Simina Crisan1,2, Cristina Vacarescu2, Alina-Ramona Nicola1, Mihai-Andrei Lazar2, Dragos Cozma1,2, Cristian Mornos1,2, Lucian Petrescu1,2
1 “Victor Babes” University of Medicine and Pharmacy, Timisoara, Romania
2 Institute of Cardiovascular Diseases, Timisoara, Romania
Abstract: Improving the outcomes of patients with acute coronary syndromes has become a matter of great importance, since worldwide coronary artery disease is still the leading cause of morbidity and mortality. Beginning with the reperfusion era, the goal of STEMI treatment is to restore blood fl ow to ischemic myocardium in order to reduce infarct size, but beyond the benefit of reperfusion, an important number of patients develop reperfusion injury and the “no-reflow” phenomenon. Taking these data into account and also the fact that coronary microvascular dysfunction increases the risk of cardiovascular events, we consider that an additional strategy in order to improve the outcomes of STEMI patients could be represented by the assessment of coronary microvascular functional and structural obstruction. Along with some issues related to the pathogenesis of coronary microvascular obstruction, some diagnostic methods as well as a few therapeutic options are been presented.
Keywords: microcirculation, coronary microvascular obstruction, repefusion injury.
THE REPERFUSION INJURY AND THE “NO-REFLOW” PHENOMENON
Since worldwide coronary artery disease is still the leading cause of morbidity and mortality, improving the outcomes of patients with acute coronary syndromes is a matter of great importance1-2. The majority of deaths following acute coronary syndromes are determined by ST segment elevation myocardial infarctions (STEMI), the result of acute thrombotic occlusion of a coronary artery. Beginning with the reperfusion era, first with fibrinolytic therapy and currently with primary PCI as gold standard, the goal of STEMI treatment is to restore blood flow to ischemic myocardium in order to reduce infarct size. The great challenge is to reduce time to reperfusion and the management of STEMI patients is now dominated by a principle that has became a
fundamental one in coronary heart disease – “time is muscle”3. In practice, this principle is represented by a “door to balloon” time of 90 minutes, meaning the interval from the fi rst ECG showing ST elevation segment to mechanical reperfusion of the occluded coronary artery. Nowadays, the “door to balloon time” is a standard part of the European Society of Cardiology Guidelines
for the management of STEMI patients, often used as a measure of quality performance4. However, even in the setting of an acceptable “door to balloon” time, the in-hospital mortality of STEMI patients is an important one (~10%) and also, two thirds of the surviving ones tend to develop chronic heart failure5. These fi ndings suggest that additional strategies are needed in order to reduce in-hospital mortality and also in order to improve the outcome of STEMI patients. Also, it becomes clear that a review of the therapeutic objectives in the setting of an acute coronary
syndrome may be of great interest, and that is why, beyond coronary reperfusion with subsequent restoration of myocardial oxygen supply, alternative approaches in order to improve survival and to reduce the burden of ischaemic heart failure are needed. Reestablishing blood flow through epicardial coronary arteries after STEMI does not always lead to the end of myocardial damage, since an important number of patients develop reperfusion injury and necrosis of myocites from the infarcted area. Some of the unsolved issues related to the treatment of STEMI patients are represented by the reperfusion injury and the “no-reflow” phenomenon. The term “no-reflow” refers to inadequate myocardial perfusion after successful repermeabilization of epicardial infarct related artery. It is estimated that 10 to 40% of patients undergoing reperfusion therapy may present the angiographic feature of “slow-fl ow” or even “no-refl ow”, both associated with impaired outcomes. The “no-reflow” phenomenon may be determined by a number of pathogenical compounds, among which: distal atherothrombotic embolization, ischemic injury, reperfusion injury, endothelial dysfunction, infl ammation and myocardial oedema6. The presence of “no-reflow” may