Edme Roxana Mustafa1, Alin Demetrian2, Cristina Florescu1, Serenada Bala3
1 Department 3 of Cardiology, University of Medicine and Pharmacy, Craiova, Romania
2 Department of Thoracic Surgery, University of Medicine and Pharmacy, Craiova, Romania
3 Department of Pathology, Emergency Hospital Craiova, Romania
Abstract: A 58 year old male diagnosed one month ago with left inferior lobe pulmonary tumor had a transthoracic echocardiography performed after a syncope. A large mass attached to the lateral wall of the the left atrium was found, with a mobile distal part protruding through the mitral valve in diastole. Thoracic CT confi rmed the mediastinal extension of the lung tumor.
Keywords: lung tumor cardiac extension left atrium
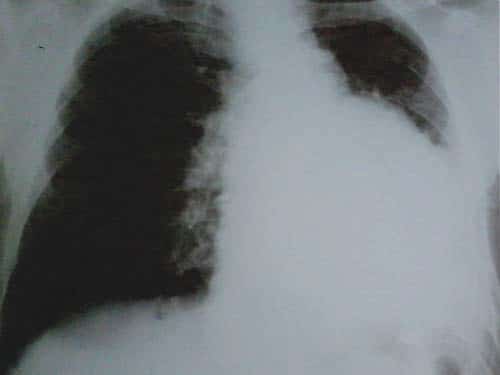
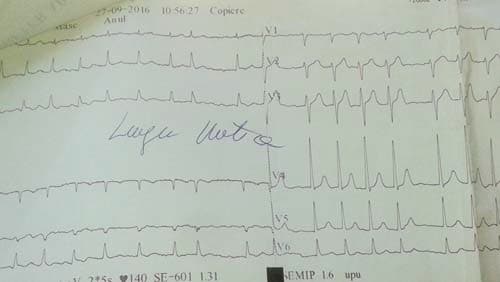
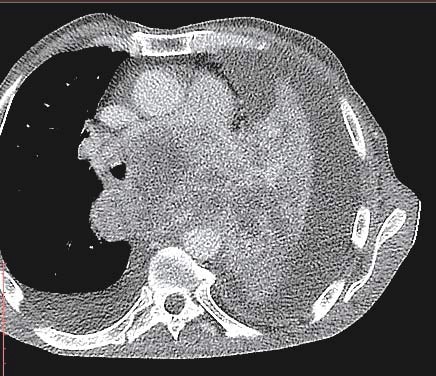
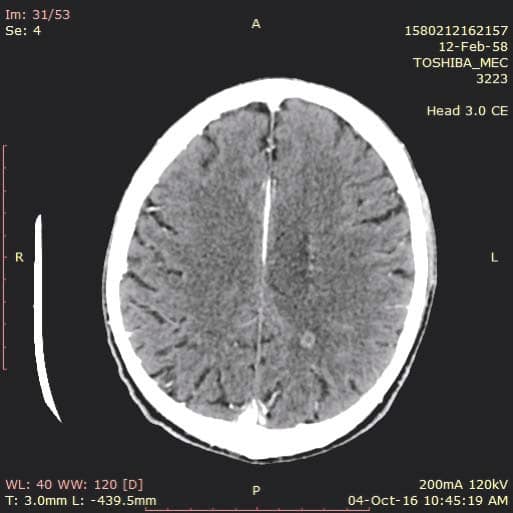
A 58 year old male presented to the emergency department for syncope, dizziness, sweating, shortness of breath when doing less than ordinary physical activity and fatigue. A former smoker, he was diagnosed 1 month ago with left inferior lobe pulmonary tumor from which a sample tissue was collected (by percutaneous transthoracic biopsy) for histological exam and which showed small cell carcinoma. Shortness of breath and fatigue intensified in the last 2 weeks and he had syncope while standing. On admission his general condition was medium, BMI = 19 kg/m2, he was conscious, with dullness at percussion of inferior 2/3 of left thorax, absent breath sounds at this level, SpO2=92%, normal heart size, heart rate was 100 beats/min, without gallop sound or murmurs, BP was 100/70 mmHg, the rest of physical exam was normal. Blood analysis revealed microcytic, hypochromic anemia (Hb=11 g/d) with normal ferritin value, high inflammation markers (ESR=70 mm/h, PCR=96 mg/ dl), hypoproteinemia (protein=5.9 g/dl), other results were normal. Tumoral markers were measured one month ago when the lung tumor was first diagnosed and had high values: CEA value=7.74 ng/ml (NV 0-0.47 ng/ml), CYFRA 21-2=18.97 ng/ml (NV=0.1-2.3 ng/ml), NSE=64.43 ng/dl (NV=0-16.3 ng/dl). Chest X ray (performed one month ago (Figure 1) showed a large opacity (with an atelectatic component and traction of the mediastinum) occupying the inferior ¾ parts of left lung, fluid accumulation in the left costophrenic sinus and enlarged pulmonary hilum (Figure 1). First chest CT (performed one month ago) revealed a large tumor of 14/14/18 cm located in the left lung with mediastinal extension, the tumor had necrotic areas and nonhomogenous contrast accumulation; it surrounded left main bronchus, invaded and occluded