Bogdan Radulescu1,2, Silvia Pieleanu2, Vlad Anton Iliescu1,2
1 University of medicine and pharmacy “Carol Davila” Bucharest
2 Emergency institute for cardiovascular diseases “Prof. Dr. C.C.Iliescu” Bucharest
Abstract: Background – Coronary artery bypass grafting (CABG) is still one of the most common major cardiac operations in the world. Recent studies confirm that it remains the gold standard for most patients with multivessel and/or left main disease. The purpose of this study was to evaluate the post operatory differences between the patients that underwent myocardial revascularization using single internal mammary artery (SIMA) and both internal mammary arteries (BIMA). Materials and methods – This study is descriptive retrospective analysis on 267 patients that underwent coronary artery bypass grafting (CABG) with one or two mammary arteries and saphenous vein graft(s) (SVG). We divided patients in two groups, group 1 (122 patients; 45,69%) included patients that underwent myocardial revascularization using SIMA, and group 2 (145 patients; 54.3%) included patients with BIMA grafts. Results – There were no statistical significant differences between the two groups in age, comorbidities, aortic clamp time, extracorporeal circulation time, left ventricle ejection fraction (LVEF), postoperative length of stay, perioperative morbidity (bleeding, deep sternal wound complications, need for reoperation, stroke, acute myocardial infarction, low cardiac output) and mortality. Conclusion – BIMA grafting is a safe method of myocardial revascularization, it increases survival, and it must be done in a skeletonized fashion.
Keywords: myocardial revascularization, CABG, BIMA, skeletonized, sternal wound infection, mediastinitis
BACKGROUND
Coronary artery bypass grafting (CABG) is still one of the most common major cardiac operations in the world. Recent studies confirm that it remains the gold standard for most patients with multivessel or/and left main disease. The left and/or right internal mammary artery (LIMA/RIMA) is overwhelmingly accepted as the first choice of conduit for grafting, particularly to the left anterior descending (LAD) and left ventricle wall arteries. Even if IMAs are the best in grafting left coronary vessels, in 2005 the Cleaveland Group has confirmed that for RCA grafting, saphenous vein patency was equivalent to or better than IMA patency within 5 years from surgery. However, by 10 years, IMA patency was better in RCAs with 70% stenosis or more1. IMA it has a superior resistance to the development of arteriosclerosis, intimal hyperplasia, medial calcification and better vasoreactive properties2. Furthermore, BIMA provide an additional survival benefit over the single LIMA3,1,4,5. BIMA use is increasingly recognized to improve cardiac free survival and reduce the need for repeat revascularization6-8. However, selection of the third choice of conduit is debated. The 10 years patency for IMA is in range of 90% to 96%, 86% to 88% for the radial artery (RA), and as low as 50% to 57% for saphenous vein grafts. Remains the most frequently used
additional conduit because of its abundance and ease of use9,10. The use of BIMA grafts was considered for many years as an important risk factor for a higher incidence of deep sternal wound complications, especially in diabetic patients. Harvesting IMA as a skeletonized conduit, together with better controlled glucose management in the postoperative period, is demonstrated to
lower the risk of sternal wound problems in patients who receive BIMA grafting, even in diabetic patients1. Even if BIMA revascularization is demonstrated to be the best choice, is not universally used due to increased operative time, usually an additional 30 minutes, technical complexity and potential heightened vulnerability of some patients to sternal wound infections11,12. The most important reasons not to use BIMA grafts, especially in diabetic patients are: fear of sternal wound infection, sternal dehiscence, and the associated risk of mediastinitis13. Studies demonstrated that BIMA harvesting compromises blood supply to the sternum, but a skeletonized manner is proved to significantly decrease the risk of deep sternal wound infection14,15. Also strict glycemic control16 accompanied by skeletonisation technique for harvesting of BIMA have been consistently shown to reduce wound complications in diabetics17,18. The skeletonisation technique
