Răzvan Constantin Șerban1,2, Silvia Lupu1,2, Irina Pintilie2, Alina Scridon1,2, Dan Dobreanu1,2
1 Physiology Department, University of Medicine and Pharmacy of Tîrgu Mureș, 540139, Tîrgu Mureș, Romania
2 Adults Cardiology Department (I), Emergency Institute for Cardiovascular Diseases and Transplantation Tîrgu Mureș, 540136, Tîrgu Mureș, Romania
INTRODUCTION
Premature ventricular contractions (PVCs) and non-sustained ventricular tachycardia (NSVT) are relatively common findings in clinical practice1. The presence of these arrhythmias is often revealed during routine ECG, ambulatory ECG monitoring, or exercise stress ECG testing performed for other reasons in asymptomatic patients. The major challenge is to establish whether these ventricular arrhythmias are benign or indicative of an increased risk of sudden cardiac death (SCD). One of the major determinants of prognosis in these patients is the presence or absence of underlying structural heart disease2. Standard ECG, exercise ECG testing, transthoracic echocardiography, and coronary angiography are usually used, collectively or in various combinations, to rule out structural heart disease3. However, even in the presence of fully normal standard diagnostic techniques, novel imaging techniques have often identified structural cardiac abnormalities, particularly in patients with right ventricular outflow tract tachycardias4. To date, it remains unclear how extensively should these patients be evaluated. Moreover, no consensus exists so far on the definition of structurally normal hearts. We report a case of high ventricular arrhythmogenicity in the absence of any apparent cardiac structural abnormalities, as assessed using standard diagnostic techniques.
CASE REPORT
A 53-year-old asymptomatic Caucasian male, without any personal cardiac history or ambulatory treatment, presented to a cardiologist for routine physical examination. At that time, all blood tests were within normal ranges and physical examination didn’t find any abnormalities. Particularly, there were no signs of anemia, hypoxia, or electrolyte imbalance. The ECG revealed frequent, polymorphic PVCs, including episodes of ventricular bigeminy and couplets. PVCs displayed at least three different morphologies, suggestive of left ventricular outflow tract, left bundle branch fascicles, and inferior septal origins (Figure 1A, arrows). Ambulatory ECG monitoring confirmed the presence and the polymorphic feature of PVCs, revealing over 20,000 PVCs / 24-h, as well as the presence of multiple NSVT episodes with left bundle branch block morphology (Figure 1B), probably originating in the inferior septal area. Remarkably, QRS morphology during tachycardia was not homogeneous, suggesting multiple exit-points, probably around a restricted area. Transthoracic echocardiogram (Figure 2) failed to show any significant structural abnormalities, but the patient had poor acoustic windows. Similarly, coronary angiogram (Figure 3) excluded significant coronary artery disease. The high ventricular arrhythmic burden prompted us to start the patient on Amiodarone. The antiarrhythmic treatment was rapidly efficient, ambulatory ECG monitoring confirming complete resolution of PVCs and NSVT episodes. Two years later, the patient developed Amiodarone-induced thyrotoxicosis of unclear mechanism. Amio darone was halted and the patient was started on Prednisone and antithyroid medication, allowing full normalization of the thyroid function within a few months. Shortly after cessation of Amiodarone, ventricular arrhythmias’ recurrence was noted.
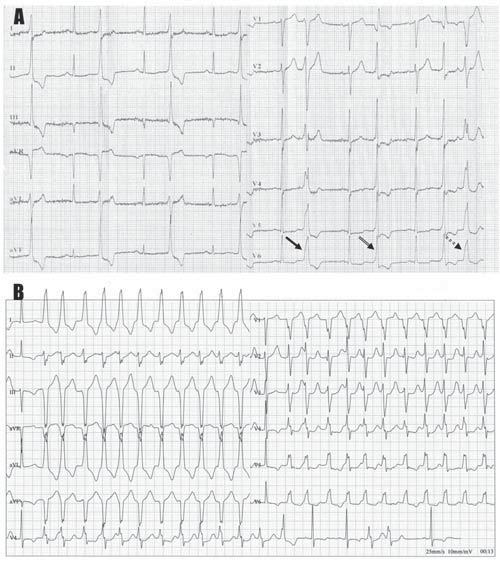
Figure 1.[A]Electrocardiographic recording depicting three different QRS morphologies of premature ventricular contractions: narrow, tall QRS complex in leads II, III, and aVF, and relatively broad R waves in leads V1-V2 indicating a left ventricular outflow tract origin (simple arrow); narrow QRS complex in the precordial leads, with right bundle branch block-like morphology, suggesting a fascicular origin (double arrow); and highly fragmented QRS complex, with left bundle branch block-like morphology (double, round dot arrow). This later morphology resembles that observed during the non-sustained ventricular tachycardia episode recorded using ambulatory ECG monitoring (B), which displays a delayed transition in lead V4, with positive QRS complexes in lead I and equibiphasic QRS complexes in leads II and aVL, indicating an inferior septal origin. Remarkably, QRS morphology during tachycardia is not homogeneous, suggesting multiple exit-points, probably around a restricted area.
