Ciprian Rezus1,2, Anca Cardoneanu3,4, Nicoleta Dima5, Adeline Josephine Funingana Cumpata6, Elena Rezus3,7
1 Emergency Clinical Hospital “Sfantul Spiridon”, 3rd Medical Clinic, Iasi, Romania
2 University of Medicine and Pharmacy “Gr.T.Popa” Iasi, Romania, Associate Professor MD, PhD, ciprianrezus@yahoo.com
3 Rehabilitation Clinical Hospital, 1st Rheumatology Clinic, Iasi, Romania
4 University of Medicine and Pharmacy “Gr.T.Popa” Iasi, Romania, Teaching assistant MD, PhD student, cardoneanu_anca84@yahoo.com
5 Emergency Clinical Hospital “Sfantul Spiridon”, 3rd Medical Clinic, Iasi, Romania, MD, nicoleta2006r@yahoo.com
6 Emergency Clinical Hospital “Sfantul Spiridon”, 3rd Medical Clinic, Iasi, Romania, MD, jcumpata22@gmail.com
7 University of Medicine and Pharmacy “Gr.T.Popa” Iasi, Romania, Associate Professor MD, PhD, elena_rezus@yahoo.com
Abstract: Rheumatic inflammatory diseases are a group of disorders characterized by damage to the vascular system and connective tissue as well as damage to the internal organs (kidneys, heart, lungs and spleen). Cardiovascular manifestations of these disorders are followed by substantial morbidity and mortality. The most important risk factor for the development of cardiac pathology, namely myocardial ischemia, is considered to be accelerated atherosclerosis. Leading cause of early atherosclerosis is the presence of chronic infl ammation, succeeded by immune and endothelial dysfunction. Keywords: myocardial ischemia, inflammation, rheumatic inflammatory diseases
Collagen is considered to be the main protein in the body that contributes to the mechanical properties of tissues. In pathology, the concept of rheumatic inflammatory diseases was introduced in the 5th decade in order to define a few diseases with vascular system and connective tissue injuries and damage to the internal organs (kidneys, heart, lungs and spleen). This group of diseases includes Rheumatoid Arthritis (RA), Systemic Lupus Erythematosus (SLE), Systemic Scleroderma (SS), Dermatomyositis, Polyarteritis, Systemic Vasculitis and mixed connective tissue disease. The causes of rheumatic inflammatory diseases are not yet fully understood. Their evolution has a chronic course, being interrupted by acute periods, followed by remissions. In the evolution of the lesions we can distinguish the following physiological phenomena: aberrant immune response – hyper--globulinemia, high titers of circulating autoantibodies, autoimmune vasculitis and fibrinoid degeneration of connective tissue. Clinically, most diseases evolve with multiple manifestations at both a mucocutaneous and a musculoskeletal level as well as at the level of other internal organs such as the cardiovascular, pulmonary, renal, nervous, ocular systems, etc. Regarding cardiovascular events, these can be more important or clinically silent but they are characterized by substantial morbidity and mortality. The most important risk factor for the development of cardiac pathology, namely myocardial ischemia, is considered to be accelerated atherosclerosis. Chronic inflammation and immune as well as endothelial dysfunction participate in the formation of vascular atherosclerotic plaque1,2. When we mention the term “inflammation” we need to refer to the recruitment of mononuclear cells found in blood, an increased expression of adhesion molecules, the production of matrix metallo-proteinase
and an increased release of proinflammatory cytokine3 (Figure 1).
Figure 1. The main pathogenic mechanisms of atherosclerosis.