independently lowers the risk of sternal wound complications in all patients and particularly in those with diabetes19,20,24,21,17,18. A meta-analysis made by Soso et al. validated that skeletonized IMA harvesting reduced the risk of all sternal wound infections by 60%, and in diabetic patients reduces this risk from 21.3% to 3.57%. In case of harvesting BIMA, the advantage
of skeletonisation was maintained with reduction of sternal wound infection from 11,7% to 2.96% for all studies, and in diabetic patients from 14.2% to 4%22. When skeletonisation technique was adopted, the risk of sternal wound infection in BIMA patients was just a little higher than that in single IMA patients, but the difference did not reach statistical significance. Despite the beneficial impact of skeletonisation on reducing the risk of sternal wound infection it is important to emphasize that skeletonisation is technically more demanding and more time consuming than pedicled IMA harvesting with a steep learning curve associated with it23. A study made by De Pauli’s and colleagues compared two groups of 450 patients, who received CABG using pedicled SIMA, or BIMA harvested in a skeletonized fashion (150 patients), to verify the impact of BIMA grafting on deep sternal wound complications. Diabetes was not found to be a risk factor, while the pedicled harvesting technique impacted on the incidence of sternal complications (odds ratio: 4.1). The incidence of sternal wound complications in the SIMA group was significantly higher compared with the skeletonized BIMA grafting group. (1.1 vs 3.3%, p=0.01). A meta-analysis of Taggart and colleagues, including seven studies with at least 100 patients, and followed for at least 4 years, showed signifi cantly better survival in the BIMA group, than in SIMA1 In another study of 400 diabetic patients that underwent CABG with one or BIMA showed an incidence of 2.25% of sternal wound complications, without significant differences between the two groups. In the pedicled IMA harvesting technique was noticed a higher incidence of sternal wound problems (6.45 vs 1.5%, p=0.0045)1. Even if there are enough arguments for better outcome in myocardial revascularization using BIMA, the actual publications and multicenter studies shows a reduced rate of these strategy of revascularization. Should it be from the risk of sternal wound problems, longer recovery time, increased in hospital morbidity and mortality, increasing mean age of patients, or is it just
another surgery that not many surgeons are willing to make? Do they have a real reason? And does that depend on the hospital possibilities? Skeletonization of the IMA it provides superiority to
the quality of grafts with fewer traumas, fewer postoperative complications, decreased sternal wound complications and achieve extra length. Very important, in the high-risk population has a potential benefi t in mortality and mainly adverse cardiac events. Because of improving outcomes with skeletonized IMA harvesting, the frequency of BIMA grafting was signifi cant elevated
after that (from 23% to 61% for diabetic patients)3.
MATERIALS AND METHODS
The purpose of this study was to evaluate the post operatory differences between the patients that underwent myocardial revascularization with SIMA and BIMA. This study is a descriptive retrospective analysis on 267 patients undergoing coronary artery bypass grafting (CABG) with one or two mammary arteries and SVG. Patients in this study were operated by a single surgical team in the period 06.2011 – 12.2015. All patients were revascularized using on-pump technique, with SIMA or BIMA, and SVG for completing the number of grafts needed. The IMA conduits were harvested in a skeletonized fashion. All the patients were evaluated pre and postoperative for left ventricle ejection fraction (LVEF). Dates were collected using patient’s electronic information from the hospital database. These data were analyzed using Microsoft Excel 2010 and SPSS. We calculated p value, with p<0.05 considered statistical significant (S), while p >/=0.05 was non-significant (NS). The inclusion criteria were: patients with angina and at least uni-vessel disease with indication for elected or emergency surgery. The exclusion criteria were valvular