generate severe arrhythmia and important haemodynamic impaired function, with a great increase of clinical complications rate. Myocardial reperfusion was considered from the beginning of its use a “double-edged sword” due to the fact that, beyond restoration of blood fl ow to ischaemic myocardium, reperfusion also promotes cardiomyocyte death and microvascular damage through the so called “myocardial ischaemia reperfusion injury” process7. First, this process is determined by the fact that the occlusion of a coronary artery quickly determines uncoupled oxidative phosphorylation and reduced mitochondrial ATP synthesis. The decrease of ATP generation leads to the increase of intracellular calcium and lactate levels, with subsequent intracellular PH decrease. Beyond this, the reduction of ATP synthesis also determines the generation of reactive
oxygen species (ROS) through a process known as “ROS-induced ROS release”. Increased ROS levels may also lead to mitochondrial damage by opening a gap in the mitochondrial permeability transposition pore. Thus, reperfusion will indeed restore blood fl ow to the myocardium, but on the other hand it will also promote mitochondrial damage through the opening of the mitochondrial permeability transposition pore, a process that will further lead to necrosis and increased infarct size5. Therefore, fi nding an effective treatment approach in order to stop the myocardial injury related to coronary occlusion without sacrifi cing the benefi t of reperfusion therapy would be the goal of STEMI therapy. Taking into account the data previously highlighted, we
consider that an additional strategy in order to improve the outcomes of STEMI patients could be represented by the assessment of coronary microvascular functional and structural obstruction.
THE ROLE OF CORONARY MICROVASCULAR DYSFUNCTION
It has been demonstrated that traditional and nontraditional cardiovascular risk factors play an important role, both in epicardial as well as in microvascular endothelial dependent dysfunction8. Moreover, coronary microvascular dysfunction increases the risk of cardiovascular events8. Related to reperfusion, patients with pre-existent microvascular dysfunction will benefit less from reopening of the epicardial vessel. Also, a pre-existent impairment of myocardial microcirculation has been showed to be associated with a greater vulnerability to PCI related myocardial injury as well as with a poorer long term outcomes9. Thus, transient or permanent microvascular dysfunction infl uences the prognosis of acute coronary patients through the reduction of coronary blood flow with altered shear stress, impaired endothelial dysfunction of epicardial arteries and enhanced thrombus formation. The pathogenesis of coronary microvascular obstruction is mainly influenced by four interacting mechanisms: ischaemia-related injury, reperfusion-related injury, distal embolization and individual susceptibility of the microcirculation to injury10. Ischaemic
injury is infl uenced by the duration and the extent of ischaemia and is associated with severe capillary damage and myocardial cell swelling determined by sodium and calcium overload. The main determinants of reperfusion injury are represented by neutrophils, endothelin-1, thomboxane-A2, and platelets. Neutro phil-platelet aggregates may lead to obliteration of vessel lumen and is associated with release of vasoconstrictors and inflammatory mediators. As mentioned before, in cardiomyocytes, reperfusion increases the production of reactive oxygen species by the mitochondria. The consequence of this process is the aggravation of microvascular function. Infarct size may also be increased due to mitochondria swelling and cell rupture, phenomenon determined by the opening of the mitochondrial membrane permeability transition. The third mechanism, distal embolization of plaque and thrombus material may lead to microcirculation injury by mechanically obstruction, beyond the fact that it also represents a source of vasoconstrictors and procoagulant substances. Finally, the last mechanism taken into consideration is represented by individual susceptibility of the microcirculation to injury, mechanism influenced by genetic variability, diabetes, acute hyperglycemia, hypercholesterolemia and the lack of pre-conditioning10.
