antero-posterior diameter in midesophageal long axis view, anterolateral-posteromedial diameter in mid-esophageal commissural view, and of mitral valve leaflets: the length of the anterior and posterior leaflets in the mid-esophageal long axis view. The morphology of every scallop was also appreciated by 3D echocardiography: from the ‘surgical view’ and from specific cut planes used in this volumetric data set (Figure 2). Based on the number of scallops affected we subdivided the group of patients with MR in limited mitral valve disease (1-2 scallops affected by prolapse/fl ail) and extensive (3-6 scallops affected by prolapse/fl ail). The mitral valve was reconstructed from the acquired 3D volume using a specific software (4D MV Assessment 2.0 TomTec imaging Systems). The mathematical model illustrates graphically the leaflet topology by colouring each point of the leaflet surface of the model according to its distance to the best fitting plane of the mitral annulus. This plane is defined as the plane that fi ts the majority of points of the mitral annulus and minimizes the distance to points that are not on the plane. Generally, this plane is located lower than the highest points of the mitral annulus and higher than the lowest points of the mitral annulus. The points of mitral valve leaflets located above this plane, in the left atrium, are coded in red, the points located below this plane, towards the left ventricle, are coded in blue, while the points located in this plane are coded in white (Figure 3). Therefore, the part of mitral valve leaflets displayed in red is considered prolapsing. The antero-posterior and anterolateral-posteromedial mitral annulus diameters, as well as the anterior and posterior leaflet areas, were automatically calculated by the software. The data obtained by 3D TEE analysis were considered the gold standard method6. The MV assessment by standard 2D TEE, complete 2D TEE, 3D TEE, and 3D mathematical model was performed remotely, blinded, by an experienced echocardiographer, holding EACVI certification in TEE (MMG).
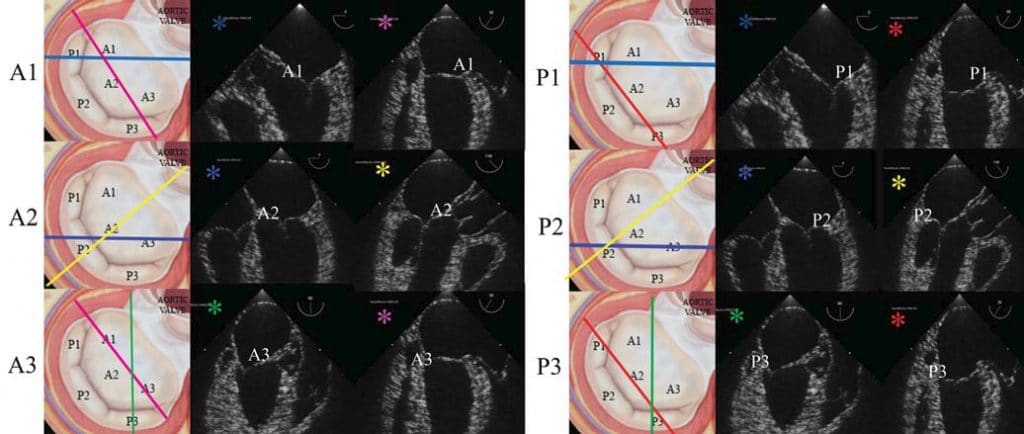
Figure 1. 2D transesophageal echocardiography complete protocol for analysing the mitral valve morphology (mid-systole still images): A1 visualisation in mid-esophageal five chamber view (light blue plane) and modified bi-commisural view (probe rotated to right for the visualisation of the entire anterior leafl et A1-A2-A3) (pink plane); A2 visualisation in mid-esophageal four chamber view (blue plane) and long-axis view (yellow plane); A3 visualisation in the modified two-chamber view (probe rotated to the right for the visualisation of A3 and P3 scallops) (green plane), and modifi ed bi-commisural view mentioned previously (pink plane); P1 visualisation in five chamber view (light blue plane) and bi-commisural view (red plane); P2 visualisation in mid-esophageal
four chamber view (blue plane) and long axis view (yellow plane); P3 visualisation in modified two chamber view mentioned previously (green plane) and bi-commsiural view (red plane). Every scallop is analysed in at least two different views and prolapse is defi ned as the systolic movement of that segments into the left atrium during systole, above the mitral annulus.
