Mihaela Grecu1, Alina Cristina Simion1, Otilia Apetrei1, Iuliana Ardeleanu1, Catalina Marina Arsenescu1, Cristian Statescu1
1 „Prof. Dr. George I. M. Georgescu” Institute of Cardiovascular Diseases, Iasi, Romania
Abstract: Introduction – Systemic scleroderma is a rare, progressive, autoimmune disease, with multi organ implicati-ons, which may affect the heart, more frequent in the diffuse form (32%) than in a localized one (23%). The ventricular ar-rhythmias have a negative impact on prognosis and global mortality of the patients with scleroderma. Case report – Female patient, 39 years old, diagnosed with systemic scleroderma, implanted with a cardiac defibrillator (ICD) for sustained ventri-cular tachycardia (VT), treated by radiofrequency ablation (RFCA), in another center in 2012, was admitted in our center in November 2014, for numerous syncope and ICD therapies (20/4 days), despite Amiodarone treatment. EKG is suggestive for VT with right ventricle (RV), posterolateral region origin. The patient was submitted to an electrophysiological study. A 3D voltage cartography of the RV was done confi rming the presence of extended basal fibrosis, with detection of abnormal potentials across the area. Radiofrequency applications were made at the borderline zone in order to isolate slow diastolic scar potentials until non-inducibility of any sustained tachycardia. During the next 40 months the patient remained asymptomatic, with an improved psychological status, without ICD therapies, under 100 mg Amiodarone. Conclusions – Radio-frequency catheter ablation can be an extremely helpful tool in the management of ventricular arrhythmias in patients with systemic scleroderma, at long term, especially in case of electrical storm. The arrhythmia freedom can improve the quality of life and survival, reducing the ICD therapies.
Keywords: ventricular arrhythmia, scleroderma, radiofrequency ablation
INTRODUCTION
Systemic scleroderma is a rare progressive connective tissue autoimmune disease, which affects the skin and internal organs. Estimated incidence is of 18-20 cases/ million/year. Cardiac symptoms are more frequent in the diffused form (32%) than in the localized form (23%)1. Morphopathological cardiac injuries are the result of microvascular conditions, overproduction of collagen from damaged fibroblasts with extracellu-lar matrix deposits and the degrading of the immune system complex. Myocardial fibrosis is uneven distri-buted without any connection with the irrigation ter-ritory of epicardial arteries2. Reports indicate that the heart is affected in about 25% of such patients. Cardi-ac manifestations include irregular heartbeat, myositis, cardiac fi brosis, pericardium defects, implication of the coronary arteries and rarely valvular pathology3. Myo-cardial fibrosis is the substrate of irregular heartbeat and cardiac heart failure, worsened by the presence of secondary pulmonary hypertension (HTP), secondary of pulmonary lesions. Cardiac arrhythmias have a negative effect on the prognosis disease being responsi-ble for 6% of the global mortality among patients with scleroderma4. A large number of cases are diagnosed with supraventricular tachycardia (32%) and ventri-cular ectopic beats (20%) and rarely with ventricular tachycardia (10%). Reentry is the main mechanism of incessant or recurrent ventricular tachycardia. Less often abnormal automatism appears to be the trigger for ventricular arrhythmia5.
Radiofrequency catheter ablation (RFCA) revolu-tionized the treatment of ventricular arrhythmia, as a unique therapeutic solution, especially in patients with multiple therapies for monomorphic ventricular tachycardia episodes, which cannot be managed by an-tiarrhythmic treatments or by programming the ICD6.
The results of RFCA in these categories of patients are often reported as case reports or included in se-ries of non-ischemic cardiomyopathy. Invasive treat-ment by RFCA seems to be less efficient at long term, with a higher rate of recurrence (50%) when compare to RFCA in ischemic cardiomyopathy (18%)7.