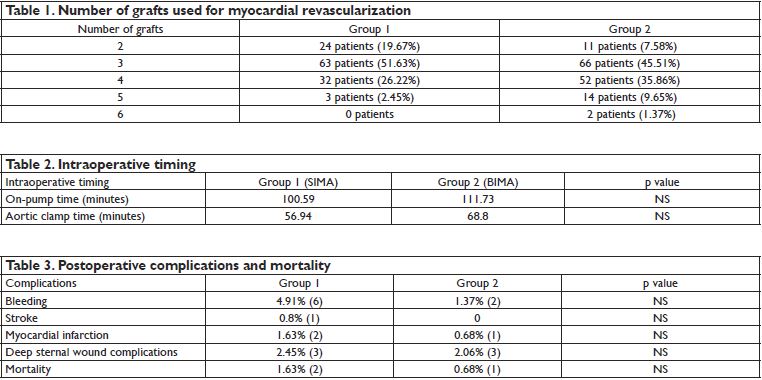
heart disease with indication for repair or replacement in the same time with revascularization, and patient with univessel disease that needed only one CABG, redo surgery, and medical conditions that limited the use of IMA. We divided patients in two groups, group 1 (122 patients; 45,69%) included patients that underwent myocardial revascularization using SIMA, and group 2 (145 patients; 54.3%) included the ones with BIMA grafts. We analyzed if there are any differences between these two groups, in age, comorbidities, aortic clamp time, extracorporeal circulation time, left ventricle ejection fraction (LVEF), postoperative days of hospitalization, perioperative complications (bleeding, deep sternal wound complications, need for reoperation, stroke, acute myocardial infarction, low cardiac output) and mortality. Commonly, in this population IMA grafts were used in situ, so in BIMA group, is very important to identify the best target vessel for each IMA. The LIMA is usually anastomosed to the LAD and the RIMA to the lateral wall, passing over or behind the aorta, through the transverse sinus. Care should be taken when passing the RIMA anteriorly across the mediastinum, because it imposes an extremely high risk of conduit injury during future iterative surgery. Also the strategy of passing RIMA behind the aorta, through the transverse sinus for anastomosis to the lateral wall, can be very dangerous, as the graft may be under unrecognized tension, distortion and obscured bleeding1,4,5. When using both IMAs for lateral wall anastomosis and RIMA is too short, it can be used as a free graft and sustured to the LIMA as a T or a Y (Figure 3)1,4,
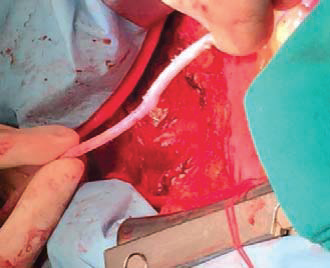
Figure 1. The left internal mammary artery (LIMA) – skeletonized harvesting.
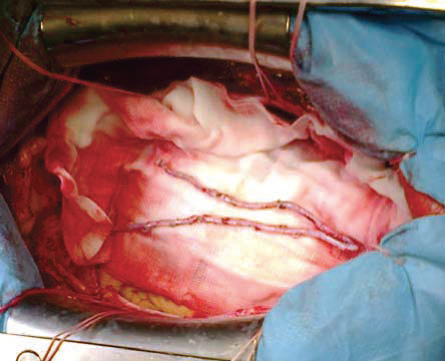
Figure 2. The saphenous vein graft (SVG).
Figure 3. Both internal mammary arteries (BIMA) – anastomosed in Y shape.
RESULTS
The study group was represented by 80.9% men (216 patients) and 19.15 females (51 patients), with mean age of 62.47 years old (between 29 to 81 years, SD: 8.92). 105 patients (39.32%) were known with diabetes type II, 197 (73.78%) patients were (ex) smokers, 124 (46.44%) of them were obese, 247 (92.5%) patients had high cholesterol or were in treatment with hypolipemiants, 117 (43.82%) had periferic multivessel atherosclerotic disease, 187 (70.03%) were known with high blood pressure, 178 (66.66%) had at least one cardiac event (acute coronary syndrome, acute
myocardial infarction). About half of patients were in NYHA class III or IV (50.56%). Mean preoperator LVEF was 51.31 (25-60%, SD: 7.7), 27.71% (74 patients) had also left main disease.
24.34% (65 patients) of patients underwent emergency CABG, and the rest of them (75.65%) elective operation. Mean extracorporeal circulation time was 106.64 minutes (40-286; SD: 35.12), and aortic clamp time was 63.38 minutes (27-171; SD: 20.57). Postoperative hospital stay was an average of 8 days (SD: 6) with a minimum stay of 4 days and a maximum of 93 days, the latter was due to neurologic complications. Patients were divided in two groups, Group 1 included patients that underwent CABG using SIMA, and Group 2 using BIMA. In the Table 1 is shown that most of patients received 3 or 4 grafts, and that can be explained by multivessel disease and also significant left main stenosis (27.71% of patients). The differences between the two groups were statistical
not significant for extracorporeal circulation time and aortic clamping time, as it is shown in Table 2. In Table 3 are shown the postoperative complications in our two groups, and the differences are not significant. We also calculated the data for elected and emergency operations, but neither in those groups were significant differences between SIMA and BIMA grafting strategy.