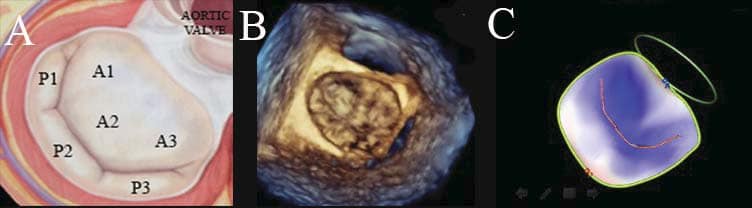
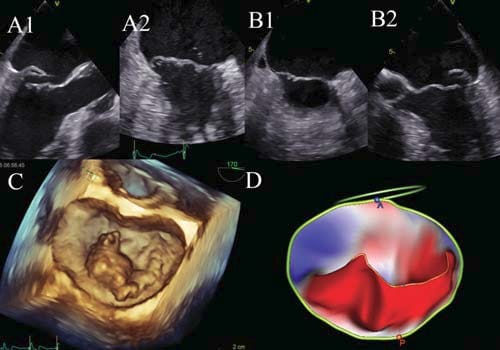
Figure 2. Mitral valve scallops based on Carpentier’s classifi cation: three scallops at the level of the posterior leaflet, separated by incisures noted P1-P2-P3, P1 being adjacent to the left atrium appendage and antero-lateral commissure, and P3 being adjacent to the postero-medial commissure and the tricuspid valve; and three corresponding anterior mitral leaflet scallops: A1-A2-A3 (A). The mitral valve can be analysed by 3D TEE using the ‘surgical view’. Looking from left atrium down to the mitral valve all the above-mentioned scallops can be easily visualized; note the normal closure and the lack of prolapsing segments in a normal subject (B). The mathematical 3D model is a colour coded model showing non-prolapsing scallops coloured in blue, and prolapsing
scallops coloured in red; note the blue appearance in a normal subject (C).
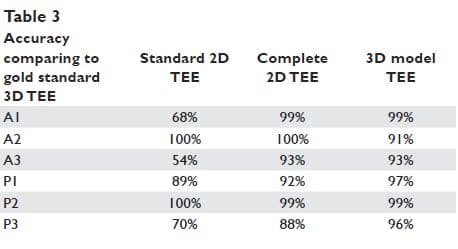
Figure 3. The graphic representation of the best fitted plane for a saddle shape structure (A). The mathematical 3D model reconstructs the mitral valve annulus (yellow line), the mitral valve leaflets (green), and the coaptation line (red line) (B). The colour coded model allows the possibility to appreciate mitral valve prolapse: the points of MV leaflets located above the best fitted plane are coloured in blue, the points of MV leafl ets located below the best fitted plane are coloured in red, while the points located at the best fitted plane level are coloured in white (C). The colour intensity (i.e. blue and red) is proportional with the distance from the best fitted plane.
Statistical Analysis
Continuous data are presented as mean +/- SD and nonparametric data as median and ranges. For testing the differences between two groups we used Student’s t-test and between three groups we used one-way ANOVA. The relationships between different parameters were assessed by correlation analysis: Pearson’s method or Spearman’s r method, as appropriate. Reproducibility of the 3D parameters measurements was assessed in a randomly chosen group of 15 patients. Intraobserver variability was asses sed using repeated measurements performed by the same observer one month apart, while interob server variability was evaluated by repeating the analysis by a second independent observer, blinded to the results of all prior measurements. The intraobserver variability was 96% for standard 2D TEE, 94% for complete 2D TEE, 98% for 3D TEE and 98% for 3D model TEE, while interobserver variability was 84% for standard 2D TEE, 84% for complete 2D TEE, 84% for 3D TEE and 96% for 3D model TEE. Statistical analyses were performed using SPSS version 21.0 (SPSS, Inc., Chicago, IL). P values <0.05 were considered significant.