DIAGNOSIS OF CORONARY MICROVASCULAR DYSFUNCTION
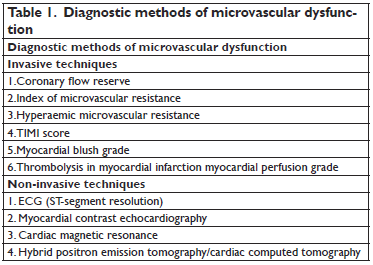
Diagnostic methods of microvascular dysfunction may be invasive or non-invasive (Table 1). When using these methods, the incidence of coronary microvascular dysfunction is variable, ranging from 10% when using angiographic assessment of thrombolysis in myocardial infarction (TIMI) flow, to 60% while using CMR or myocardial contrast echocardiography11. The gold standard method for invasive assessment of coronary microvascular dysfunction and obstruction assessment is the direct measurement of coronary flow reserve using intracoronary Doppler wire, with
a typical flow pattern characterized by: systolic retrograde flow, diminished systolic anterograde flow, and rapid deceleration of diastolic flow10. Still, this method has some major disadvantages, such as the need for special equipment and the use of pharmacological interventions. Other invasive methods are represented by the index of microvascular resistance and the hyperaemic
microvascular resistance index.
The first one is providing an assessment of microcirculation, independent of haemodynamic parameters whereas the hyperaemic microvascular resistance index is associated with ventricular recovery and clinical outcomes after acute coronary syndromes12. Also, another invasive tool for the assessment of microvascular dysfunction is the score grading system that describes the rate of blood flow in the epicardial vessels, the TIMI (thrombolysis in myocardial infarction) flow. The range of TIMI fl ow is quantified between grade 0 (no flow at all) and grade 3 flow (normal flow), while a TIMI fl ow <3 is considered as a marker of microvascular dysfunction10. Other invasive methods to be taken into consideration are the myocardial blush
grade, that is assessing the intensity of contrast medium in the microcirculation and the TIMI myocardial perfusion grade that assesses microvascular clearance of contrast medium10. The non-invasive tools that allow us to assess myocardial dysfunction are the monitoring in a single lead of the ST segment resolution, myocardial contrast echocardiography, and cardiac magnetic resonance. Other methods are represented by myocardial scintigraphy and hybrid positron emission tomographycomputed tomography. Among these methods, ST-segment resolution is a useful method for the assessment of coronary microvascular dysfunction, with an important prognostic value. Myocardial contrast echocardiography can also be used in order to assess coronary
microvascular dysfunction, by the use of ultrasound to visualize contrast microbubbles, with a typical pattern that is represented by the lack of intra-myocardial contrast opacification. Cardiac magnetic resonance (CMR) is another non-invasive method that allows accurate quantification and localization of coronary microvascular dysfunction as well as of the infarct size relative to the entire left ventricle. Typical signs of coronary microvascular dysfunction and obstruction in CMR are represented by the lack of gadolinium enhancement during first pass and the lack of gadolinium enhancement within a necrotic region. Myocardial scintigraphy and the hybrid positron emission tomography are noninvasive techniques that may allow the assessment of
inflammatory reactions after reperfusion10.
TREATMENT STRATEGIES
A few treatment strategies have been taken into consideration in order to ameliorate microvascular dysfunction. Therapies that have showed good results when used before catheterization laboratory are represented by ongoing statin therapy at the time of STEMI, with a better functional recovery of myocardial function after 6 months of follow-up in compare with patients without statin therapy13. Other therapies with evidences in the fi eld of microvascular dysfunction that may be useful before catheterization laboratory procedures are the use of beta-blockers such as carvedilol or nebivolol, pre-hospital abciximab administration as well as remote ischaemic pre-conditioning10. Also before PCI, therapies with controversial results are represented by the use of the glucose–insulin–potassium (GIK) in the setting of STEMI as well as the chronic treatment with ACE inhibitors or nitrates14,15. No amelioration of microvascular dysfunction was found in
association with the use of ticagrelor, COX inhibitors or hypothermia10. In the catheterization laboratory, there are several therapies with results tested in large trials with clinical endpoints. One of these is represented by the use of adenosine (the AMISTAD trial)16. Other therapeutic options are the use of atrial natriuretic peptide, cyclosporine and exenatide (a glucagon-like peptide-1
agonist), all known to have cardioprotective effects10. Controversial therapies in the setting of catheterization laboratory are represented by manual thrombus aspiration and the administration of vasodilators such as verapamil, diltiazem, and nitroprusside17. Inadequate therapies are the intracoronary administration of abciximab witch failed to improve the rate of MACEs (allcause
mortality, recurrent MI, and new heart failure) as well as the administration of nicorandil, a hybrid drug of ATP-sensitive K-channel opener10.Finally, a few therapeutic strategies in order to improve microvascular dysfunction and obstruction need to be taken into consideration after the catheterization laboratory. Among these, the aggressive risk factors modifications and rehabilitation measures had a significant impact on the recurrence of acute coronary syndromes and re-hospitalization rate10. Therapies that are still controversial and need further research are
represented by the administration of cilostazol (for 1 month) to double antiplatelet therapy with aspirin and clopidogrel, as well as the use of vasodilators (calciumchannel antagonist, dypiridamole) or metabolic drugs (ranolazine)18,19. The use of intra-aortic balloon pumping (IABP) did not show favorable results in amelioration of microvascular dysfunction20,21.
