Adela Serban1,2, Nicoleta Batrana1, Adrian Molnar1,2, Emese Kovacs1
1 „Niculae Stăncioiu” Heart Institute, Cluj-Napoca, Romania
2 „Iuliu-Hatieganu” University of Medicine and Pharmacy, Cluj-Napoca, Romania
Abstract: Introduction – Papillary fibroelastoma is the most common cardiac tumor with valvular localization, involving more frequently the aortic or mitral valve and much rarely the tricuspid or pulmonary valve. It is the second most common primary cardiac tumor, after myxoma. Many patients are asymptomatic, but the tumor represents a potential source of embolism which explains its clinical importance. Case presentation – We report the case of a 60-year-old woman with nonspecific symptoms in whom a mass attached to the tricuspid valve was found during a routine transthoracic echocar-diography, as part of her cardiac evaluation for an elective orthopedic surgical procedure. Multimodality imaging including transesophageal echocardiography and cardiac MRI was afterwards performed. Since the patient had a high global risk of pulmonary embolism, the tumor was surgically removed with preservation of the tricuspid valve and an uneventful posto-perative course. The histopathological examination confirmed the diagnosis of papillary fibroelastoma. Conclusion – This case emphasizes the importance of echocardiography as a first-line imaging modality in the diagnosis of cardiac tumors. The embolic risk for papillary fibroelastomas is high and even if the discovery of the tumor is incidental, excision is advisable as surgery has excellent long-term results.
Keywords: cardiac tumor, embolism, papillary fibroelastoma, echocardiography
INTRODUCTION
Primary cardiac tumors are rare. Their incidence is 0.1% on pathological studies, significantly lower com-pared to secondary cardiac tumors (metastases) that affect the heart 20 times more frequently1. Cardiac tumors can be symptomatic or may be discovered incidentally by various imaging techniques (echocardi-ography, MRI, CT) during a routine evaluation. Symp-toms are nonspecifi c and may mimic other cardiovas-cular diseases1,2.
Most primary cardiac tumors are benign. 10% of them are represented by papillary fi broelastoma, whi-ch is the second most common primary cardiac tumor in adults, after myxoma, but the most common tu-mor with valvular localization, accounting for 85% of cardiac valvular tumors3,4. Papillary fi broelastoma can occur at any age but is most commonly seen after the fourth decade of life, usually the diagnosis is made at an average age of 60 years. It can be found everywhere on the endothelial surface, but in 80% of the cases is situated on the valves, more frequently on the valves of the left heart. The location on the tricuspid valve is relatively rare and according to literature involves 17% of the cases3,4,5.
Papillary fi broelastoma can cause symptoms, but up to a third of patients are asymptomatic and the tumor is an incidental finding. The clinical manifesta-tions depend on the location of the tumor and most commonly symptoms are due to embolic events; only in rare cases symptoms are caused by interference of the mass with cardiac function. The papillary fi broelas-tomas of the right heart are usually asymptomatic, but sometimes, as they may generate tricuspid regurgitati-on, arrhythmias, intermittent obstruction of the right ventricular outflow tract or pulmonary embolism, they can cause chest discomfort, exertional dyspnea, palpi-tations, syncope, right heart failure, sudden death3,6,7.
We present a case of papillary fi broelastoma of the tricuspid valve in a woman admitted to our medical centre in whom the tumor was diagnosed during pre-operative cardiac evaluation for a noncardiac surgical procedure.
CASE PRESENTATION
A 60- year-old woman was reffered to our hospital for cardiological assessment prior to undergoing an electi-ve orthopedic surgery (bilateral hip arthroplasty). The patient had multiple cardiovascular risk factors (active smoking, hypertension, dyslipidemia). She complained of palpitations and exertional dyspnea. Her exercise capacity was also severely limited by the coexistence of the orthopedic pathology. No significant abnormali-ties were found on the physical examination.