The patient was started on Propafenone 450 mg/day and was scheduled 48-h later for an ECG stress testing. By that time, complete resolution of PVCs was noted at rest. Treadmill stress ECG testing using the standard Bruce protocol revealed the reappearance of PVCs and NSVT at a heart rate of 114 bpm, with rapid resolution when the heart rate decreased bellow 100 bpm (Figure 4), suggesting the involvement of sympathetic activation in the etiology of ventricular arrhythmias. This finding prompted us to supplement the patient’s treatment with a beta-blocker. The patient was scheduled for cardiac magnetic resonance imaging (MRI), which revealed left ventricular volumes at the upper normal range and increased left ventricular mass index, as well as moderate global hypokinesia, with mild impairment of the left ventricular ejection fraction (LVEF; 58%). Left ventricular anterior and lateral segments displayed multiple base-to-apex trabeculae, fulfilling the Petersen criteria5 for left ventricular noncompaction (LVNC) over four segments (Figure 5A-D).
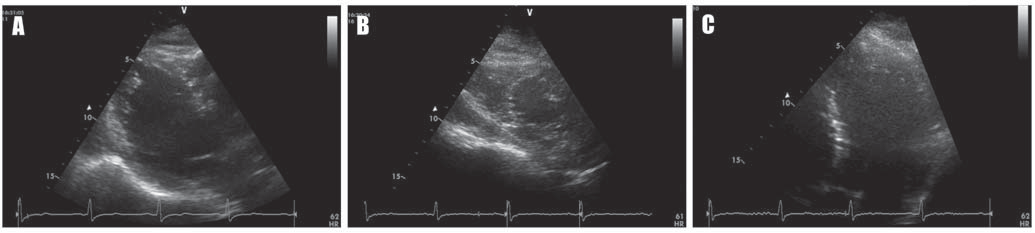
Figure 2. Echocardiographic B-mode images of the left ventricle in (A) long-axis view, (B) short-axis view, and (C) apical view, showing no significant structural abnormalities.
The right ventricle presented normal volumes and normal systolic function. The free wall of the right ventricle displayed crenelated appearance, but no significant kinetic abnormalities or aneurismal changes were noted. On delayed enhancement imaging, there was late gadolinium enhancement involving the basal region of the infero-lateral segment of the left ventricle, affecting the endocardial layer of the myocardium, limited to an area of noncompaction (Figure 5E and F, arrows). Six months later, clinical and echocardiographic follow-up of the patient revealed no progression of the myocardial disease. Resting and stress ECG under combined beta-blocker and Propafenone treatment showed complete resolution of ventricular arrhythmias.
DISCUSSION
Non-sustained ventricular tachycardia and PVCs have been recorded in a wide range of conditions, from apparently healthy individuals to patients with significant heart disease6. Although in the vast majority of patients PVCs entail a favorable benign prognosis7, in patients with structural heart disease the presence of PVCs has been associated with a higher risk of SCD8,9, particularly in patients with prior myocardial infarction9. Accordingly, current management of patients with frequent PVCs is designed to identify an underlying structural substrate of these arrhythmias. Although resting ECG, exercise ECG testing, transthoracic echocardiography, and coronary angiography are commonly used to exclude myocardial disease7, in some cases, particularly in patients with poor acoustic windows, other techniques may be necessary for definitive exclusion of an underlying disease2.