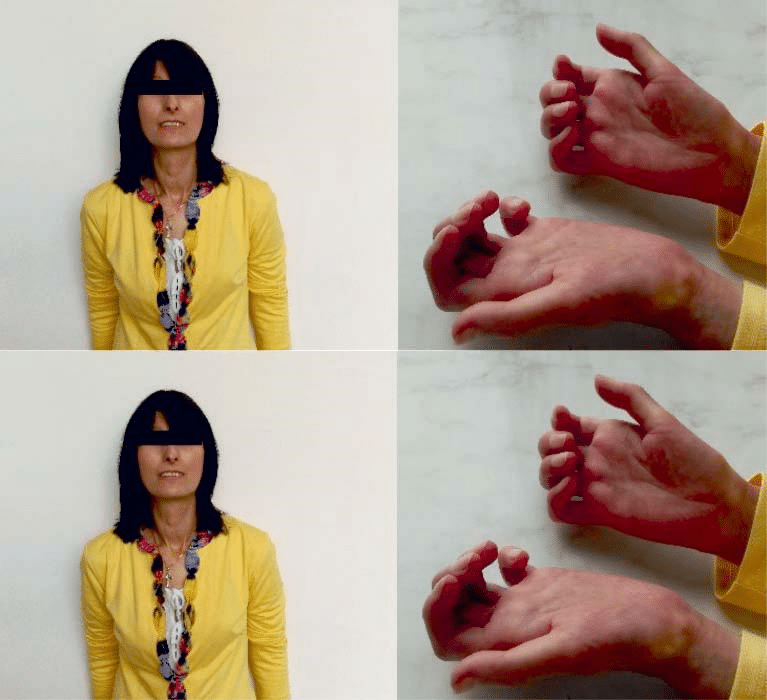
Figure 1. Byzantine icon facies with thin, rigid and retracted lips, who partial uncovered dental arch and form transverse wrinkles (left); sclerodactyly, cutaneous calcinosis (right).
CASE REPORT
A female patient of 39 years old was admitted in Car-diovascular Diseases Institute ”Prof. Dr. George I. M. Georgescu”, in November 2014, for heart palpitations with fast rhythm, repetitive and multiple syncopal epi-sodes which started 3 months before admission. The patient was diagnosed with systemic scleroderma in 2009, with cutaneous and cardiac symptoms. An au-tologous stem cell transplant was done in 2012. She remained under mycophenolate mofetil treatment. Same year a radiofrequency ablation (RFCA) procedu-re for sustained ventricular tachycardia (VT) with left ventricle septal origin was done, in an Italian center (Torino), and a Boston DDD cardiac defibrillator was implanted afterwards. Between 2012 and 2014 the pa-tient did not present any VT episode even though the signs of heart failure increased and the obvious evo-lution of the cutaneous scleroderma was noted. The recurrences of ventricular rhythm irregularities began 3 months before admission in our center, despite of high doses of antiarrhythmic medication (Amiodarone 400 mg).
At clinical exam the patient showed systemic scle-roderma symptoms, with general affected status, un-derweight (BMI 16,9 kg/m2), asthenic constitution, waxy skin, indurated, immobile facies with aspect of “byzantine icon”, sclerodactyly, cutaneous calcinosis (Figure 1). Intermittent arrhythmic sounds, apparently without any overlapped sounds, without signs of car-diac heart failure. Mental and emotional status showed severe depression, without motivation to live, functi-onal impotency and incapacity to assume her role as a mother, in accordance to her desire.
Basal EKG showed sinus rhythm, 60/min, right axial deviation, major right bundle branch block, QRS 120 ms (Figure 2). Event EKG showed sustained ventricular tachycardia with left bundle branch block (LBBB), 150 beats/min rate, which required external cardioversion. Thoracic radiography showed an enlarged heart (car-diothoracic index 0.56), lungs without evolving lesi-ons, free pleura. We noted a nondilated left ventricle, at transthoracic echocardiography, with diminished global systolic function, LVEF 44%, moderate tricuspid regurgitation, moderate pulmonary hypertension (45 mmHg), and normal right ventricle ejection fraction with a normal tricuspid anulus motion about 24 mm.
The interrogation of ICD showed 17 shocks/24h for ventricular tachycardia (VT) the day of admission. Adjusting of the antiarrhythmic treatment with amio-darone, beta blockers, and sedation, the patient has a favorable in hospital follow-up, without malignant ventricular arrhythmias. Ten days after discharge the patient was readmitted with electrical storm, numero-us ICD therapies (20 events/4 days), two of them with syncope. Event ECG showed monomorphic sustained TV with LBBB aspect, intermediate axis and positive QRS complex in DI, aVL, V3-V4 transition, suggesting right ventricle origin, in the posterolateral region (Figure 3).