CONCLUSIONS
The role of the microcirculation in determining the outcomes of patients with acute coronary syndromes is still a matter of great debate. From the mechanisms by which reperfusion damage contributes to the microvascular abnormalities to novel aspects of the complex role of coronary microcirculation in acute coronary syndromes, future trials should explore the effects of integrated treatments aimed to ameliorate the outcomes of reperfusion strategies following primary PCI. Conflict of interest: none declared.
References
1. Nichols M, Townsend N, Luengo-Fernandez R, Leal J, Gray A, Scarborough P, Rayner M. European Cardiovascular Disease Statistics 2012. Brussels/Sophia Antipolis: European Heart Network/European Society of Cardiology, 2012.
2. World Health Organisation. Cardiovascular disease. http://www. who.int/cardiovascular_diseases/en/ (15 March 2015).
3. Niccoli G, Scalone G, Lerman A, Crea F. Coronary microvascular obstruction in acute myocardial infarction. Eur Heart J. 2016 Apr 1; 37(13):1024-33.
4. Steg PG, James SK, Atar D, Badano LP, Lundqvist CB, Borger MA, Di Mario C, Dickstein K, Ducrocq G, Fernandez-Aviles F, Gershlick AH, Giannuzzi P, Halvorsen S, Huber K, Juni P, Kastrati , Knuuti J, Lenzen MJ, Mahaffey KW,Valgimigli M, van’t Hof A, Widimsky P, Zahger D. ESC Guidelines for the management of acute myocardial infarction in patients presenting with ST-segment elevation: The Task Force on the management of ST-segment elevation acute myocardial infarction of the European Society of Cardiology (ESC). Eur Heart J 2012;33:2569–2619.
5. Kapur NK, Karas RH. A new shield from the double-edged sword of reperfusion in STEMI. Eur Heart J. 2015 Nov 21;36(44):3058-60.
6. Verma S, Fedak PW, Weisel RD, Butany J, Rao V, Maitland A, Li RK, Dhillon B, Yau TM. Fundamentals of reperfusion injury for the clinical cardiologist. Circulation. 2002 May 1;105(20):2332-6.
7. Braunwald E, Kloner RA. Myocardial reperfusion: a double-edged sword? J Clin Invest. 1985 Nov;76(5):1713-9.
8. Lerman A, Holmes DR, Herrmann J, Gersh BJ. Microcirculatory dysfunction in ST-elevation myocardial infarction: cause, consequence, or both? Eur Heart J 2007;28:788–797.
9. Albertal M, Voskuil M, Piek JJ, de Bruyne B, Van Langenhove G, Kay PI, Costa MA, Boersma E, Beijsterveldt T, Sousa JE, Belardi JA, Serruys PW. Coronary flow velocity reserve after percutaneous interventions is predictive of periprocedural outcome. Circulation 2002;105:1573–1578.