DISCUSSIONS
The progress in coronary surgery is slowly, particularly in the choice of conduits to be used as coronary grafts. In the beginning, SVG and internal mammary arteries appeared almost at the same time. It’s obviously that all the surgeons who were performing Vineberg’s operation were also able to harvest mammary arteries, but the knowledge was not translated in direct myocardial
revascularization1. The SVG became the most used graft and, for a long time, it remained the main conduit used for coronary surgery. It took a long time and a lot of work until the’80s, when it was proven that the use of the LIMA on the LAD was able to prevent, in most patients, further ischemic events and, increase survival. Then the use of BIMA was becoming the best solution for any
patient with a reasonable life expectancy, but it was still a problem due to the complications following the pedicled technique of harvesting the graft. At the end of the ‘90s arterial revascularization became obviously superior to standard revascularization (LIMA and SVGs), but still today’s surgeons are reluctant to use BIMA grafting and its use is limited to a few centers and global percentage of patients revascularized with this technique is approximately 10% of overall surgical experience1. Increasing of the life expectancy did not help to increase the mean age and the presence of dangerous comorbidities, which often limit life expectancy, all contributed to maintaining the “status quo”. The achievements of clinical research were not applied and the application of multiple arterial conduits remains a niche in coronary surgery. Least, but not last, interventional cardiology is competing with coronary surgery, and maybe will force cardiac surgeons to become better, to improve the quality of their long term results, because early results of interventional myocardial revascularization are surely less traumatic for the patient than surgery1.
CONCLUSIONS
For a long time the diabetic population represented a subgroup of patients in whom the double mammary was not used due to a high incidence of deep sternal infections. For these reason, the long term results of BIMA vs LIMA had not been reported in the literature. In recent years, with the routine use of skeletonized BIMA in some cardiac centers and the consequent decline in sternal problem rate, more surgeons are also using the double mammary in this subset of patients1. Accordingly also to this study, there are no significant differences between the SIMA and BIMA groups,
not even for diabetics, if the conduits are harvested in a skeletonized fashion, together with better controlled glucose management in the postoperative period. There are few studies in the literature that have clearly demonstrated the superiority of BIMA vs LIMA grafting in diabetic patients. Hirotani and colleagues did not find any benefit, Endo and colleagues found a non-significant difference for 10 year survival between BIMA (80.2%) and LIMA (75.4%) patients (p=0.46). Another study on 8 year outcome for diabetic patients that underwent revascularization with BIMA or LIMA and SVG showed signifi cantly better 8 year freedom from any cause of death, cardiac death, AMI in any area or in a grafted area. Cox analysis confirmed that the use of LIMA and SVG(s) was an independent predictor for lower freedom from death (HR: 1.8), cardiac death (HR: 1.9), AMI (HR: 9.7). These fi ndings clearly demonstrated that BIMA grafting offers the possibility to improve late outcome of patients undergoing myocardial revascularization, even if they are diabetics1. Conflict of interest: none declared.
References
1. Calafiore, M. d. M. Antonio Maria, “Bilateral internal mammary artery grafting,” Expert Rev. Cardiovasc. Ther, vol. 4, pp. 395-403, 2006.
2. P. Ruengsakulrach, R. Sinclair, M. Komeda, J. Raman, I. Gordon, B. Buxton, “Comparative histopathology of radial artery versus internal thoracic artery and risk factors for development of intimal hyperplasia and atherosclerosis,” Circulation, vol. 100 Supl, pp. 139-144, 1999.
3. Hu, Q.Z.Xiang, “Skeletonized internal thoracic artery harvest improves prognosis in high-risk population after coronary artery bypass surgery for good quality grafts,” Ann Thorac Surg, pp. 48-58, 2011.
4. Lawrence H. Cohn, Jon-Cecil M. Walkes, Enrique Gongora, Thoralf M. Sundt, “Myocardial revascularization with cardioplumonary bypass,” in Cardiac surgery in the adult, The McGraw-Hill, 2008, pp. 599-624.
5. Kirklin, Barratt-Boyes, Nicholas T. Kouchoukos, Eugene H. Blackstone, Frank L. Hanley, James K. Kirklin, “Ischemic heart disease,” in Cardiac surgery, Morphology, Diagnostic Criteria, Natural History, Techniques, Results and Indications, Elsevier, 2013, pp. 367-413.
6. P. A. Kurlansky, E.A. Traad, M. J. Dorman, D. L. Galbut, M. Zucker, G. Ebra, “Thirtyyear follow-up defi nes survival benefi t for second internal mammary artery in propensity-matched groups,” Ann Thorac. Surg., vol. 90, pp. 101-108, 2010.
7. B. W. Lytle, E. H. Blackstone, J. F. Sabik, P. Houghtaling, F. D. Loop, D. M. Cosgrove, “The effect of bilateral internal thoracic artery grafting on survival during 20 postoperative years,” Ann. Thorac. Surg., vol. 78, pp. 2005-2012, 2004.