This time the patient was submitted to an electro-physiological study, previous fasting, after program-ming the ICD OFF therapy. The procedure was done with local anesthesia through right femoral approach followed by superficial sedation with fractionated dose of Midazolam. Right ventricle cartography was done using the irrigated, 4 mm, Biosense Webster ablati-on catheter, noncontact force, D curve. To stabilize the mapping catheter a non-deflectable Preface sheath was used. Intraprocedural objective was to identify scar tissue zone by bipolar voltage mapping of the RV (0,5-1,5 mV parameters). Extended area of low ampli-tude, fragmented potentials was detected in the pos-terior region of RV (Figure 4). The apex and the lateral region of the RV were not affected by the endocardial fibrosis. Areas of slow conduction were diffi cult to identify because of severe micro-voltage and the dif-ficulty of maintaining the mapping catheter in contact with the walls of right ventricle.
Repetitive radiofrequency applications were done especially at borderline zone, guided by abnormal po-tentials suggesting entrance points into the scar zone, or slow conduction zone, followed by testing induci-bility (Figure 4). At the end of the procedure, non-in-ducibility of any sustained ventricular tachyarrhythmia was obtained. Only repetitive answers of 1-2 ven-tricular extra beats, with LBBB aspect, superior axis occurred, with different EKG morphology of clinical tachycardia. Post ablation the atrioventricular conduc-ting parameters remained unchanged (AH 78 ms, HV 54 ms). At the end of the procedure the defibrillator was reprogramed with ON therapy. ATP was progra-med in VT1 zone 140/min and VT2 160/min, shocks in VF zone 180/min. Total duration of RFCA procedure was 210 min with a total exposure time of 26.01 min, radiation dose 2824 μmGy/m2mn. The radiofrequency applications time was 24.01 min.
During following 40 months the patient came regu-larly in ambulatory visits for DEF interrogation, every 6 months. The patient remained asymptomatic, witho-ut any ICD therapy, under antiarrhythmic treatment (200 mg amiodarone/day 12 months and 100 mg/day till present) associated with beta blocker.
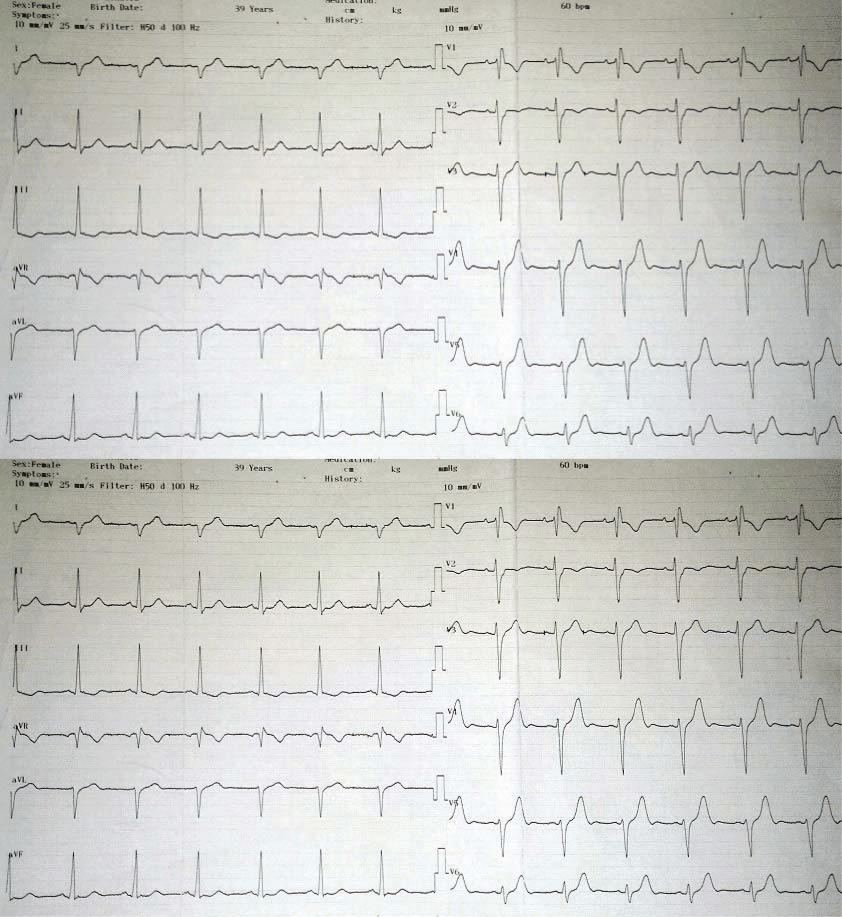
Figure 2. Basal EKG: Sinus rhythm, 60/min, right axial deviation, right bundle branch block, signs of right ventricular overload.