left inferior lobar bronchus, left superior lobar bronchus was compressed without invasion; limphangitic tracts and subsegmentary atelectasis were present in the superior ventral segment; enlarged mediastinal nodes (up to 20-26 mm) were seen on both sides of the trachea, in the right and left pulmonary hillum; both pulmonary arteries were surrounded by tumor; a small pericardial effusion, an intracardiac extension of the tumor measuring 2.8/2.1 cm and a large left pleural effusion were noticed. According to the CT exam the tumor stage was T4N3. The pleural effusion was drained, it was hemorrhagic fl uid with high protein count (exsudate), with mesothelial cells, free nuclei, frequent lymphocytes, rare polymorphonuclear neutrophils and frequent RBC but no tumoral cells. Tumor tissue sample could be collected by bronchoscopy or by percutaneous transthoracic biopsy, the last one was preferred. On current admission the patient had an episode of paroxysmal atrial fi brillation (Figure 2) which converted spontaneously to sinus rhythm (sinus tachycardia), then the ECG was normal. Chest CT was repeated and showed a slight increase in tumor size (Figure 3) and invasion of mediastinum, the tumor didn’t had a clear separation from pulmonary artery trunk, aortic arch and descending thoracic aorta or the left ventricle, with infiltration of left main bronchus and compression of the esophagus; there were blocks of mediastinal lymph nodes with necrosis and pleural effusion measuring 4 cm thickness in left and 1.7 cm in the right thorax Brain CT performed for tumor staging and after syncope showed a nodular metastatic lesion 9.4/5.7 mm in the left posterior parietal region with ring pattern contrast accumulation and with surrounding edema (Figure 4); the differential diagnosis includes a
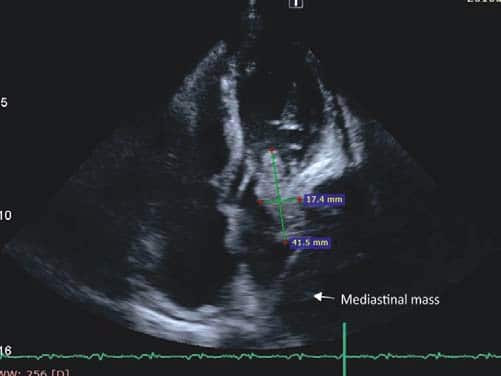
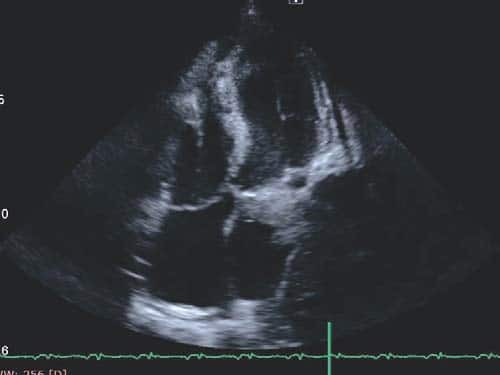
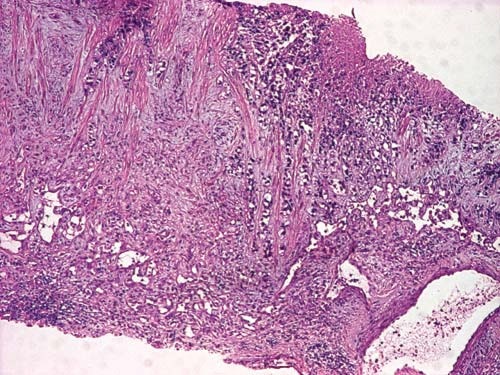
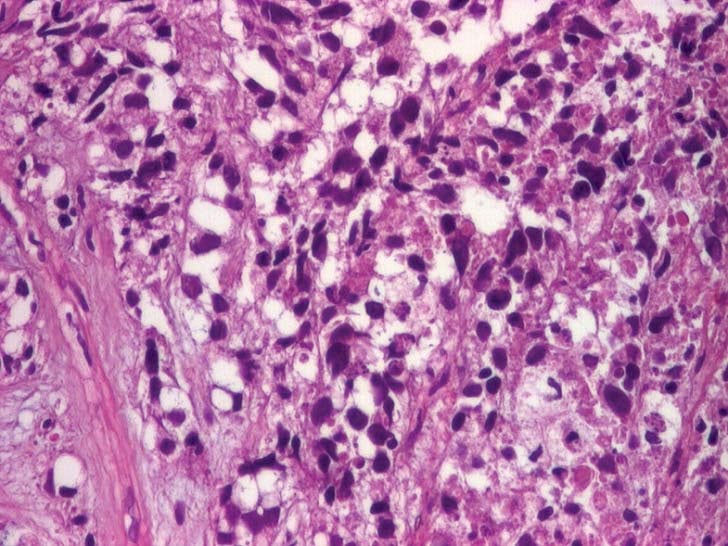
primary cerebral tumor or an abscess. The transthoracic echocardiogram showed an homogenous mass (tumor – Figure 5 a+b) in the left atrium (LA) measuring 4/1.7 cm attached to the lateral wall (possibly arising from the region of left pulmonary veins drainage), the distal part of the mass being mobile and protruding through mitral valve in diastole creating a functional moderate mitral stenosis (transmitral pressure gradient at rest was 8/5 mmHg); the LA lateral wall was pushed by a mediastinal mass. Spontaneous contrast was present in the left ventricle. A small pericardial effusion was also noticed. LV systolic function was preserved (EF=60%). The LA mass was considered to be the intracardiac extension of