RESULTS
One hundred and fifty patients were included: 110 patients with organic mitral regurgitation due to myxomatous mitral valve disease, and 40 normal subjects. Thirteen patients were excluded after the echo analysis due to poor image quality and inadequate temporal resolution. Therefore, 137 patients were included in the final study group: 38 normal subjects and 99 patients with mitral regurgitation (MR). The mean age (54.9+/-10.6 vs 58.3+/-11.7, p=0.12) and gender distribution (57% men vs 57% men, p=0.9) were similar in the group of patients with mitral regurgitation and in the normal group. The normal subjects had no symptoms, while in the MR group there were 52 patients in NYHA I class, 37 patients NYHA II class and 10 patients NYHA III class. Using the 3D TEE protocol in the MR group 28 patients (28%) had just one scallop affected by prolapse, 22 patients (22%) had two scallops, 12 patients (12%) had three scallops, 9 patients (9%) had four scallops, 5 patients (5%) had five scallops and 21 patients (21%) had all scallops affected. Two patients with severe mitral regurgitation had an isolated lesion of the posterior commissure, with no evidence for prolapse. In 6 patients (6%) the pathology was seen only at the level of the anterior mitral leaflet, in 49 patients (49%) the pathology was seen only at the level of the posterior mitral leaflet, while in 44 patients (44%) both mitral leaflets were affected.
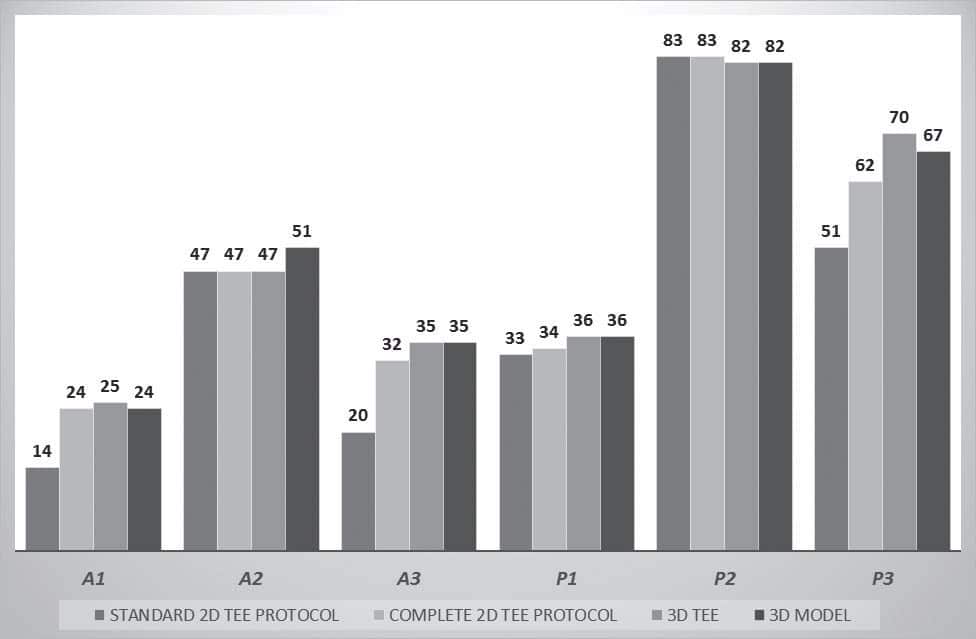
Figure 4. The graphic representation of number of affected scallops by the four methods: standard 2D TEE, complete 2D TEE, 3D TEE and 3D TEE model. The main difference relates to the A1, A3 and P3 scallops.