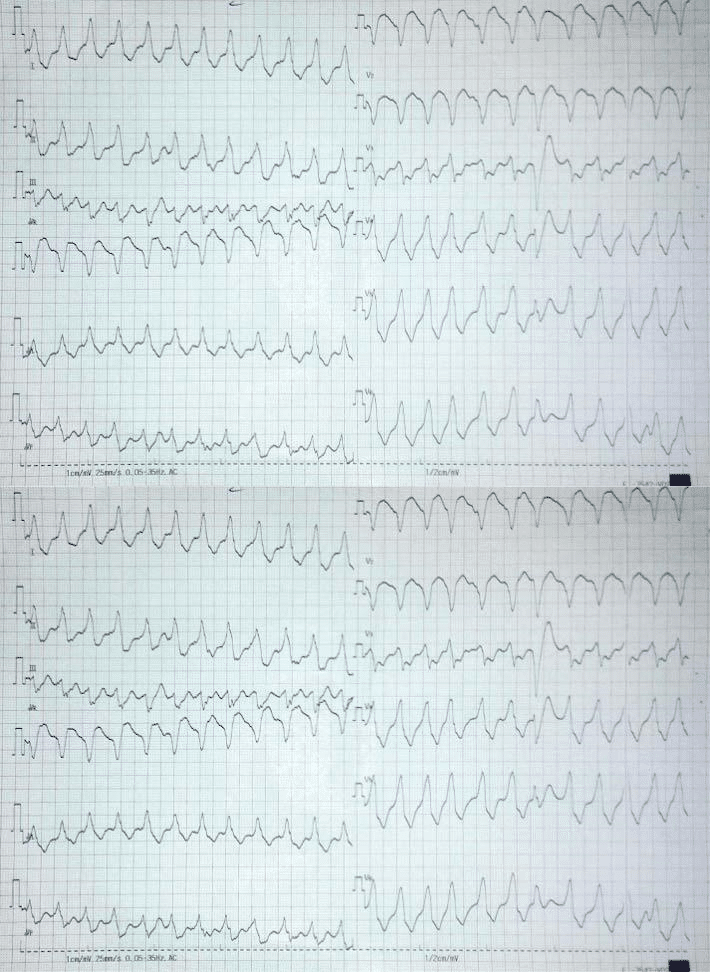
Figure 3. Event EKG: sustained monomorphic ventricular tachycardia with LBBB aspect, intermediate QRS axis, positive QRS complex in DI, aVL, V3-V4 transition, suggestive for right ventricle infero-lateral VT origin. Ventricular beat capture in precordial leads.