As underlined by the guidelines, because the pati-ent was symptomatic we started the evaluation of her perioperative risk with the performance of a 12-lead ECG and a transthoracic echocardiography. The main focus was the assessment of the left ventricular func-tion, as left ventricular systolic dysfunction represents the most important predictor of major cardiac events in patients undergoing noncardiac surgery. The ECG was normal and contrary to initial expectations, tran-sthoracic echocardiography did not reveal any signi-ficant changes in the left heart (the left ventricle was mildly hypertrophied with normal ejection fraction), but described instead the presence of an 13/14 mm-size homogeneous echogenic mass on the anterior leaflet of the tricuspid valve; the mass had gelatinous structure, was pedunculated, mobile and did not cause valvular dysfunction (Figures 1, 2). At this point, the differential diagnosis between a cardiac tumor and valvular vegetation was taken into consideration, but in the absence of clinical and biolo-gical evidence of infection, we thought that the descri-bed mass most likely represented a primary cardiac tumor.
For a more comprehensive and accurate assess-ment, multimodality imaging was performed. The tran-sesophageal echocardiography confi rmed the feature of the mass described transthoracically and highlighted a mild tricuspid regurgitation, unrelated to the presen-ce of the tumor on the valve as the cooptation of the tricuspid leaflets was not influenced by the punctiform insertion of the tumor’s pedicle (Figure 3). We con-tinued the evaluation with MRI which offers in a sin-gle examination the assessment of morphology, ana-tomy and functional impact of a cardiac tumor, with a unique ability of tissue characterization, compared to 3D-Echo and cardiac CT. The contrast-enhanced MRI described on the anterior leaflet of the tricuspid valve a 10/6 mm well defi ned nodular structure, with intermediate signal in the T1- and T2- weighted sequences and intense enhancement in the delayed phase. The mass was located at the free edge of the valve, on its atrial side, was mobile with no impact on the closure or opening of the valve (Figures 4, 5). The characteris-tics of the tumor were suggestive of papillary fi broe-lastoma. There were no signs of pulmonary embolism (D-dimer levels were normal, there were no clinical, ECG or echocardiographic signs suggestive of pulmo-nary embolism and no thrombus was described in the main pulmonary arteries on MRI). The patient had a high global risk of pulmonary em-bolism, determined on the one hand by the orthope-dic pathology (arthroplasty of the lower limb requi-ring prolonged bed rest) and on the other hand by the intrinsic embolic risk of the tumor (mobile pedun-culated mass around 1 cm-size localized on the free edge of the leaflet) combined with the possibility of thrombus formation on the surface of the tumor. For these reasons we recommended surgical removal. No coronary artery disease was found on the preoperati-ve coronary angiography.
The excision of the mass was performed followed by the repair of the anterior leaflet of the tricuspid valve (Figure 6). At the macroscopical histopatholo-gical examination, the tumor measured 1.3/1 cm and resembled a sea-anemone after its placing in saline so-lution (Figure7). Microscopically the tumor was com-posed of multiple dense, hyaline branches formed by crowded elastic laminae; at the surface the branches were covered by CD 31-positive endothelial cells and there was a reduced infl ammatory infi ltrate composed of lymphocytes, plasmocytes, rare neutrophils locali-zed at the implantation base of the tumor (Figure 8 and 9). Histological and immunohistochemical charac-teristics confirm the diagnosis of papillary fibroelas-toma. The postoperative course was uneventful. Tran-sthoracic echocardiography described a normal tri-cuspid valve and a small intrapericardial hematoma lo-cated next to the right atrium with no hemodynamic impact (Figure 10) which disappeared on follow-up echocardiographic exams. Clinical outcome was favo-rable, so the patient was discharged 14 days later. The patient is scheduled for echocardiographic reevaluation 6 months later and annually afterwards.
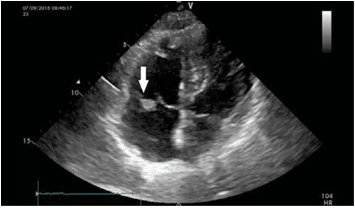
Figure 1. The transthoracic echocardiography (apical 4 chambers view) identifies an echo dense mass (arrow), on the anterior cusp of the tricuspid valve.
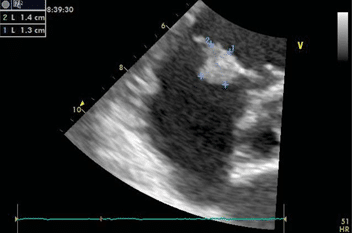
Figure 2. Transthoracic echocardiography (parasternal short axis view) with measuring of the echo dense mass, sized 13/14 mm.