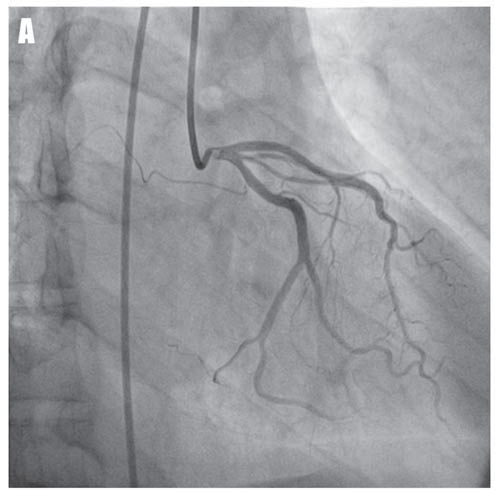
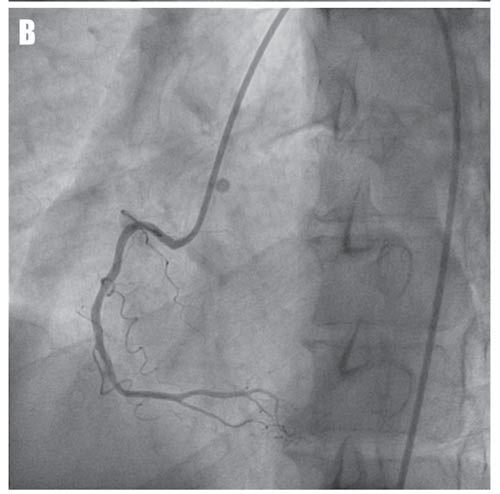
Figure 3. Coronary angiograms of the left coronary artery in right-anterior-oblique caudal view (A) and of the right coronary artery in left -anterior-oblique view (B) showing the absence of significant coronary artery disease.
However, to date, it remains unclear how extensively should these patients be evaluated. Additional ECG criteria, such as ventricular arrhythmias’ relationship with exercise and/or the polymorphic appearance of the arrhythmias, have been proposed to facilitate this decision.
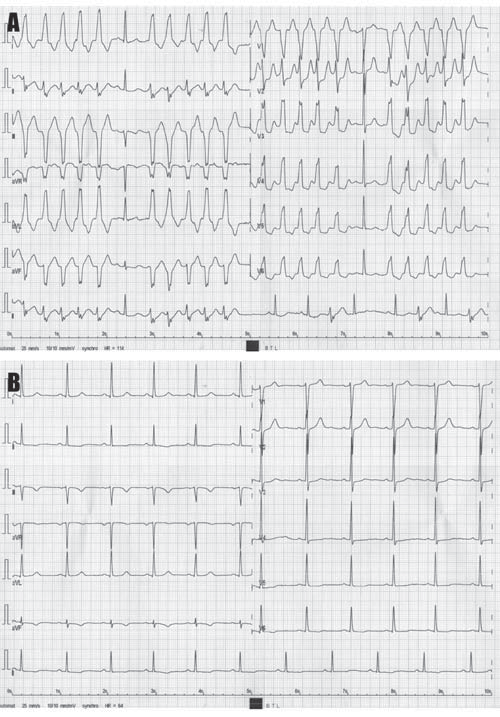 Figure 4.[A] Electrocardiographic tracing recorded during treadmill ECG testing showing the occurrence of non-sustained ventricular tachycardia episodes at a heart rate of 114 bpm. Note that the morphology of ectopic QRS complexes is similar to that of the QRS complexes recorded during the spontaneous non-sustained ventricular tachycardia episode depicted in Fig. 1B. (B) Post-effort, at lower heart rates, complete resolution of ventricular ectopic beats is noticed.
Figure 4.[A] Electrocardiographic tracing recorded during treadmill ECG testing showing the occurrence of non-sustained ventricular tachycardia episodes at a heart rate of 114 bpm. Note that the morphology of ectopic QRS complexes is similar to that of the QRS complexes recorded during the spontaneous non-sustained ventricular tachycardia episode depicted in Fig. 1B. (B) Post-effort, at lower heart rates, complete resolution of ventricular ectopic beats is noticed.