Among proinfl ammatory cytokines, a crucial role is played by the tumor necrosis factor (TNF) and interleukin 6 (IL-6). These determine the activation and dysfunction of the endothelium with the help of the nuclear factor k-B (NFk-B)4, followed by leukocyte infiltration, activation and proliferation in the subendothelial layer3,5. Also, TNF is closely related to the appearance of insulin resistance, dyslipidaemia, to the stimulation of other inflammatory molecules or to the development of a prothrombotic status4,6. Studies proved that toll-like receptors (TLR) were highlighted at the level of atherosclerotic plaques, thus making the link between atherosclerosis and autoimmunity3,7. Regarding the role of leukocytes, first place is occupied by activated T cells. These T cells were found both in stable and unstable atherosclerotic plaques, mentioning that a subset of CD25 positive T cells were found in an increased titer in unstable lesions8. Inflammation is also characterized by an increased expression of adhesion molecules. An activated endothelium determines the expression of a large number of adhesion molecules which favors early atherosclerosis through leukocyte recruitment into the subendothelial layer3. Clinical trials on patients with large-vessel vasculitis, SLE or RA sustained that vascular cell adhesion protein 1 (VCAM-1) is directly involved in the occurrence of cardiovascular events5. Monocyte-chemotactic protein-1 (MCP-1) is expressed by activated endothelium cells under the influence of TNF and promote atherosclerosis by attracting leukocytes and monocytes to the vascular endothelium9. The presence of MCP-1 correlated with coronary calcifications in patients diagnosed with SLE can
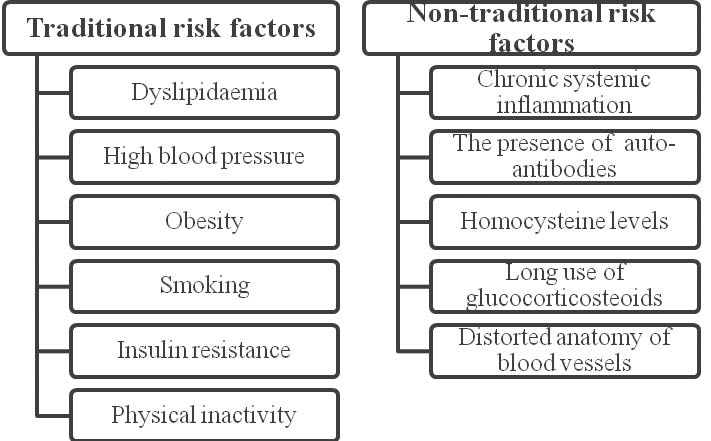
even be observed in healthy people with an increased risk of coronary artery disease3,10. Figure 2 reiterates both common and rare cardiovascular risk factors in inflammatory rheumatic diseases.
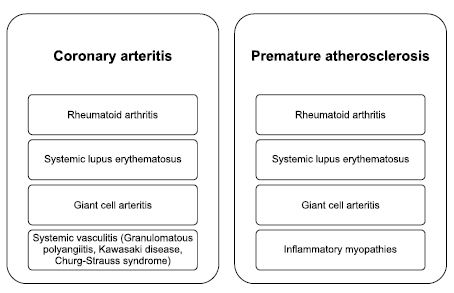
Due to the presence of inflammation, many inflammatory rheumatic disorders are associated with the inflammation of coronary arteries and with cardiovascular events such as: myocardial infarction, angina, coronary angioplasty and stoke (Figure 3)11-13. Systemic lupus erythematosus is a complex disease associated with premature atherosclerosis and early deaths due to cardiovascular events or severe infections14. In the pathogenesis of SLE a very important role is played by type 1 interferon which is considered to determine the instability of atherosclerotic lesions, aortic stiffness or dysfunction of the endothelium15. Studies demonstrated that 40% of patients have myocardial perfusion defects16 and 50% of patients have impaired endothelial vasodilatation17. Clinical trials using ultrasound at carotid artery level, sustained the early appearance of atherosclerotic plaques which were related to SLE activity which was quantifi ed by the SLEDAI index18.
Figure 2. Common and rare cardiovascular risk factors in inflammatory rheumatic diseases.
Figure 3. Inflammatory rheumatic diseases and coronary involvement.