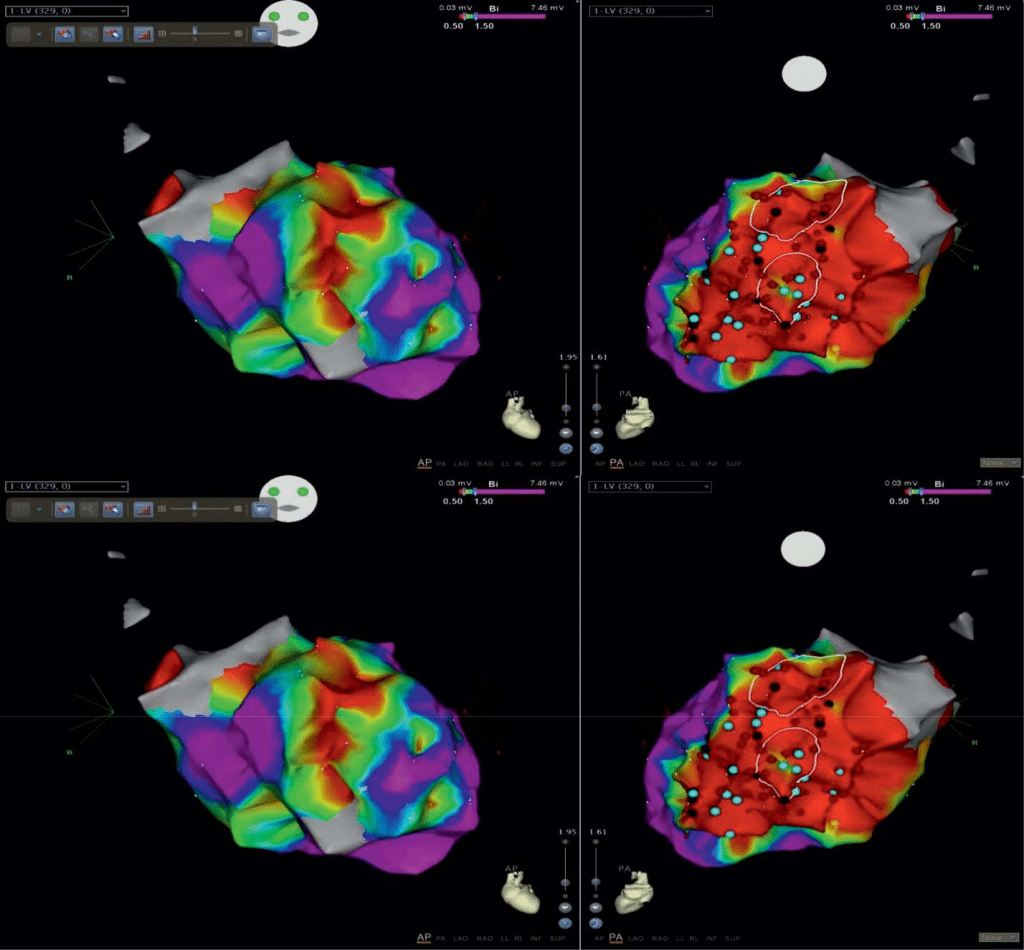
Figure 4. Endocardial bipolar voltage map of the right ventricle anterior (left) and posterior view (right). Posterior scar area (low voltage 0,5-1,5 mV) of the RV (red), borderline area (green-blue) and normal myocardium (mauve) of the apex and lateral zone. Bleu points represent pathological potentials of slow conduction, with low amplitude, fractionated and long duration. Red points represent points of RF lesion.
DISCUSSION
Our case shows the positive results of the radiofre-quency 3D ablation procedure in a patient with seve-re scleroderma with systemic sclerosis. We take into consideration to report this case because it illustrates well the magnitude and malignancy of cardiac effect in scleroderma.
There are few publications in the literature show-ing the results of ventricular arrhythmias ablation and these are only case reports8,9. All cases that have been already published show an effective 3D ablation guided procedure of right ventricular tachycardias. These cases show a moderate affected right ventricle with small amount of fibrosis. Our patient had an extensive right ventricular fi brosis, as voltage mapping showed, with septal fibrosis which explains the origin of left septal ventricular arrhythmias treated two years befo-re admission in our center.
Obviously the first RFCA procedure, done in the Italian center, was a very successful one because the patient remained completely asymptomatic almost two years. The recurrence of ventricular arrhythmias was a consequence of progression of disease. Unlike ischemic cardiomyopathy when myocardial scar suffers minimal modifications in time, in sleroderma there is a progression of the myocardial substrate, which can make difficult to fi nd any therapeutic solution, especi-ally in electrical storm. ICD is used to prevent sudden death, but even an acceptable rate of survival about 80% in the first year after implantation, these patients have a greater risk for cardiac death at long term be-cause of cardiac insufficiency. Defibrillator shocks not only increase mortality but also worsen quality of life, so radiofrequency ablation remains a unique alternati-ve in many cases10.