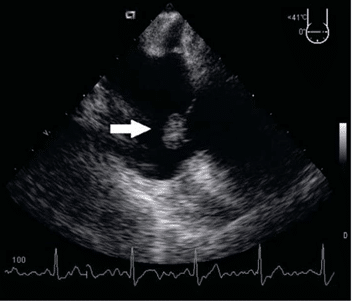
Figure 3. Transesophageal echocardiogram: the mass (arrow) was round, with well-demarcated borders, echo dense, with a homogenous structure, pedunculated, features typical of a papillary fi broelastoma. It was confirmed the presence of the tumor on the anterior cusp of the tricuspid valve.
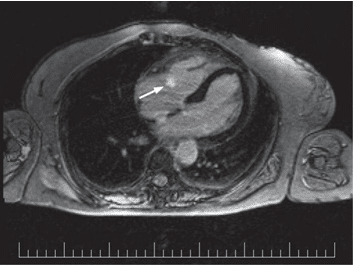
Figure 4. MR examination, T1-weighted sequence with phase sensitive in-version recovery (PSIR) in transversal view: one endocavitary mass (arrow) can be identified on the tricuspid valve, small, well-outlined lesion
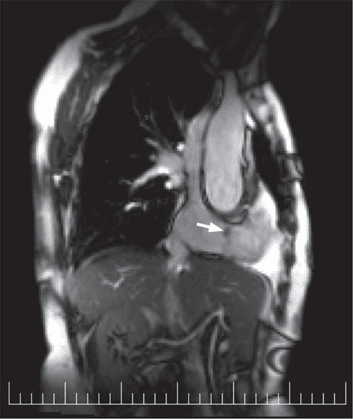
Figure 5. MR examination, T2-weighted sequence in sagittal view: papillary fibroelastoma (arrow) originating in the anterior cusp of the tricuspid valve.
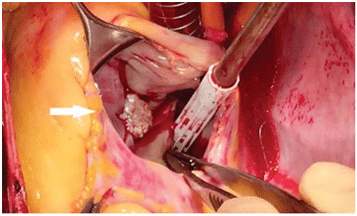
Figure 6. Intraoperative finding shows the pedunculated mass and with multiple papillary fronds (arrow) attached to the anterior cusp of the tri-cuspid valve.

Figure 7. Gross view of the tumor with characteristic frond-like appear-ance and resemblance to a sea anemone, after immersion in saline solution.
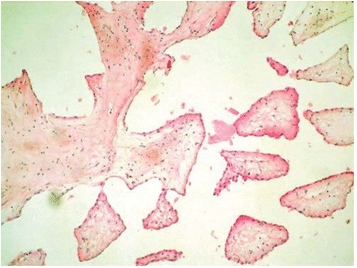
Figure 8. The histological specimen of the papillary fibroelastoma (he-matoxilin-eosin stain, magnification x10): multiple dense, hyaline branches covered by endothelial cells.
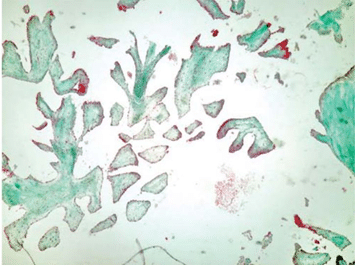
Figure 9. The tumor was histologically diagnosed as a papillary fibroelas-toma covered with CD31-positive endothelial cells (immunohistochemis-try CD31 staining, magnifi cation x10).
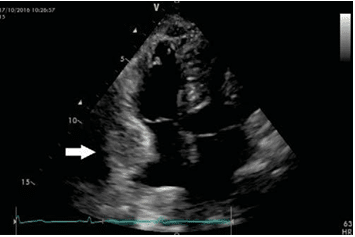
Figure 10. Postoperative transthoracic echocardiogram (apical 4 cham-bers view): tricuspid valve with normal appearance, pericardial effusion lo-cated next to the right atrium (arrow).