The cardiovascular events occurred with a higher frequency in people under 40 years of age, the risk of myocardial infarction being 50-fold greater in women ages 35 to 44 than the general population19. Another important fact related to cardiovascular risk is corticosteroid use which is considered to be dosedependent (a 5-fold increase in cardiovascular risk at a dose more than 20mg/day)20. Other factors involved in the occurrence of cardiac events are considered to be: high titer of anti DNA double-stranded antibodies, renal complications such as lupus nephritis, duration and disease activity21. Rheumatoid arthritis, characterized by chronic inflammation, shows premature atherosclerosis even without common risk factors as well as a high prevalence
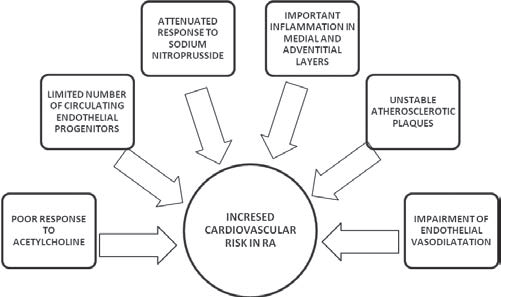
of ischemic heart disease, namely myocardial infarction22-24. Moreover, events such as sudden cardiac death and silent myocardial infarction are more frequent among these patients25. Many factors are thought to increase mortality in RA patients like: specific antibodies- rheumatoid factor, anti-citrullinated peptide antibodies, the presence of rheumatoid nodules and the level of erythrocyte sedimentation rate, female sex, erosions, synovitis or associated lung disease26-28. Clinical trials have highlighted a poor response to acetylcholine which leads to an impairment of endothelial vasodilatation and a limited number of circulating endothelial progenitors29. One study found a defective relaxation of arterial smooth muscle due to an attenuated response to sodium nitroprusside30. Studies analyzing the morphology of atherosclerotic plaques in patients with RA had important inflammation in the medial and adventitial layers, making the
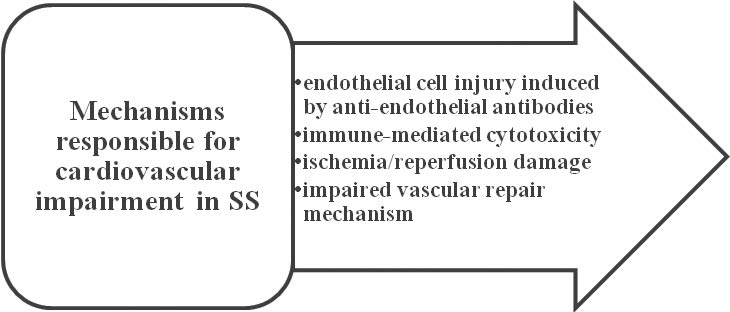
atherosclerotic plaques more unstable which caused an increased number of thromboses31,32. So, patients with RA are at high risk to develop silent cardiac ischemia, myocardial infarction or heart failure. Figure 4 illustrates the main factors that increase the cardiovascular risk in patients with RA. Systemic sclerosis is also part of the umbrella group of inflammatory rheumatic diseases, being characterized by an increased risk of premature atherosclerosis. The main mechanisms responsible for cardiovascular impairment are: endothelial cell injury induced by antiendothelial antibodies, ischemia/reperfusion damage, immune-mediated cytotoxicity and an impaired vascular repair mechanism (Figure 5)33. The excess of the oxidative phenomena causes the release of proinflammatory cytokines, activation and damage of endothelium and inflammation in the vascular wall34,35. Studies highlighted an increased stiffness in the carotid artery wall36 and showed the positive role of statins in the treatment of vascular damage33. Systemic vasculitis of small, medium or large blood vessels is a chronic infl ammatory disease in the rheumatic
disorders subgroup and is characterized by accelerated atherosclerosis and an increased premature mortality rate due to cardiovascular events. Vascular injury has multiple mechanisms such as, activation of endothelial cells which causes increased expression of monocyte adhesion molecules and autoantigens, accumulation in an increased number of oxidized low density lipoprotein (oxLDL) molecules and the formation of foam cells37,38. In patients with Takayasu Arteritis, studies highlighted an important infl ammation in the endothelial layer and a distorted arterial anatomy which led to a disturbed arterial blood fl ow39. Also, this giant arteritis shows early accelerated atherosclerosis, increased aortic stiffness and a high prevalence of silent myocardial infarction40,41. Regarding ANCA-associated vasculitis, a very important role is played by specific antibodies which can activate neutrophils which can result in the formation of reactive oxygen species that determine increased aortic stiffness and dysfunction of the endothelium42-44. Concerning Spondylarthropaties, especially Ankylosing Spondilitis (SA) and Psoriatic Arthritis (PsA), systemic infl ammation remains the most important cardiovascular risk factor for developing a cardiac event. In AS patients, aortic regurgitation and aortic disease, was found but could not be correlated with the progression of AS45. The genetic background represented by the HLA-B27 antigen increases the risk of a first-degree atrioventricular block46. Regarding PsA, we can find accelerated atherosclerosis and arterial dysfunction due to prolonged inflammation leading to an increased secretion of Th1 cytokines, to the formation of foam cells or to endothelial dysfunction47,48. Also, it has been revealed that persistently elevated erythrocyte sedimentation rate (ESR) is associated with the extension of atherosclerotic lesions49. PsA is also characterized