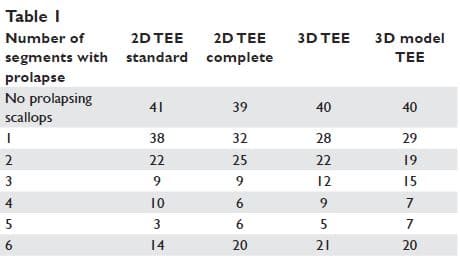
The patients’ classification based on the extension of mitral valve prolapse by the four methods is presented in Table 1. We overall analysed 137 segments of each type (A1, A2, A3, P1, P2, P3) by the four methods: standard 2D TEE, complete 2D TEE, 3D TEE, and 3D model TEE. The results are presented in Figure 4 and Table 2. The advantages of complete 2D TEE compared to standard 2D TEE are demonstrated by the high number of segments classified as normal by standard 2D TEE and reclassified as prolapsing by complete 2D TEE (34 segments: 4,1% of all the scallops analysed). In fact, 29% of prolapsing A1 scallops, 37% of prolapsing A3 scallops and 17% of prolapsing P3 scallops were not detected by sole analysis of standard 2D TEE views. 3D TEE provides new incremental information and 15 segments classified as normal by complete 2D TEE were reclassified as prolapsing by 3D TEE, while one scallop considered prolapsing by complete 2D TEE was considered normal by 3D TEE. Of note, 8 P3 scallops considered normal by complete 2D TEE were considered prolapsing after 3D TEE analysis (i.e. 5% of all P3 scallops analysed). Compared to complete 2D TEE, 3D modelling reclassified as prolapsing 4 A2 scallops, 3 A3 scallops and 5 P3 scallops. Comparing 3D TEE data with 3D model TEE data 8 segments from 822 have been reclassified based on 3D model (4 A2 scallops were considered prolapsing and 3P3 scallops were considered non-prolapsing by 3D model TEE) (Figure 5).
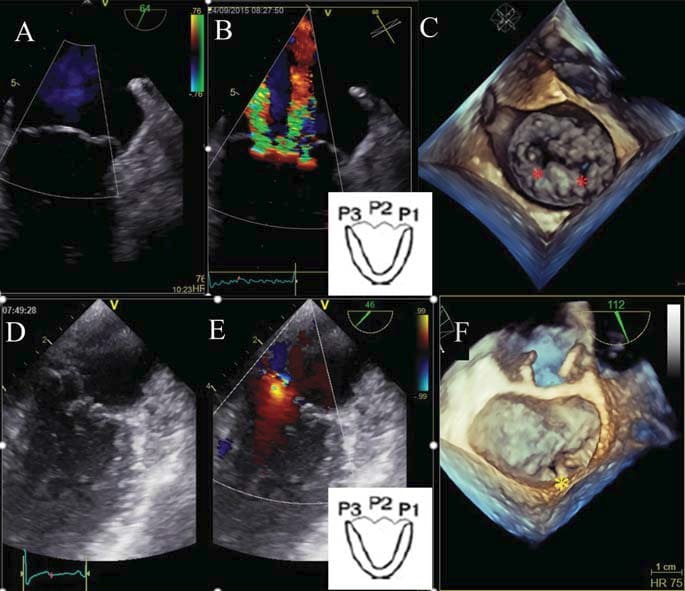
Figure 5. Mitral valve analysis in a patient with myxomatous mitral valve disease. The 2D standard TEE showed P2 prolapse visualised in long axis view (A1) and P3 prolapse visualised in bicommisural view (A2); The 2D TEE complete examination proved also A3 prolapse visualised in modified bicommisural view (B1) and P1 prolapse visualised in fi ve chamber view (B2). The 3D TEE surgical view of the mitral valve suggests prolapsing of P2, P3 and A3 scallops (C), while the 3D mathematical model shows that the body of A2 is above the mitral annulus as well (D).