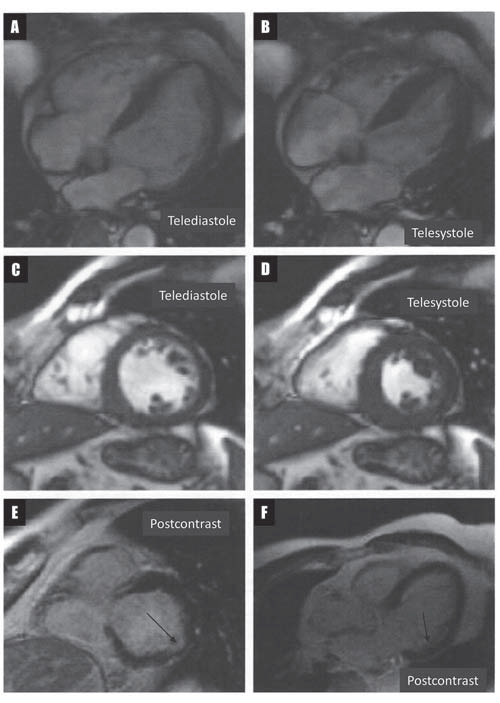 Figure 5. Long-axis (A and B) and short-axis (C and D) cardiac magnetic resonance images showing multiple base-to-apex trabeculae involving the left ventricular anterior and lateral segments, and crenelated appearance of the free wall of the right ventricle. Short-axis (E) and long-axis (F) delayed enhancement cardiac magnetic resonance images showing late gadolinium enhancement involving the basal region of the infero-lateral segment of the left ventricle, affecting the endocardial layer of the myocardium, limited to an area of noncompaction (arrows).
Figure 5. Long-axis (A and B) and short-axis (C and D) cardiac magnetic resonance images showing multiple base-to-apex trabeculae involving the left ventricular anterior and lateral segments, and crenelated appearance of the free wall of the right ventricle. Short-axis (E) and long-axis (F) delayed enhancement cardiac magnetic resonance images showing late gadolinium enhancement involving the basal region of the infero-lateral segment of the left ventricle, affecting the endocardial layer of the myocardium, limited to an area of noncompaction (arrows).
Thus, it has been demonstrated that PVCs that mostly occur at rest and suppress with exercise are usually benign, whilst those detected during exercise, and especially at recovery, may be indicative of increased cardiovascular mortality within the next decades10. In the same vein, polymorphic PVCs usually arise in the presence of structural heart disease and often entail an increased risk of sustained ventricular arrhythmias and SCD2. These exact features, the increase in ventricular ectopy at exercise and the polymorphic feature of ventricular arrhythmias, prompted us to investigate further for the presence of an underlying myocardial disease, using one of the modern, more sensitive imaging techniques. This allowed the diagnosis of LVNC in the absence of any apparent structural abnormalities with echocardiography. To date, the morphological substrate and the prognostic significance of ventricular arrhythmias in patients with LVNC are far from clear11. Perfusion defects in areas of noncompaction may provide a substrate for reentrant arrhythmias, explaining the often polymorphic appearance of the arrhythmias, as well as the occurrence of the arrhythmias at exercise in these patients12-15. However, in the present case, the recorded QRS morphologies of PVCs and NSVTs can hardly be related to any area of noncompaction, as indicated by cardiac MRI, suggesting that other factors may also be involved in the high ventricular arrhythmogenicity observed in this population. Indeed, in the study of Shan et al. the frequency of SCN5A variants, encoding for the alpha-subunit of the voltage-gated sodium channel, was significantly higher in patients with LVNC that presented ventricular arrhythmias than in those who did not, suggesting that genetic factors may also represent a risk factor for arrhythmias in this population16. In the present case, combined beta-blocker and Propafenone treatment allowed complete resolution of ventricular arrhythmias. However, suppression of arrhythmic events with antiarrhythmic drugs could not be associated with improved survival in various clinical scenarios17,18, while in studies such as CAST and CAST II, class Ic antiarrhythmics were actually associated with a significant increase in mortality in post-myocardial infarction patients, despite a significant reduction in arrhythmia burden19,20. To date, no specific data are available in LVNC patients. Accordingly, the decision of initiating antiarrhythmic treatment in this patient was driven by the high arrhythmic burden and the consequent risk of tachycardiomyopathy, and not by the rationale that this might reduce the risk of SCD. In patients with structural heart disease, Amiodarone is usually the preferred antiarrhythmic drug. The occurrence of Amiodarone-induced thyrotoxicosis prompted us to stop the treatment and to change the patient to Propafenone. However, Propafenone treatment carries a significant proarrhythmic risk in patients with heart failure, imposing regular follow-up of the patients’ left ventricular systolic function21,22, as well as the need to regularly monitor the duration of the QRS complex. Galizio et al. recently proposed a number of criteria useful for identifying patients at