by increased arterial stiffness through: reduced levels of endothelial progenitor cells, oxidative stress, a decreased release of nitric oxide, advanced glycation end products or increased activation of lytic enzymes such as matrix metalloproteinases50,51. Also, the presence of psoriatic skin lesions is associated with an increased cardiovascular risk.
Figure 4. Factors that increase the cardiovascular risk in RA patients.
Figure 5. Mechanisms responsible for cardiovascular impairment in SS.
Pertaining to the treatment of rheumatic diseases, the use of synthetic modifying antirheumatic drugs, mainly Methotrexate, can reduce mortality and cardiovascular risk52. It decreases carotid thickening (intimamedia thickness) and improves endothelial vasodilatation after one year of treatment53. Concerning biological therapy, particularly anti tumor necrosis factor (TNF) antibodies, clinical trials revealed that their use reduces aortic stiffness54, improves endothelial function55, decreased the risk of stoke, myocardial infarction and heart failure56. Symptomatic therapy used for pain relief and for improving the patient’s quality of life (non-steroidal antiinfl ammatory drugs or corticotherapy) is closely linked to the appearance of cardiovascular
complications, being a dose-dependent risk26, 57. In the treatment of such patients, the benefits and risks associated with specific drugs should be assessed. Regarding hydroxychloroquine in SLE patients, it is well known that it can improve the cardiovascular outcome due to its antithrombotic, hypolipidemic, hypoglycemic and immunomodulatory effects. This drug has the possibility
to decrease Toll-like receptor activation and the systemic infl ammation58-61. Methotrexate, in RA, can reduce mortality with approximately 70% due to the decrease of inflammation and cardiovascular events (a decline up to 21%)62,63. Studies highlighted that methotrexate favors the efflux of cholesterol from macrophages thereby decreasing the formation of foam cells and improving the dyslipidaemic profile64. The use of corticosteroids increases the risk of cardiovascular events up to 47%65. This can be explained by the effects on blood pressure, on lipid and glucose metabolisms, on body mass index66, even though they are used for their antiproliferative and antiinflammatory effects. Both NSAIDs and steroids can cause a dose-dependent risk of cardiovascular manifestations. Among NSAIDs, due to the antithrombotic outcome, naproxen is considered to have the best cardiovascular profile67. Patients diagnosed with inflammatory rheumatic diseases require special attention from both rheumatologists and cardiologists, due to the high cardiovascular risk associated with these diseases. So far, we don’t have special methods of active screening in order to prevent cardiovascular events in rheumatic diseases. Depending on the case, we can use low doses of aspirin for an anti-platelet effect or statins to improvethe lipid profile. The RORA-AS statin intervention study demonstrated that rosuvastatin can improve endothelial function in patients with infl ammatory joint diseases, leading to a decreased mortality and lowering adverse cardiac events68. In conclusion, we strongly support that inflammatory rheumatic diseases have an important role in the development of atherosclerotic cardiovascular disorders and ischemic events due to the presence of systemic infl ammation and immune hyperactivity. The rheumatologic patient should be considered as having an elevated cardiovascular risk which requires a well rounded approach in the management of the disease; the treatment pertaining to the patient’s comorbidities. A proper and effective collaboration between a cardiologist
and a rheumatologist is necessary for optimal treatment of these patients.