DISCUSSIONS
Papillary fibroelastoma was fi rst described by Yater in 1931. It is an avascular structure composed of dense connective tissue lined by endocardium. It is the most common primary tumor of the heart located on the valves, mostly involving the aortic (29% of cases) and the mitral valve (25% of cases), rarely the tricuspid valve (17% of cases) and the pulmonary valve (13% of cases). Its size may range from a few mm up to 5 cm, the average size is 1 cm, as was the case in our pati-ent. It is usually a solitary tumor (90% of cases), but occasionally can have multiple location, on the same valve, on multiple valves or on any endocardial sur-face (mural endocardium, papillary muscles, chordae tendineae)4,8. Papillary fibroelastoma rarely causes sig-nifi cant valvular dysfunction, but it is a potential sour-ce of embolism which explains its clinical importance. Emboli may come directly from the tumor, but more commonly from thrombus attached to its surface. The tumor itself is slow growing, but can be a very good substrate for deposition of a large quantity of throm-botic material in a relatively short period of time and can cause life threatening major embolic complicati-ons. In our case, this was the main reason we conside-red it necessary to excise the tumor9,10.
The surgical treatment of symptomatic papillary fibroelastoma is widely accepted in literature, but asymptomatic tumor excision, especially for those lo-cated in the right heart, is controversial11,12,13. Once discovered, many authors recommend excision of left-sided fibroelastomas, regardless of tumor charac-teristics (size, mobility), especially if the patient is a good surgical candidate (Society of Thoracic Surgeons score <1%), because there is no imagistic characteris-tic based on which the embolic risk of the tumor can be determined with certainty (although, according to some observations, the embolic risk is higher for lar-ge, mobile and pediculated tumors)4,14,15. This attitude is supported by the fact that the surgery in experien-ced centers has excellent results, with high likelihood of valve preservation (in 98% of cases)14,16. The recur-rence rate of the tumor is small (1.6%), but it is not null as reported in previous studies and patients re-quire lifetime echocardiographic follow-up14. Surgical excision of asymptomatic right-sided fibroelastomas is not unanimously recommended in the literature. We believe that the decision should be individualized for each case, taking into account the global embolic risk (which was considered high for our patient), the sur-gical risk and not least the patient’s preferences1,11,12,13. If non-surgical approach is chosen, some authors re-commend treatment with anticoagulant or antiplatelet agents in order to lower the risk of thrombotic ma-terial overlay on the surface of the tumor and con-sequently its embolic potential14.
Finally we would like to emphasize the importance of echocardiography as a first-line imaging modality in the diagnosis of cardiac tumors. Echocardiography can provide valuable information on tumor location and characteristics (size, shape, mobility), on its he-modynamic impact and can guide to a certain point the differential diagnosis (for papillary fibroelastomas the differential diagnosis should include valvular vege-tations, Lambl’s excrescence, valvular strands, other types of valvular tumors, such as myxomas with valve location,)10,17,18,19. The sensitivity of echocardiography for detecting papillary fibroelastomas is 62% for the transthoracic examination and 77% for the transeso-phageal examination8,20, explained by the fact that the tumor is generally small and may go unnoticed, espe-cially at a superfi cial examination or in patients with poor acoustic windows. In the case of our patient, the echocardiographic finding of a papillary fibroelasto-ma changed the entire initial therapeutic strategy and surgical removal of the heart tumor was considered necessary before the surgical treatment of orthopedic pathology.
CONCLUSIONS
The papillary fibroelastoma of the tricuspid valve may be an incidental fi nding during echocardiographic exa-mination performed for a completely different reason. Its embolic risk is high, so if the patient is not a high risk surgical patient, the surgical excision of the tumor is recommended as surgery has excellent long-term results.
Acknowledgements: none declared.
Funding: none declared.
Conflicts of interest: none declared.
References
1. Sydow K, Willems S, Reichenspurner H, Meinertz T. Papillary Fibro-elastomas of the Heart. Thorac Cardiov Surg 2008; 56: 9–13.
2. Ibrahim S, Patel N, Al-Saffar F, Touchan J. Coinciding anomalous cor-onary artery and papillary fibroelastoma. J Geriatr Cardiol 2016; 13: 924-926.
3. Li W, Zheng J, Zhao H, Xu H, Ni Y. Beating-heart surgical treatment of tricuspid valve papillary fibroelastoma. Medicine 2016; 95(34): e4690. doi:10.1097/MD.0000000000004690.
4. Gowda RM, Khan IA, Nair CK, Mehta NJ, Vasavada BC, Sacchi TJ. Cardiac papillary fibroelastoma: A comprehensive analysis of 725 cases. Am Heart J, 2003; 146(3): 404-410.
5. Parthenakis F, Nyktari E, Patrianakos A, Pitsis A, Asimaki A, Vardas P. Asymptomatic papillary fibroelastoma of the aortiv valve in a young woman – a case report. Cardiovasc Ultrasound 2009; 7:43.