Because of the multifocal myocardial effects and the progression of the disease, RFCA of the ventricular tachycardias has a lower success rate when compare to ischemic cardiomyopathy.
We tried to target the clinical arrhythmia, using the surface EKG criteria to localize VT and identifying the abnormal potentials by 3D ventricular mapping. We planned to treat first the ventricular substrate and not to induce arrhythmia taking into consideration that many clinical events were syncopal and also because the sedation during procedure was only superfi cial with no anesthesiologist in the room to support a ge-neral anesthesia just in case.
Imagistic DE-MRI investigation would have been ex-tremely helpful to identify the distribution and locati-on of fi brotic zones. The presence of an ICD non-MRI safe was a contra-indication for DE-MRI, so MRI could not be done in this case. Even though DE-MRI is safe in patients with implanted ICD MRI compatible, the presence of the artefacts of the defibrillation channel limits the acquisition of quality images in many situati-ons. So, we used CT imaging as an anatomical support to reconstruct RV.
Even though the arrhythmia substrate cannot be completely treated, RFCA was a unique opportunity to control the threatening events. The positive impact of RFCA procedure in psychological status and quality of life was huge. The patient opted for palmar surgical reconstruction 20 months later and she is now profes-sionally active in a kindergarten.
CONCLUSIONS
RFCA can be an extremely helpful tool in the mana-gement of ventricular arrhythmias in patients with systemic scleroderma, especially in case of electrical storm. Even though the underlining substrate cannot be integrally treated, a successful procedure can be done, with a good long-term result. The arrhythmia freedom can improve the quality of life and survival by reducing the number of ICD therapies.
Conflict of interest: none declared.
References
1. Meune C, Vignaux O, Kahan A, et al. Heart involvement in systemic sclerosis: evolving concept and diagnostic methodologies. Arch Car-diovasc Dis. 2010 Jan;103(1):46-52.
2. Ferri C, Di Bello V, Martini C, et al: Heart involvement in systemic sclerosis: an ultrasonic tissue characterization study. Ann Rheum Dis 1998; 57: 296–302.
3. Rankin AC. Arrhythmias in systemic sclerosis and related disorders. Card Electrophysiol Rev 2002; 6: 152–154.
4. Tyndall AJ, Bannert B, Vonk M, et al. Causes and risk factors for death in systemic sclerosis: a study from the EULAR Scleroderma Trials and Research (EUSTAR) database. Ann Rheum Dis 2010;69:180915
5. Rankin CA, Osswald S, Brian A, et al. Mechanism of sustained mono-morphic ventricular tachycardia in systemic sclerosis. Am J Cardi-ol. 1999 Feb 15;83(4):633-6, A11.
6. Wissner E, Stevenson WG, Kuck KH. Catheter ablation of ven-tricular tachycardia in ischaemic and non-ischaemic cardiomyopa-thy: where are we today? A clinical review European Heart Journal (2012),33,1440–1450.
7. Lubitz SA, Goldbarg SH, Mehta D. Sudden cardiac death in infiltra-tive cardiomyopathies: sarcoidosis, scleroderma, amyloidosis, hema-chromatosis. Prog Cardiovasc Dis. 2008;51:58-73.
8. Lacroix D, Brigadeau F, Marquie C, et al: Electroanatomic mapping and ablation of ventricular tachycardia associated with systemic scle-rosis. Europace 2004; 6:336–342.
9. Nakano Y, Ogi H, Miyoshi M, et al. Ablation of Ventricular Tachy-cardia Originating from the Right Ventricle Associated with Sclero-derma Cardiomyopathy. J Arrhythmia 2005; 21: 542–545.
10. Al-Khatib MS, Stevenson WG, Ackerman MJ, et al. 2017 AHA/ACC/ HRS Guideline for Management of Patients With Ventricular Ar-rhythmias and the Prevention of Sudden Cardiac Death. Circulation. 2017; 000:e000–e000.
 This work is licensed under a
This work is licensed under a