pulmonary tumor and probably included some thrombus attached, at least the mobile extension. This mass severely reduced the inflow in LA and LV which could explain the syncope and could be a source of embolism. A second LA independent tumor is unlikely. The histological exam of lung tumor (tissue obtained by transthoracic needle biopsy) revealed small cell tumor proliferation (Figure 5 – HE, 40x, islands and cords of tumor cells, tumor necrosis was noticed), (Figure 6 – HE, 200x, tumor cells- marked with arrow- with nuclear polymorphism, some nuclei having incisions, tumor necrosis was also noticed). The imunohistological exam could not be done because of insufficient tissue sample. The diagnosis is: Left lung small cell carcinoma (T4N2M1b). Left atrium mass (suggestive of intracardiac tumor extension). Syncope. Paroxysmal atrial fibrillation. Pericarditis (with small pericardial effusion). Anemia (of chronic inflammatory disease). Syncope could be due to reduced cardiac output caused by mechanical interference of tumor with ventricular filling, which was also reduced by atrial fibrillation. The irregular rate in atrial fibrillation could interfere with baroreceptor signaling and cause syncope. Cerebral embolism with thrombus from LA or a tumor fragment is another cause of syncope, but CT brain exam does not support this etiology. The metastatic brain lesion could cause partial simple or complex seizure with autonomic signs ( sweating) with impaired consciousness which must be differentiated from syncope. Other symptoms of parietal mass could be numbness, tingling, burning sensation in the opposite limbs. Other causes of syncope seemed less likely. The lung tumor was considered inoperable (small cell carcinoma -T4N2M1b- extensive disease) and the patient was referred to oncology department for palliative chemotherapy. He started therapy with cisplatin 60 mg/m2 iv (on day 1) plus etoposide 80 mg/m2 iv (on days 1-3) and received only the first cycle of therapy. He also received anticoagulation with low molecular weight heparin (enoxaparin 1 mg/kg bid). He was programmed for whole brain radiation therapy for brain metastasis. The patient died at home after 2 weeks.
Figure 1. Left pulmonary opacity.
Figure 2. ECG – Atrial fibrillation with HR=140 b/min.
Figure 3. Left pulmonary tumor with mediastinal extension.
Figure 4. Brain CT – Nodular metastatic lesion in the left posterior parietal region.
Figure 5A. TTE – Mass attached to the lateral wall of LA and protruding through the mitral valve in diastole.
Figure 5B. TTE- Mass in LA.
Figure 5bis. Tumor cells visible in lung tissue sample (HE, X40).
Figure 6. Tumor cells in lung tissue (HE, X200).