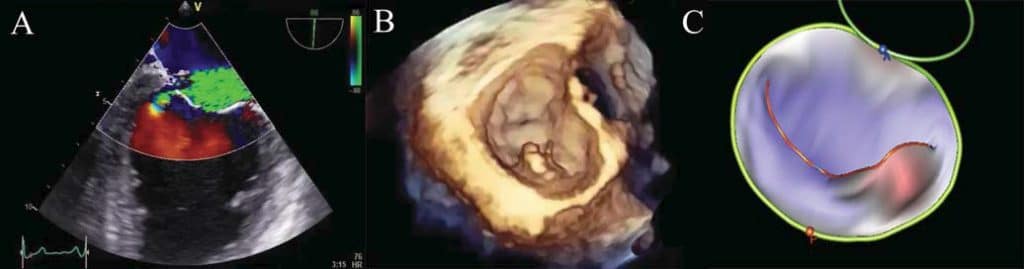
Figure 6. Severe mitral regurgitation visualised in the bi-commisural view, the origin of mitral regurgitation jet being adjacent to the P3 scallop (A). The 3D surgical view (B) and the 3D model (C) proved that the P3 scallop is prolapsing and the posterior commissure is also affected.
Figure 7. 2D color Doppler TEE in a patient with mixomatous mitral valve disease: in the standard bi-commisural view (A) there is no mitral regurgitation jet, while in the modified bi-commisural view at P1-P2-P3 level there are three jets of mitral regurgitation (B). The 3D atrial view of the mitral valve is very helpful for establishing the diagnosis: one jet is central at the level of A2-P2 that are prolapsing, and the other two jets are located at the level of pseudocommisures between P1-P2 and P3-P2 (C). In another case of mitral regurgitation,the modified bi-commisural view at P1-P2-P3 level suggests mitral valve prolapse and mitral regurgitation jet at the level of P2 scallop (D, E); the 3D surgical view of the mitral valve is very helpful for establishing the diagnosis: P1-P2 cleft (F).
The accuracy of the other methods compared with gold standard 3D TEE is: 98% for complete 2D TEE, 93% for standard 2D TEE, and 98% for 3D modelling. Patients with scallops reclassified from non-prolapsing to prolapsing by complete vs standard 2D TEE had more often severe mitral regurgitation (73.1% of reclassified patients vs 40.3% of non-reclassified patients have severe mitral regurgitation, p<0.001) and extensive mitral valve disease (61.5% of reclassifi ed patients vs 51.4% of non-reclassified patients have more than 3 scallops affected, p<0.001). Patients with scallops reclassified from non-prolapsing to prolapsing by 3D vs complete 2D TEE have more frequent severe mitral regurgitation (53.2% from reclassified patients vs 45.1 % from non-reclassified patients have severe mitral regurgitation, p<0.001) and limited mitral valve disease (92.3 % from reclassified patients vs 43.4 % from nonreclassified patients have less than 2 scallops affected). Based on complete 2D TEE exam, 20 patients have the anterior commissure affected, and 38 patients have the posterior commissure affected. The anterior commissure was generally affected in patients with extensive mitral valve disease (just in one case less than two scallops were affected) and the posterior commissure was affected in 11 cases with limited mitral valve disease and 27 cases with extensive mitral valve disease. Using 3D echocardiography one case with anterior commissure involvement and three more cases with posterior commissure involvement were detected (Figure 6). 3D TEE was useful for the detection of one case with P1-P2 and P2-P3 pseudo-commissures, and one case with posterior mitral valve cleft (Figure 7). Mitral valve fl ail was detected by complete 2D TEE for 55 scallops (four A2 scallops, one A3 scallop, one P1 scallop, 46 P2 scallops and 3 P3 scallops). Using standard 2D TEE we detected fl ail in 44 cases (2 A2 scallops and 42 P2 scallops) while using 3D TEE fl ail was observed in 40 segments (4A2 scallops, 4P3 scallops, and 32 P2 scallops). The length of the anterior and the posterior mitral leaflets measured by 2D echocardiography in the long axis view had a weak correlation with the area of the anterior and respectively posterior mitral leaflet, measured by 3D echocardiography (r=0.27, p=0.01 and r=0.17, p=0.05, respectively). The antero-posterior MA diameter measured by 2D TEE in the long axis view, and the anterolateralposteromedial MA diameter measured by 2D TEE in bi-commissural view had moderate correlations with the values of these diameters obtained by 3D geometrical reconstruction (r=0.39, p<0.001 and=”” r=”0.53,<br”> p<0.001). However, the values obtained by 2D versus 3D echocardiography are significantly different for the antero-posterior diameter of the mitral annulus: 3.4+/- 0.54 cm vs 3.5+/-0.61 cm, p=0.002.