6. Yandrapalli S, Mehta B, Montal P, Gupta T, Khattar P, Fallon J, Gold-berg R, Sule S, Aronow WS. Cardiac papillary fibroelastoma: The need for a timely diagnosis. World J Clin Cases 2017; 5(1):9-13.
7. Rohani A, Bigdelu L, Nezafati M, Akbari V. Three-dimensional echo-cardiography of a tricuspid valve papillary fibroelastoma. J Saudi Heart Assoc 2017; 29:57-59.
8. Sun JP, Asher CR, Yang XS, Cheng GG, Scalia GM, Massed AG, Grif-fin BP, Ratliff NB, Stewart WJ, Thomas JD. Clinical and Echocardiographic Characteristics of Papillary Fibroelastomas: a retrospective and prospective study in 162 patients. Circulation 2001;103:2687-2693.
9. Baikoussis NG, Dedeilias P, Argiriou M, Argiriou O, Vourlakou C, Prapa E, Charitos C. Cardiac papillary fibroelastoma; when, how, why?. Ann Card Anaesth 2016; 19:162-165.
10. O’Laughlin JP, Verma G, Gulkarov I. Cardiac fi broelastoma versus thrombus: echocardiographic evidence can be misleading. Case Rep Cardiol 2016; Article ID 2896056. doi:10.1155/2016/2896056.
11. Anastacio MM, Moon MR, Damiano RJ Jr, Pasque MK, Maniar HS, Lawton JS. Surgical experience with cardiac papillary fi broelastoma over a 15-year period. Ann Thorac Surg 2012; 94(2): 537-41.
12. Abu Saleh WK, Al Jabbari O, Ramlawi B, Reardon MJ. Cardiac papil-lary fibroelastoma: Single-institution experience with 14 surgical pa-tients. Tex Heart Inst J, 2016; 43(2): 148-51.
13. Elbardissi AW, Dearani JA, Daly RC, Mullany CJ, Orszulak TA, Puga FJ, Schaff HV. Survival after resection of primary cardiac tumors: A 48-year experience. Circulation, 2008; 118(14 Suppl.): S7-15.
14. Tamin SS, Maleszewski JJ, Scott CG, Khan SK, Edwards WD, Bruce CJ, Oh JK, Pellikka PA, Klarich KW. Prognostic and bioepidemiolog-ic implications of papillary fibroelastomas. J Am Coll Cardiol 2015; 65(22):2420-2429.
15. Mkalaluh S, Szczechowicz M, Torabi S, Dib B, Sabashnikov A, Mash-hour A, Karck M, Weymann A. Surgery for Cardiac Papillary Fibro-elastoma: A 12-Year Single Institution Experience. Med Sci Monit Basic Res 2017;23:258-263.
16. Ngaage DL, Mullany CJ, Daly RC, Dearani JA, Edwards WD, Taze-laar HD, McGregor CG, Orszulak TA, Puga FJ, Schaff HV, Sundt TM 3rd, Zehr KJ. Surgical treatment of cardiac papillary fibroelastoma: a single center experience with eightyeight patients. Ann Thorac Surg 2005;80:1712-1718.
17. Choi KB, Kim HW, Kim DY, Jo KH, Choi HJ, Hong SB. Tricus-pid Papillary Fibroelastoma Mimicking Tricuspid Vegetation in a Pa-tient with Severe Neutropenia. Korean J Thorac Cardiovasc Surg 2016;49(3):195-198.
18. Kim M, Kim SH, Moon SY, Jeong EG, Jung EH, Nam HS, Choi JH, Park K. Native aortic valve thrombosis resembling papillary fibro-elastoma. J Cardiovasc Ultrasound 2014;22(3):148-150.
19. Prifti E, Ademaj F, Ikonomi M, Demiraj A. Papillary fibroelastoma of the anterior leaflet of the mitral valve mimicking vegetation. J Surg Case Rep 2015; 7:1-3.
20. Diaz Angulo C, Mendez Diaz C, Rodriguez Garcia E, Soler Fernan-dez R, Rois Siso A, Marini Diaz M. Imaging fi ndings in cardiac masses (Part I): Study protocol and benign tumors. Radiologia 2015;57:480-488.
 This work is licensed under a
This work is licensed under a