Conflict of interests: none declared.
References
1. Full LE, Ruisanchez C, Monaco C. The inextricable link between atherosclerosis and prototypical inflammatory diseases rheumatoid arthritis and systemic lupus erythematosus. Arthritis Res. Ther. 2009; 11:217.
2. Şerban M, Jurcuţ R, Cosmin M, Bucşă A, Jurcuţ C, Ginghină C. Afectare aterosclerotică la o pacientă cu poliartrită reumatoidă – Inflamaţia şi riscul cardiovascular. Revista Română de Cardiologie. Vol. XXIII, Nr. 2, 2008; 157-161.
3. Bartoloni E, Shoenfeld Y, Gerli R. Inflammatory and autoimmune mechanisms in the induction of atherosclerotic damage in systemic rheumatic diseases: two faces of the same coin. Arthritis Care Res. (Hoboken). 2011; 63:178–183.
4. Montecucco F, Mach F. Common inflammatory mediators orchestrate pathophysiological processes in rheumatoid arthritis and atherosclerosis. Rheumatology (Oxford). 2009; 48:11–22.
5. Breland UM, et al. Infl ammatory markers in patients with coronary artery disease with and without inflammatory rheumatic disease. Rheumatology (Oxford). 2010; 49:1118–1127.
6. Kotani T, et al. Serum levels of matrix metalloproteinase (MMP) 9, a risk factor for acute coronary syndrome, are reduced independently of serum MMP-3 by anti-TNF-alpha antibody (infliximab) therapy in patients with rheumatoid arthritis. J. Pharmacol. Sci. 2012; 120:50–53.
7. Lai MH, et al. Effect of abdominal acupuncture therapy on the endocrine and metabolism in obesity-type polycystic ovarian syndrome patients [Chinese]. Zhen Ci Yan Jiu. 2010; 35:298–302.
8. van der Wal AC, et al. Recent activation of the plaque immune response in coronary lesions underlying acute coronary syndromes. Heart. 1998; 80:14–18.
9. Libby P, Okamoto Y, Rocha VZ, Folco E. Infl ammation in atherosclerosis: transition from theory to practice. Circ. J. 2010; 74:213–220.
10. Symmons DP, Gabriel SE. Epidemiology of CVD in rheumatic disease, with a focus on RA and SLE. Nat. Rev. Rheumatol. 2011; 7:399–408.
11. Dregan A, Charlton J, Chowienczyk P, Gulliford MC. Chronic inflammatory disorders and risk of type 2 diabetes mellitus, coronary heart disease, and stroke: a population-based cohort study. Circulation 2014;130:837–844.
12. Soto ME, Melendez-Ramirez G, Kimura-Hayama E, Meave-Gonzalez A, Achenbach S, Herrera MC, Guering EL, Alexanderson-Rosas E, Reyes PA. Coronary CT angiography in Takayasu arteritis. JACC Cardiovasc Imaging 2011;4:958–966.
13. Tervaert JWC. Cardiovascular disease due to accelerated atherosclerosis in systemic vasculitides. Best Prac Res Clin Rheum 2013;27: 33–44.
14. Nossent J, Cikes N, Kiss E, Marchesoni A, Nassonova V, Mosca M, Olesinska M, Pokorny G, Rozman B, Schneider M, Vlachoyiannopoulos PG, Swaak A. Current causes of death in systemic lupus erythematosus in Europe, 2000–2004: relation to disease activity and damage accrual. Lupus 2007;16:309–317.
15. Kahlenberg JM, Kaplan MJ. Mechanisms of premature atherosclerosis in rheumatoid arthritis and lupus. Annu Rev Med 2013;64:249–263.
16. Bruce IN, Burns RJ, Gladman DD, Urowitz MB. Single photon emission computed tomography dual isotope myocardial perfusion imaging in women with systemic lupus erythematosus. I. Prevalence and distribution of abnormalities. J Rheumatol 2000;27:2372–2377.