DISCUSSION
Mitral valve prolapse was described by John Brereton Barlow7 already 50 years ago, based on clinical auscultation and cine-angiographic exam. However, even after five decades, it remains a difficult to define entity8. It was initially recognized as a clinical entity, the most important auscultatory element being the late systolic click, with or without a late systolic murmur9. The first echocardiographic features described by M mode echo (late systolic negative fl ow)10 had low sensitivity and specificity compared to clinical and angiographic data11. Bi-dimensional echocardiography defined mitral valve prolapse as the superior systolic movement of mitral valve leaflet in relation to the mitral annulus12. Therefore, the early studies reported a high incidence
of mitral valve prolapse13,14, considering abnormal the superior displacement of mitral valve leaflets even in the four-chamber view. The description of the 3D saddle shape of the mitral annulus15 changed the understanding and assessment of mitral valve prolapse. This new information suggested that mitral valve prolapse should be diagnosed only in the long-axis view, but in
this view only the A2 and P2 scallops can be analysed. The current agreement is that diagnosis of mitral valve prolapse can be made in any view as long as billowing of the anterior mitral leaflet of less than 5 mm is not considered16. The 3D reconstruction of the mitral valve offers the possibility to overcome these problems and to diagnose and localise accurately the mitral valve prolapse17.
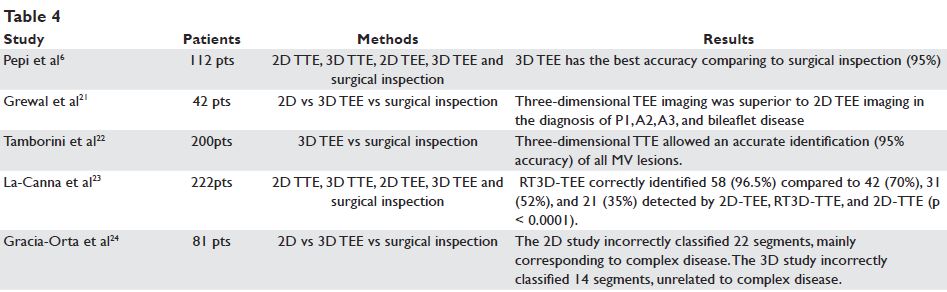
The usefulness of 3D TEE in the correct identification of prolapsing scallops has been proved previously18,19, with an accuracy of this method compared to intraoperative results of >95%6. The advantages of 3D TEE over 2D TEE in localisation of mitral valve prolapse are even more important for inexperienced echocardiographers, compared to expert echocardiographers20. In our study, the accuracy of 2D vs 3D TEE in the diagnosis of MV prolapse was 98%, higher than previously reported, probably due to multiple views utilised for MV examination. The advantage of 3D TEE vs complete 2D TEE was important mainly for the diagnosis of A3, P1 and P3 prolapse. The detection of mitral valve fl ail and cordal rupture was higher for complete 2D TEE comparing to 2D TEE probable due to the higher temporal resolution. Therefore, a complete 2D TEE exam of the MV remains of great value for the diagnosis of prolapse25. Conversely, the standard 2D TEE examination has a low sensitivity for MV prolapse diagnosis, mainly concerning the A3 and P3 scallops. The new method analysed in our study was the identification of prolapsing scallops based on colour coded mathematical modelling. The differences between 3D TEE and the mathematical model are mainly explained by the model considering the distance from every point of MV leaflets to the best fitting plane, and not to the mitral annulus plane. The accuracy of 3D TEE model vs 3D TEE is 98%. Of note, this difference is mainly related to the A2 scallop: the basal part of A2 may be superior to the best fitted plane, but still inferior to the highest point of the mitral annulus (the anterior point). The use of this colour coded mathematical model may overcome some of the difficulties in making the diagnosis of MV prolapse15,26,27 and may be very useful in everyday clinical practice28, especially for interpreters with limited experience29. The method needs further validation against intraoperative findings. Three-dimensional TEE is superior in the diagnosis of MV indentations (i.e. grooves separating the posterior mitral leaflets <50% of leaflet length), or clefts indentations (i.e. grooves separating the posterior mitral leaflets less than 50% of leaflet length)30.