10. Niccoli G, Scalone G, Lerman A, Crea F. Coronary microvascular obstruction in acute myocardial infarction. Eur Heart J. 2016 Apr 1;37(13):1024-33.
11. Niccoli G, Cosentino N, Spaziani C, Fracassi F, Tarantini G, Crea F. No-reflow: incidence and detection in the cath-lab. Curr Pharm Des 2013;19:4564–4575.
12. Van de Hoef TP, Bax M, Meuwissen M, Damman P, Delewi R, de Winter RJ, Koch KT, Schotborgh C, Henriques JP, Tijssen JG, Piek JJ. Impact of coronary microvascular function on long-term cardiac mortality in patients with acute ST-segment-elevation myocardial infarction. Circ Cardiovasc Interv 2013;6: 207–215.
13. Kim JS, Kim J, Choi D, Lee CJ, Lee SH, Ko YG, Hong MK, Kim BK, Oh SJ, Jeon DW, Yang JY, Cho JR, Lee NH, Cho YH, Cho DK, Jang Y. Efficacy of high-dose atorvastatin loading before primary percutaneous coronary intervention in ST-segment elevation myocardial infarction: the STATIN STEMI trial. JACC Cardiovasc Interv 2010;3:332–339.
14. Mehta SR, Yusuf S, Diaz R, Zhu J, Pais P, Xavier D, Paolasso E, Ahmed R, Xie C,Kazmi K, Tai J, Orlandini A, Pogue J, Liu L; CREATE-ECLA Trial Group Investigators. Effect of glucose-insulin-potassium infusion on mortality in patients with acute ST-segment elevation myocardial infarction: the CREATE-ECLA randomized controlled trial. JAMA 2005;293:437–446.
15. Zhao JL, Yang YJ, You SJ, Jing ZC, Wu YJ, Cheng JL, Gao RL. Pre-treatment with fosinopril or?valsartan reduces myocardial no-reflow after acute myocardial infarction and reperfusion. Coron Artery Dis 2006;17:463–469.
16. Kloner RA, Forman MB, Gibbons RJ, Ross AM, Alexander RW, Stone GW. Impact of time to therapy and reperfusion modality on the efficacy of adenosine in acute myocardial infarction: the AMISTAD-2 trial. Eur Heart J 2006;27:2400–2405.
17. Huang D, Qian J, Ge L, Jin X, Jin H, Ma J, Liu Z, Zhang F, Dong L, Wang X, Yao K, Ge J. REstoration of coronary flow in patients with no-reflow after primary coronary intervention of acute myocardial infarction (RECOVER). Am Heart J 2012;164:394–401.
18. Tanzilli G, Greco C, Pasceri V, Pelliccia F, Arrivi A, Placanica A, Mangieriet E. Dipyridamole versus verapamil for treatment of no-reflow during primary angioplasty. Catheter Cardiovasc Interv 2010;76:787– 793.
19. Pelliccia F, Pasceri V, Marazzi G, Rosano G, Greco C, Gaudio C. A pilot randomized study of ranolazine for reduction of myocardial damage during elective percutaneous coronary intervention. Am Heart J 2012;163:1019–1023.
20. Maekawa K, Kawamoto K, Fuke S, Maekawa K, Kawamoto K, Fuke S, Yoshioka R, Saito H, Sato T, Hioka T. Effects of intra-aortic balloonpumping on the angiographic no-reflow phenomenon after percutaneous coronary intervention in patients with anterior myocardial infarction. Circ J 2006;70:37–43.
21. Patel MR, Smalling RW, Thiele H, Barnhart HX, Zhou Y, Chandra P, Chew D,Cohen M, French J, Perera D, Ohman EM. Intra-aortic balloon counterpulsation and infarct size in patients with acute anterior myocardial infarction without shock: the CRISP AMI randomized trial. JAMA 2011;306:1329–1337.
 This work is licensed under a
This work is licensed under a