17. El-Magadmi M, Bodill H, Ahmad Y, Durrington PN, Mackness M, Walker M, Bernstein RM, Bruce IN. Systemic lupus erythematosus: an independent risk factor for endothelial dysfunction in women. Circulation 2004;110:399–404.
18. Roman MJ, Shanker BA, Davis A, Lockshin MD, Sammaritano L, Simantov R, Crow MK, Schwartz JE, Paget SA, Devereux RB, Salmon JE. Prevalence and correlates of accelerated atherosclerosis in systemic lupus erythematosus. N Engl J Med 2003;349:2399–2406.
19. Manzi S, Meilahn EN, Rairie JE, Conte CG, Medsger TA Jr, Jansen-McWilliams L, D’Agostino RB, Kuller LH. Age-specific incidence rates of myocardial infarction and angina in women with systemic lupus erythematosus: comparison with the Framingham Study. Am J Epidemiol 1997;145:408–415.
20. Magder LS, Petri M. Incidence of and risk factors for adverse cardiovascular events among patients with systemic lupus erythematosus. Am J Epidemiol 2012;176: 708–719.
21. Skaggs BJ, Hahn BH, McMahon M. Accelerated atherosclerosis in patients with SLE-mechanisms and management. Nat Rev Rheumatol 2012;8:214–223.
22. Van Doornum S, McColl G, Wicks IP. Accelerated atherosclerosis: an extraarticular feature of rheumatoid arthritis? Arthritis Rheum. 2002; 46:862–873.
23. Michaud K, Vera-Llonch M, Oster G. Mortality risk by functional status and health-related quality of life in patients with rheumatoid arthritis. J. Rheumatol. 2012; 39:54–59.
24. del Rincon ID, Williams K, Stern MP, Freeman GL, Escalante A. High incidence of cardiovascular events in a rheumatoid arthritis cohort not explained by traditional cardiac risk factors. Arthritis Rheum. 2001; 44:2737–2745.
25. Maradit-Kremers H, et al. Increased unrecognized coronary heart disease and sudden deaths in rheumatoid arthritis: a population-based cohort study. Arthritis Rheum. 2005; 52:402–411.
26. Gabriel SE. Cardiovascular morbidity and mortality in rheumatoid arthritis. Am J Med 2008;121:S9–S14.
27. Goodson NJ, Wiles NJ, Lunt M, Barrett EM, Silman AJ, Symmons DP. Mortality in early inflammatory polyarthritis: cardiovascular mortality is increased in seropositive patients. Arthritis Rheum 2002;46:2010– 2019.
28. Elena Rezus, Alexandra Burlui, Georgiana Stamate. Rheumatoid Arthritis in “Essential Rheumatology for Trainees and Medical Students”. Coordonator Conf. Univ. Dr. Elena Rezus. ed. “Gr. T. Popa” Iasi 2016;41-50.
29. Herbrig K,Haensel S, Oelschlaegel U, Pistrosch F, Foerster S, Passauer J. Endothelial dysfunction in patients with rheumatoid arthritis is associated with a reduced number and impaired function of endothelial progenitor cells. Ann Rheum Dis 2006;65:157–163.
30. Bergholm R, Leirisalo-Repo M, Vehkavaara S, Makimattila S, Taskinen MR, Yki-Jarvinen H. Impaired responsiveness to NO in newly diagnosed patients with rheumatoid arthritis. Arterioscler Thromb Vasc Biol 2002;22:1637–1641.
31. Semb AG, Rollefstad S, Provan SA, Kvien TK, Stranden E, Olsen IC, Hisdal J. Carotid plaque characteristics and disease activity in rheumatoid arthritis. J Rheumatol 2013; 40:359–368.
32. Tanasescu C, Jurcut C, Jurcut R, Ginghina C. Vascular disease in rheumatoid arthritis: from subclinical lesions to cardiovascular risk. Eur J Intern Med. 2009 Jul;20(4):348-54.