STUDY LIMITATIONS
The main limitation of our study was that the mitral valve was evaluated only by echocardiography and no surgical or anatomical information was available for comparison.
CONCLUSION
Three-dimensional echocardiography becomes an essential tool for the precise diagnosis of mitral valve prolapse in everyday clinical practice because certain areas of the mitral valve cannot be seen completely without a full, careful and experienced evaluation (A1, A3, P3, commissurres). The 3D colour-coded mathematical model generated semi-automatically may be very useful for less experienced echocardiographers. Two-dimensional TEE remains an important diagnostic tool in experienced hands, able to perform a complete mitral valve examination. The standard 2D TEE exam has a low sensitivity for the diagnosis of mitral valve prolapse and should not be recommended as the only method for mitral valve morphology analysis.
Acknowledgment: We would like to gratefully thank our colleagues who have referred patients for this study.
Funding: This work was supported by a grant from the Romanian Ministry of National Education, CNCSUEFISCDI, project number PN-II-ID-PCE-2012-4-0560 (contract 21/2013), and by the Sectorial Operational Programme Human Resources Development (SOP HRD), financed by the European Social Fund and by the Romanian Government under the contract number POSDRU/159/1.5/S/132395.
References
1. Pellerin D, Breker S, Veyrat C. Degenerative mitral valve disease with emphasis on mitral valve prolapse. Heart 2002;88:iv 20-iv.
2. Delling F, Vasan R. Epidemiology and pathophysiology of mitral valve prolapse: new insights into disease progression, genetics and molecular basis Circulation, 2014;129:2158-2170.
3. Devereux RB, Kramer-Fox R, Shear MK, et al. Diagnosis and classification of severity of mitral valve prolapse: methodologic, biologic, and prognostic considerations. Am Heart J. 1987;113:1265-1280.
4. Hahn RT, Abraham T, Adams MS et al. Guidelines for performing a comprehensive transesophageal echocardiographic examination: recommendations from the American Society of Echocardiography and the Society of Cardiovascular Anesthesiologists.J Am Soc Echocardiogr. 2013 Sep;26(9):921-64.
5. Carpentier A. Cardiac valve surgery — the ‘‘French correction’’. J Thorac Cardiovasc Surg 1983;86:323-37.
6. Pepi M, Tamborini G, Maltagliati A, et al. Head-to-head comparison of two- and three-dimensional transthoracic and transesophageal echocardiography in the localization of mitral valve prolapse. J Am Coll Cardiol. 2006 Dec19;48(12):2524-30.
7. Barlow JB, Bosman CK. Aneurysmal protrusion of the posterior leaflet of the mitral valve. An auscultatory-electrocardiographic syndrome. Am Heart J. 1966 Feb;71(2):166-78.
8. Călin A, Ginghină C. Regurgitarea mitrală. [book auth.] Ginghină C. Mic tratat de cardiologie. Editura Academiei Române, Bucureşti, 2010: 451-468.
9. Hancock EW, Cohn K. The syndrome associated with mid-systolic click and late systolic murmur. Am J Med 1966; 41:183-96.
10. Kerber RE, Isaeef OM, Hancock EW . Echocardiographic patterns in patients with the syndrome of systolic click and late systolic murmur. N Engl J Med 1971;3:284:691.
11. Abbasi A, DeCristofaro D, Anabtawi J, Irwin L.Mitral Valve Prolapse: Comparative Value of M-Mode, Two-Dimensional and Doppler Echocardiography. JACC 1983;2 (6):1219-1223.