8. A.J. Weiss, S. Zhao, D. H. Tian, D. P. Taggart, T. D. Yan, “A meta-analysiscomparing bilateral internal mammary artery with left internal mammary for coronary artery bypass grafting,” Ann. Cardiothorac. Surg., vol. 2, pp. 390-400, 2013.
9. Rehman, G. Y. D. P. T. Syed M., “The radial artery: Current Concepts on its use in coronary artery revascularization,” Ann. Thorac. Surg., 2013.
10. Jorapur, A.C.-G. C. A. C. Vinod, “Should saphenous vein grafts be the conduits of last resort for coronary artery bypass surgery?,” Cardiology in Review, vol. 17, pp. 235-242, 2009.
11. J. Tatoulis, B. F. Buxton, J.A. Fuller, “The right internal thoracic artery: is it underutilized?,” Curr. Opin. Cardiol., vol. 26, pp. 528-535, 2011.
12. S. Mastrobuoni, N. Gawad, J. Price, V. Chan, M. Ruel, T. G. Mesana, “Use of bilateralinternal thoracic artery during coronary artery bypass graft surgery in Canada: the bilateral internal thoracic artery survey,” J. Thorac. Cardiovasc. Surg., vol. 144, pp. 874-879, 2012.
13. A. U. Momin, R. Deshpande, J. Potts, A. El-Gamel, M. T. Marrinan, J. Omigie, “Incidence of sternal infections in diabetic patients undergoing bilateral internal thoracic artery grafting,” Ann. Thorac. Surg., vol. 80, pp. 1765,1767, 2005.
14. S. G. Raja, K. Salhiyyah, M. U. Rafi q, M Navaratnarajah, D. Chudasama, C. P. Walker, “In hospital outcomes of pedicled bilateral internal mammary aertery use in diabetic and nondiabetic patients undergoing off-pump coronary artery bypass grafting: single surgeon, single center experience,” Heart Surg. Forum, vol. 16, pp. 1-7, 2013.
15. Raja, S.G., “Sternal wound infection and bilateral internal mammary artery harvest: there’s more to it than the harvesting technique,” Ann. Thorac. Surg, vol. 97, pp. 736-737, 2014.
16. H.L. Lazar, S.R. Chipkin, C.A. Fitzgerald, Y. Bao, H. Cabral, C.S. Apstein, “Tight glycemic control in diabetic coronary artery bypass graft patients improves perioperative outcomes and decreases recurrent ischemic events,” Circulation, vol. 109, pp. 1497,1502, 2004.
17. M.S. Uva, E. Braunberger, M. Fisher, Y. Fromes, P.H. Deleuze, J.A. Celestin, “Does bilateral internal thoracic artery grafting increase surgical risk in diabetic patients?,” Ann. Thorac. Surg., vol. 66, pp. 2051-2055, 1998.
18. R. Milani, P.R. Brofman, M. Guimaraes, L. Barboza, R.M. Tchaick, H. Meister Filho, “Double skeletonized internal thoracic artery vs. double conventional internal thoracic artery in diabetic patients submitted to OPCAB,” Rev. Bras. Cir. Cardiovasc., vol. 23, pp. 351-357, 2008.
19. M. Matsa, Y. Paz, J. Gurevitch, I. Shapira, A. Kramer, D. Pevny, “Bilateral skeletonized internal thoracic artery grafts in patients with diabetes mellitus,” J. Thorac. Cardiovasc. Surg., vol. 121, pp. 668-674, 2001.
20. T. Hirotani, T. Nakamichi, M. Munakata, S. Takeuchi, “Risks and benefi ts of bilateral internal thoracic artery grafting in diabetic patients,” Ann. Thorac. Surg., vol. 76, pp. 2017-2022, 2003.
21. O.M. Bical, W. Khoury, Y. Fromes, M. Fischer, M. Sousa Uva, G. Boccara, “Routine use of bilateral skeletonized internal thoracic artery grafts in middleaged diabetic patients,” Ann. Thorac. Surg., vol. 78, pp. 2050- 2053, 2004.
22. Z. L. H. Z. S. X. F. L. C. Dai, “Bilateral internal mammary artery grafting and risk of sternal wound infection: evidence from observational studies,” Ann. Thorac. Surg., vol. 95, pp. 1938-1945, 2013.
23. G. D. S.G. Raja, “Internal thoracic artery: to skeletonize or not to skeletonize?,” Ann. Thorac. Surg., vol. 79, pp. 1805-1811, 2005.

 This work is licensed under a
This work is licensed under a