33. Shoenfeld Y, et al. Accelerated atherosclerosis in autoimmune rheumatic diseases. Circulation. 2005; 112:3337–3347.
34. Sattar N, et al. Are markers of infl ammation more strongly associated with risk for fatal than for nonfatal vascular events? PLoS Med. 2009; 6:e1000099.
35. Simonini G, et al. Oxidative stress in systemic sclerosis. Mol. Cell. Biochem. 1999; 196:85–91.
36. Cheng KS, et al. Carotid and femoral arterial wall mechanics in scleroderma. Rheumatology (Oxford). 2003; 42:1299–1305.
37. Wick G, et al. Atherosclerosis, autoimmunity, and vascular-associated lymphoid tissue. FASEB J. 1997; 11:1199–1207.
38. Libby P. Pathophysiology of vasculitis. In: Creager MA, Beckman JA, Loscalzo J, eds. Vascular Medicine: A companion to Braunwald’s Heart Disease. 2nd ed. 2012. p126–132.
39. Mason JC. Takayasu arteritis – advances in diagnosis and management. Nat Rev Rheumatol 2010;6:406–415.
40. Ng WF, Fantin F, Ng C, Dockery F, Schiff R, Davies KA, Rajkumar C, Mason JC. Takayasu’s arteritis: a cause of prolonged arterial stiffness. Rheumatology (Oxford) 2006;45:741–745.
41. Keenan NG, Mason JC, Maceira A, Assomull R, O’Hanlon R, Chan C, Roughton M, Andrews J, Gatehouse PD, Firmin DN, Pennell DJ. Integrated cardiac and vascular assessment in Takayasu arteritis by cardiovascular magnetic resonance. Arthritis Rheum 2009;60:3501–3509.
42. Jennette JC, Falk RJ, Hu P, Xiao H. Pathogenesis of antineutrophil cytoplasmic autoantibody-associated small-vessel vasculitis. Annu Rev Pathol 2013;8:139–160.
43. Booth AD, Jayne DRW, Kharbanda RK, McEniery CM, Mackenzie IS, Brown J, Wilkinson IB. Infl iximab improves endothelial dysfunction in systemic vasculitis: a model of vascular infl ammation. Circulation 2004;109:1718–1723.
44. Booth AD,Wallace S, McEniery CM, Yasmin, Brown J, Jayne DRW, Wilkinson IB. Infl ammation and arterial stiffness in systemic vasculitis. A model of inflammation. Arthritis Rheum 2004;50:581–588.
45. Roldan CA, Chavez J, Wiest PW, Qualls CR, Crawford MH. Aortic root disease and valve disease associated with ankylosing spondylitis. J. Am. Coll. Cardiol. 1998; 32:1397–1404.
46. Bergfeldt L, Vallin H, Edhag O. Complete heart block in HLA B27 associated disease. Electrophysiological and clinical characteristics. Br. Heart J. 1984; 51:184–188.
47. Park S, Lakatta EG. Role of infl ammation in the pathogenesis of arterial stiffness. Yonsei Med J. 2012;53:258–61.
48. Hansson GK. Inflammation, atherosclerosis, and coronary artery disease. N Engl J Med. 2005;352:1685–95.
49. Eder L, Thavaneswaran A, Chandran V, Cook R, Gladman DD. Increased burden of infl ammation over time is associated with the extent of atherosclerotic plaques in patients with psoriatic arthritis. Ann Rheum Dis. 2014. doi: 10.1136/annrheumdis-2014-205267.
50. Westerweel PE, Verhaar MC. Endothelial progenitor cell dysfunction in rheumatic disease. Nat Rev Rheumatol. 2009;5:332–40.
51. Zieman SJ, Melenovsky V, Kass DA. Mechanisms, pathophysiology and therapy of arterial stiffness. Arterioscler Thromb Vasc Biol. 2005;25: 932–43.
52. Micha R, Imamura F, Wyler von Ballmoos M, Solomon DH, Hernan MA, Ridker PM, Mozaffarian D. Systematic review and meta-analysis of methotrexate use and risk of cardiovascular disease. Am J Cardiol 2011;108:1362–1370.