increased risk of SCD23. Based on these criteria, the authors propose cardiac defibrillator implantation for the primary prevention of SCD in patients with LVNC and LVEF <30%, or at least two of the following criteria: family history of SCD, syncope or NSVT. Out of the 80 patients included in the study 28.75% beneficiated of cardiac defibrillator implantation for the primary prevention of SCD. Only one of these patients beneficiated of an appropriate shock during the 28 ± 22 months of follow-up; this patient was implanted based on a low LEVF. These results underline the role of decreased LVEF as main predictor of mortality in these patients. Electrophysiological stuies and assessment of malignant ventricular arrhythmias induction may seem attractive for identifying patients at increased risk for SCD. However, arrhythmia induction in 24 LVNC patients could not predict the occurrence of SCD during the 61.4 ± 50 months of follow-up 24. Accordingly, although the ACC/AHA/HRS 2008 Guidelines for Device-Based Therapy of Cardiac Rhythm Abnormalities recommended cardiac defibrillators implantation in all LVNC patients25, in the light of the current data, it does not seem reasonable to formulate recommendations applicable for the entire population of LVNC patients. Until additional studies are available and definitive criteria formulated, it seems reasonable to approach these patients based on parameters with established prognostic roles. Accordingly, the strongest predictor of SCD and the most widely used parameter in deciding cardiac defibrillator implantation for the primary prevention of SCD in patients with structural heart disease remains a low LVEF26. Although cardiac MRI allowed the identification of ventricular noncompaction as substrate for arrhythmias in this patient, at present, there is little evidence to recommend the routine use of such imaging techniques in the workup of PVCs27. Moreover, it remains to be established if subtle structural abnormalities, undetected using the standard techniques, carry the same prognosis as the ‘gross’ structural abnormalities revealed by echocardiography. Further studies will have to be conducted in order to make definitive recommendations on the need and frequency of using these novel imaging techniques for the diagnosis and follow-up of these patients.
CONCLUSION
This report illustrates a case of highly arrhythmogenic LVNC undetected with the standard techniques used in the workup of patients with frequent PVCs. Additional ECG criteria, such as the occurrence of the arrhythmias during exercise and/or the polymorphic appearance of the arrhythmias, proved useful in deciding to perform further imaging workup. It remains to be established if these subtle structural abnormalities, undetected using the standard techniques, carry the same prognosis as those revealed by echocardiography. Financial support: This work was partially supported by the University of Medicine and Pharmacy of Tîrgu Mureș Research Grant number 16/11.12.2013. Conflict of interest: none declared.
References
1. Buxton AE, Calkins H, Callans DJ, DiMarco JP, Fisher JD, Greene HL, Haines DE, Hayes DL, Heidenreich PA, Miller JM, Poppas A, Prystowsky EN, Schoenfeld MH, Zimetbaum PJ, Heidenreich PA,
Goff DC, Grover FL, Malenka DJ, Peterson ED, Radford MJ, Redberg RF; American College of Cardiology; American Heart Association Task Force on Clinical Data Standards; (ACC/AHA/HRS Writing
Committee to Develop Data Standards on Electrophysiology). ACC/AHA/HRS 2006 key data elements and defi nitions for electrophysioetlogical studies and procedures: a report of the American College of
Cardiology/American Heart Association Task Force on Clinical Data Standards (ACC/AHA/HRS Writing Committee to Develop Data Standards on Electrophysiology). Circulation 2006; 48: 2360-96.
2. Prystowsky EN, Padanilam BJ, Joshi S, Fogel RI. Ventricular arrhythmias in the absence of structural heart disease. J Am Coll Cardiol 2012; 59: 1733-44.
3. Miles WM. Idiopathic ventricular outfl ow tract tachycardia: where does it originate? J Cardiovasc Electrophysiol 2001; 12: 536-7.
4. Carlson MD, White RD, Trohman RG, Adler LP, Biblo LA, Merkatz KA, Waldo AL. Right ventricular outfl ow tract ventricular tachycardia: detection of previously unrecognized anatomic abnormalities
using cine magnetic resonance imaging. J Am Coll Cardiol 1994; 24: 720-7.