DISCUSSION
Lung cancer is an aggressive tumor with the highest mortality. In 20% of cases, lung cancer is with small cells (SCLC), an undifferentiated neoplasia that originates from the precursors of neuroendocrine cells1. This is an aggressive type with high proliferation rate, early metastasis and more than 2/3 of patients have extensive stage disease since diagnosis1,2. The median survival without therapy in extensive disease is 7 weeks, while after current chemotherapy it increases to 5.5- 10 months1. SCLC is sensitive to cytotoxic therapy and ionizing radiation but develops early resistance to conventional therapy1. The usual chemotherapy scheme is etoposide and platinum – cisplatin (4-6 cycles), while for refractory or resistant patients antracyclines or topotecan are used1. In SCLC 10% of cases have brain metastasis from the moment of diagnosis and another 40-50% will develop this severe complication some time during the course of their disease and the survival in these cases is 3-4 months2. They need whole brain radiation therapy (30 Gy in 10-15 fractions) after which complete response can be expected in 1/3 of cases and a partial response in another 1/32. In the case of a single or few brain metastasis stereotactic radiosurgery (gamma knife radiosurgery) is more efficient2. Lung cancer can invade heart by hematogenous, lymphatic pathway, by direct invasion or by extension through the pulmonary veins. Heart metastases can be found in autoptic studies in up to 25% of patients with lung malignancy, although symptoms and diagnosis in clinical practice are rarely found (2-4% of cases)5. Pericardial invasion by lymphatic spread is most often found. Involvement of the proximal portion of pulmonary veins and extension into the left atrium is uncommon being found in 4.2% and 0.9% of patients examined with 3D magnetic resonance angiography5,6. The same morphological extension was found in 0.7% and 0.5% of a large group (4668 pts) who underwent surgery analysis for lung cancer7. Tumor size is not a definite risk factor for pulmonary vein invasion nor is histology, different types of malignant cells can invade heart squamous cell, adenocarcinoma, large cell, mucoepidermoid, leiomyosarcoma, chondrosarcoma etc)4,8. Tumors with high vascularity and scarce fibrous tissue have increased affinity for large vein invasion8. Reports in the literature found the cardiac extension to be a tumor mass or rarely a thrombus3. Cardiac extension severely affects prognosis and surgery to remove tumor is only for palliation.
Conflict of interest: none declared
References
1. Gabriela Alvarado-Luna, Daniele Morales Espinosa, Treatment of small lung cell carcinoma, where are we now?- a review, Transl Lung Cancer Res., 2016 feb; 5(1):26-38
2. A.L. Quan, G.M.M. Videtic, J.H. Suh, Brain metastasis in small lung cell cancer, Oncology ( Cancer Network) 2004
3. M.-T. Lin, S.-C Ku, M.-Z. Wu, C- J Yu, Intracardiac extension of lung cancer via the pulmonary vein, Thorax 2008; 63:1122
4. Wey Syun Hu, Huey-Ming Lo, Yu-Wung Yeh, Ming-Jen Lu Yei-San Hsieh, Tong- Jong Chen Intracardiac extension of a pulmonary pleomorphic carcinoma, Acta Cardiol Sin 2010; 26:41-3
5. P. Ballo, R. Laureano, M. Briganti, M.T. Passaleva, F. Piani, C. Piga, S. Tatini, G.M. Santoro, Case reports in Medicine, Left Atrial Mass Invasion from Pulmonary Neoplasm Extension via the Right Upper Pulmonary Vein Presenting as Ipsilateral Stroke, Hindawi Publishing Corporation, vol 2016, article ID 7084234
6. K. Takahashi, M. Furusa, H. Hanaoka et al., Pulmonary vein and left atrium invasion by lung cancer assessment by breath hold gadolinium enhanced 3D MR angiography, Journal of Computer Assisted Tomography, 2000, vol 24, no4, p 557-561,
7. M. Riquet, B. Grand, A. Arame, Lung cancer invading the pericardium – quantum of lymph nodes, Annals of thoracic surgery, vol 90, no 6, p 1773-1777, 2010
8. Aya Ishii, Shinsuke Tanaka, Tokuhiro Kimura, Koshiro Moritani, Dan Cui, Shinsuke Tanaka, Hiroo Kawano, Eiji Ikeda, Unexpectedly long intravenous and intracardiac extension of a small sized pulmonaty pleomorphic carcinoma, J Thorac Dis 2014; 6(12):E 259-E263.
 This work is licensed under a
This work is licensed under a