53. Guin A, Chatterjee Adhikari M, Chakraborty S, Sinhamahapatra P, Ghosh A. Effects of disease modifying anti-rheumatic drugs on subclinical atherosclerosis and endothelial dysfunction which has been detected in early rheumatoid arthritis: 1-year follow-up study. Semin. Arthritis Rheum. 2013; 43:48–54
54. Angel K, Provan SA, Fagerhol MK, Mowinckel P, Kvien TK, Atar D. Effect of 1-year anti-TNF-alpha therapy on aortic stiffness, carotid atherosclerosis, and calprotectin in inflammatory arthropathies: a controlled study. Am J Hypertens 2012;25: 644–650.
55. Hurlimann D, Forster A, Noll G, Enseleit F, Chenevard R, Distler O, Bechir M, Spieker LE, Neidhart M, Michel BA, Gay RE, Luscher TF, Gay S, Ruschitzka F. Antitumor necrosis factor-alpha treatment improves endothelial function in patients with rheumatoid arthritis. Circulation 2002;106:2184–2187.
56. Barnabe C, Martin BJ, GhaliWA. Systematic review and meta-analysis: anti-tumor necrosis factor alpha therapy and cardiovascular events in rheumatoid arthritis. Arthritis Care Res (Hoboken) 2011;63:522–529.
57. Lindhardsen J, Gislason GH, Jacobsen S, Ahlehoff O, Olsen AM, Madsen OR, Torp-Pedersen C, Hansen PR. Non-steroidal anti-inflammatory drugs and risk of cardiovascular disease in patients with rheumatoid arthritis: a nationwide cohort study. Ann Rheum Dis 2014;73:1515–1521.
58. Costedoat-Chalumeau N, Dunogué B, Morel N, Le Guern V, Guettrot- Imbert G. Hydroxychloroquine: a multifaceted treatment in lupus. Presse Med 2014; 43: 167-80.
59. Olsen NJ, Schleich MA, Karp DR. Multifaceted effects of hydroxychloroquine in human disease. Semin Arthritis Rheum 2013; 43: 264-72.
60. Wallace DJ, Gudsoorkar VS, Weisman MH, Venuturupalli SR. New insights into mechanisms of therapeutic effects of antimalarial agents in SLE. Nat Rev Rheumatol 2012; 8 (9): 522-33.
61. Petri M. Hydroxychloroquine use in the Baltimore Lupus Cohort: effects on lipids, glucose and thrombosis. Lupus 1996; 5 Suppl 1: S16- 22.
62. Wasko MC, Dasgupta A, Hubert H, et al. Propensity-adjusted association of methotrexate with overall survival in rheumatoid arthritis. Arthritis Rheum 2013;65:334–42.
63. Micha R, Imamura F, Wyler von Ballmoos M, et al. Systematic review and meta-analysis of methotrexate use and risk of cardiovascular disease. Am J Cardiol 2011;108:1362–70.
64. Robertson J, Peters MJ, McInnes IB, Sattar N. Changes in lipid levels with inflammation and therapy in RA: a maturing paradigm. Nat Rev Rheumatol 2013;9:513–523.
65. Wei L, MacDonald TM, Walker BR. Taking glucocorticoids by prescription is associated with subsequent cardiovascular disease. Ann Intern Med 2004;141:764–70.
66. Panoulas VF, Douglas KM, Stavropoulos-Kalinoglou A, et al. Long-term exposure to medium-dose glucocorticoid therapy associates with hypertension in patients with rheumatoid arthritis. Rheumatology (Oxford) 2008;47:72–5.
67. Trelle S, Reichenbach S,Wandel S, Hildebrand P, Tschannen B, Villiger PM, Egger M, Juni P. Cardiovascular safety of non-steroidal anti-inflammatory drugs: network meta-analysis. BMJ 2011;342:c7086.
68. Ikdahl et al. Rosuvastatin improves endothelial function in patients with inflammatory joint diseases, longitudinal associations with atherosclerosis and arteriosclerosis: results from the RORA-AS statin intervention study. Arthritis Research & Therapy (2015) 17:279.

 This work is licensed under a
This work is licensed under a