5. Petersen SE, Selvanayagam JB, Wiesmann F, Robson MD, Francis JM, Anderson RH, Watkins H, Neubauer S. Left ventricular non-compaction: insights from cardiovascular magnetic resonance imaging. J Am Coll Cardiol 2005; 46: 101-5.
6. Messineo FC. Ventricular ectopic activity: prevalence and risk. Am J Cardiol 1989; 64: 53J-6J.
7. European Heart Rhythm Association; Heart Rhythm Society, Zipes DP, Camm AJ, Borggrefe M, Buxton AE, Chaitman B, Fromer M, Gregoratos G, Klein G, Moss AJ, Myerburg RJ, Priori SG, Quinones
MA, Roden DM, Silka MJ, Tracy C, Smith SC Jr, Jacobs AK, Adams CD, Antman EM, Anderson JL, Hunt SA, Halperin JL, Nishimura R, Ornato JP, Page RL, Riegel B, Priori SG, Blanc JJ, Budaj A, Camm AJ,
Dean V, Deckers JW, Despres C, Dickstein K, Lekakis J, McGregor K, Metra M, Morais J, Osterspey A, Tamargo JL, Zamorano JL; American College of Cardiology; American Heart Association Task Force; European Society of Cardiology Committee for Practice Guidelines. ACC/ AHA/ESC 2006 guidelines for management of patients with ventricular arrhythmias and the prevention of sudden cardiac death: a report of the American College of Cardiology/American Heart Association Task Force and the European Society of Cardiology Committee for Practice Guidelines (Writing Committee to Develop Guidelines for Management of Patients With Ventricular Arrhythmias and the Prevention of Sudden Cardiac Death). J Am Coll Cardiol 2006; 48: e247- 346.
8. Abdalla IS, Prineas RJ, Neaton JD, Jacobs DR Jr, Crow RS. Relation between ventricular premature complexes and sudden cardiac death in apparently healthy men. Am J Cardiol 1987; 60: 1036-42.
9. Bikkina M, Larson MG, Levy D. Prognostic ventricular arrhythmias: the Framingham Heart Study. Ann Intern Med 1992; 117: 990-6. 10. Katritsis DG, Zareba W, Camm AJ. Nonsustained ventricular tachycardia. J Am Coll Cardiol 2012; 60: 1993-2004.
11. Ikeda U, Minamisawa M, Koyama J. Isolated left ventricular noncompaction cardiomyopathy in adults. J Cardiol 2014. Doi: 10.1016/j.
jjcc.2014.10.005. [Epub ahead of print].
12. Junga G, Kneifel S, Von Smekal A, Steinert H, Bauersfeld U. Myocardial ischaemia in children with isolated ventricular non-compaction. Eur Heart J 1999; 20: 910-6.
13. Soler R, Rodriguez E, Monserrat L, Alvarez N. MRI of subendocardial perfusion defi cits in isolated left ventricular noncompaction. J Comput Assist Tomogr 2002; 26: 373-5.
14. Rugină M, Predescu LM, Sălăgean M, Coman IM, Bubenek-Turconi Ș. Left ventricular noncompaction. Romanian Journal of Cardiology 2013; 23: 148-53.
15. Laky D, Parașcan L, Cândea V. Hypoxic myocardium – interstitial alterations, histochemical and ultrastructural studies (Abstr.). Romanian Journal of Cardiology 2014; Suppl: 106.
16. Shan L, Makita N, Xing Y, Watanabe S, Futatani T, Ye F, Saito K, Ibuki K, Watanabe K, Hirono K, Uese K, Ichida F, Miyawaki T, Origasa H, Bowles NE, Towbin JA. SCN5A variants in Japanese patients with left ventricular noncompaction and arrhythmia. Mol Genet Metab 2008;93: 468-74.
17. Singh SN, Fletcher RD, Fisher SG, Singh BN, Lewis HD, Deedwania PC, Massie BM, Colling C, Lazzeri D. Amiodarone in patients with congestive heart failure and asymptomatic ventricular arrhythmia. Survival Trial of Antiarrhythmic Therapy in Congestive Heart Failure. N Engl J Med 1995; 333: 77-82.
18. Buxton AE, Lee KL, Fisher JD, Josephson ME, Prystowsky EN, Hafley G. A randomized study of the prevention of sudden death in patients with coronary artery disease. Multicenter Unsustained Tachycardia Trial Investigators. N Engl J Med 1999; 341: 1882-90.