12. Perloff J, Child J. Mitral valve prolapse – Evolution and refi nement of diagnostic techniques Circulation 1989;80:710-711.
13. Sasaki H, Ogawa S, Handa S, Nakamura Y, Yamada R: Two-dimensional echocardiographic diagnosis of mitral valve prolapse syndrome in presumably healthy young students. J Cardiogr 1982;12:23-31.
14. Warth DC, King ME, Cohen JM, Tesoriero VL, Marcus E, Weyman AE: Prevalence of mitral valve prolapse in normal children. JAm Coll Cardiol 1985;5:1173-1177.
15. Levine RA, Triulzi MO, Harrigan P, Weyman AE. The relationship of mitral annular shape to the diagnosis of mitral valve prolapse. Circulation. 1987;75(4):756-67.
16. Lancellotti P, Tribouilloy C, Hagendorff A, Popescu BA, Edvardsen T, Pierard LA, Badano L, Zamorano JL. Recommendations for the echocardiographic assessment of native valvular regurgitation: an executive summary from the European Association of Cardiovascular Imaging. Eur Heart J Cardiovasc Imaging. 2013 Jul;14(7):611-44.
17. Shiota T. 3D echocardiography: the present and the future J Cardiol, 2008;2:169-185.
18. Ahmed S, Nanda NC, Miller AP et al. Usefulness of transesophageal three-dimensional echocardiography in the identifi cation of individual segment/scallop prolapse of the mitral valve. Echocardiography. 2003 Feb;20(2):203-9.
19. Chauvel C, Bogino E, Clerc P et al. Usefulness of three-dimensional echocardiography for the evaluation of mitral valve prolapse: an intraoperative study. 2000: May;9(3):341-9.
20. Hien MD, Großgasteiger M, Med C et al. Experts and beginners benefit from three-dimensional echocardiography: a multicenter study on the assessment of mitral valve prolapse. J. Am. Soc. Echocardiogr. 2013;26(8):828–834.
21. Grewal J, Suri R, Mankad S, et al. Mitral annular dynamics in myxomatous valve disease: new insights with real-time 3-dimensional echocardiography. Circulation. 2010;121(12):1423-31.
22. Tamborini G, Muratori M, Maltagliati A et al. Pre-operative transthoracic real-time three-dimensional echocardiography in patients undergoing mitral valve repair: accuracy in cases with simple vs. complex prolapse lesions. Eur. J. Echocardiogr. 2010;11(9):778.
23. La Canna G, Arendar I, Maisano F et al. Real-time three-dimensional transesophageal echocardiography for assessment of mitral valve functional anatomy in patients with prolapse-related regurgitation. Am. J. Cardiol. 2011;107(9):1365-1374.
24. García-Orta R, Moreno E, Vidal M et al. Three-dimensional versus two-dimensional transesophageal echocardiography in mitral valve repair. J. Am. Soc. Echocardiogr. 2007;20(1):4-12.
25. McCarthy PM. Three-dimensional echocardiography is not essential for intraoperative assessment of mitral regurgitation. Circulation. 2013;128(6):653-8.
26. Benenstein R, Saric M. Mitral valve prolapse: role of 3D echocardiography in diagnosis.Curr Opin Cardiol. 2012;27(5):465-76.
27. Freed LA, Levy D, Levine RAet al. Prevalence and clinical outcome of mitral-valve prolapse. N Engl J Med. 1999; 341(1):1-7.
28. Valocik G, Kamp O, Visser CA. Three-dimensional echocardiography in mitral valve disease. Eur J Echocardiogr. 2005;6(6):443-54.
29. Tsang W, Weinert L, Sugeng L, et al. The value of three-dimensional echocardiography derived mitral valve parametric maps and the role of experience in the diagnosis of pathology. J Am Soc Echocardiogr. 2011;24:860-867.
30. Tsang W, Lang RM. Three-dimensional echocardiography is essential for intraoperative assessment of mitral regurgitation. Circulation. 2013;128(6):643-52.

 This work is licensed under a
This work is licensed under a