19. Preliminary report: eff ect of encainide and fl ecainide on mortality in a randomized trial of arrhythmia suppression aft er myocardial infarction. The Cardiac Arrhythmia Suppression Trial (CAST) Investigators. N Engl J Med 1989; 321: 406-12.
20. The Cardiac Arrhythmia Suppression Trial-II Investigators. Effect of antiarrhythmic agent moricizine on survival aft er myocardial infarction. The Cardiac Arrhythmia Suppression Trial-II. N Engl J Med 1992; 327: 227-33.
21. Flaker GC, Blackshear JL, McBride R, Kronmal RA, Halperin JL, Hart RG. Antiarrhythmic drug therapy and cardiac mortality in atrial fibrillation. The Stroke Prevention in Atrial Fibrillation Investigators. J Am Coll Cardiol 1992; 20: 527-32.
22. Stevenson WG, Stevenson LW, Middlekauff HR, Fonarow GC, Hamilton MA, Woo MA, Saxon LA, Natterson PD, Steimle A, Walden JA, Tillisch JH. Improving survival for patients with atrial fibrillation and advanced heart failure. J Am Coll Cardiol 1996; 28: 1458-63.
23. Galizio NO, Gonzalez JL, Favaloro LE, Diez M, Fernandez A, Guevara E, Palazzo AA, Robles F, Casabé JH. Non-compaction cardiomyopathy. Risk stratification of sudden death for auomatic cardioverter defibrillator implantation. Rev Argent Cardiol 2011; 79: 14-20.
24. Steff el J, Kobza R, Namdar M, Wolber T, Brunckhorst C, Luscher TF, Jenni R, Duru F. Electrohysiological findings in patients with isolated left ventricular non-compaction. Europace 2009; 11: 1193-200.
25. Epstein AE, DiMarco JP, Ellenbogen KA, Estes NA 3rd, Freedman RA, Gettes LS, Gillinov AM, Gregoratos G, Hammill SC, Hayes DL, Hlatky MA, Newby LK, Page RL, Schoenfeld MH, Silka MJ, Stevenson
LW, Sweeney MO, Smith SC Jr, Jacobs AK, Adams CD, Anderson JL, Buller CE, Creager MA, Ettinger SM, Faxon DP, Halperin JL, Hiratzka LF, Hunt SA, Krumholz HM, Kushner FG, Lytle BW, Nishimura RA,
Ornato JP, Page RL, Riegel B, Tarkington LG, Yancy CW; American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Writing Committee to Revise the ACC/AHA/
NASPE 2002 Guideline update for Implantation of Cardiac Pacemakers and Arrhythmia Devices); American Association for Thoracic Surgery; Society of Th oracic Surgeons. ACC/AHA/HRS 2008 Guidelines
for Device-Based Th erapy of Cardiac Rhythm Abnormalities: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Writing Committee
to Revise the ACC/AHANASPE 2002 Guideline Update for Implantation of Cardiac Pacemakers and Antiarrhythmia Devices) developed in collaboration with the American Association for Thoracic Surgery
and Society of Th oracic Surgeons. J Am Coll Cardiol 2008; 51: e1-62.
26. Bardy GH, Lee KL, Mark DB, Poole JE, Packer DL, Boineau R, Domanski M, Troutman C, Anderson J, Johnson G, McNulty SE, Clapp- Channing N, Davidson-Ray LD, Fraulo ES, Fishbein DP, Luceri RM,
Ip JH; Sudden Cardiac Death in Heart Failure Trial (SCD-HeFT) Investigators. Amiodarone or an implantable cardioverter-defi brillator for congestive heart failure. N Engl J Med 2005; 352: 225-37.
27. Pedersen CT, Kay GN, Kalman J, Borggrefe M, Della-Bella P, Dickfeld T, Dorian P, Huikuri H, Kim YH, Knight B, Marchlinski F, Ross D, Sacher F, Sapp J, Shivkumar K, Soejima K, Tada H, Alexander ME, Triedman JK, Yamada T, Kirchhof P. EHRA/HRS/APHRS expert consensus on ventricular arrhythmias. Europace 2014; 16: 1257–83.
 This work is licensed under a
This work is licensed under a