Bianca Moise1, Monica Rosca2,3, Dragos Alexandru4, Carmen Ginghina2,3
1 Sanador Hospital, Bucharest, Romania
2 University of Medicine and Pharmacy „Carol Davila,” Euroecolab, Bucharest, Romania
3 Emergency Institute of Cardiovascular Diseases „Prof. Dr. C. C. Iliescu,” Bucharest, Romania
4 University of Medicine and Pharmacy of Craiova, Faculty of Medicine, Romania
Abstract: Objectives – The aim of this study was to assess left atrial dimensions and function in patients with non-severe mitral stenosis. Study population – Fifty-one patients (mean age of 58,8 ± 12,25 years, 82,35% women) with non-severe mitral stenosis were evaluated by standard, speckle tracking and exercise echocardiography. LA phasic function was assessed by using both volumetric and speckle-tracking derived parameters. Patients were divided into 2 subgroups – according to the mitral valve area and cardiac rhythm. Results – Compared with the control group, patients with mitral stenosis had significantly larger dimensions and all parameters regarding LA function were altered, with a strong correlation between BNP level and LA dimensions and reservoir function. LA dimensions were higher and the reservoir function was altered in patients with AF compared with patients in sinus rhythm. Except for LA active emptying fraction that was correlated with the mean transvalvular gradient measured during peak exercise and symptom intensity, there were no correlations between LA dimensions and function and exercise parameters. Echographic parameters useful in predicting AF occurrence during followup were LA dimensions, mean transvalvular gradient, systolic pulmonary artery pressure and peak systolic pulmonary artery pressure during exercise. Conclusions – Mitral stenosis leads to extensive LA remodeling, having important structural and functional consequences with implications over LA mechanical, electrical (occurrence of AF) and neurohormonal function. The LA assessment can therefore provide further insights in patients with mitral stenosis, independent of its severity, adding prognostic and clinical value.
Keywords: left atrium, mitral stenosis, echocardiography
In patients with mitral stenosis, the narrowing of the valvular orifice restricts the blood flow through the valve, thus increasing the pressure gradient between the left atrium (LA) and the left ventricle (LV) during diastole1. Therefore, we must take into account the importance of the LA in maintaining normal left ventricular filling pressures by ensuring a steady blood flow through the stenotic valve at the cost of increased left atrial pressure. In time, elevated atrial pressures lead to left atrial remodeling, resulting in progressive morphologic and functional alterations, as well as LA dilation2. The aim of this study is to assess left atrial remodeling in patients with mitral stenosis.
METHODS
Study population
We included 51 patients with mitral stenosis and preserved left ventricular systolic function. Patients were divided into 2 subgroups – according to the estimated mitral valve orifice area (over/under 1.5 cm2) and cardiac rhythm (normal sinus rhythm or atrial fibrillation). Exclusion criteria were: class IV NYHA patients, severe mitral stenosis referred for surgical correction, other significant valvular diseases, known ischemic heart disease or inductibile ischemia during exercise echocardiography and patients with contraindications for performing an exercise echocardiography. An informed consent was obtained from each patient, as well as the approval of the Ethics Committee. The following clinical data were collected for each patient: age, sex, NYHA class, associated comorbidities, as well as the cardiac rhythm and current medication.
Echocardiographic assessment
We performed the echocardiographic studies on VIVID 7 and VIVID 9 stations (GE Healthcare Horten Norway). The image acquisition and data storage were entirely digital, to allow for offline analysis. Data form each measurement was the mean result of calculations performed on three cardiac cycles for patients in sinus rhythm or five cardiac cycles for patients in atrial fibrillation.
We used a control group of 20 individuals without known cardiovascular diseases.
Standard 2D Echocardiography
We assessed mitral stenosis severity according to the latest guidelines3, based on calculated mitral valve orifice area (by 2D planimetry – form the mitral valve parasternal short axis view and from the PHT-derived formula – PHT was obtained by tracing the deceleration slope of the E-wave on color-oriented Doppler spectral display of transmitral flow. The mean transmitral gradient was calculated by manual tracing of the same anvelopes3. The pulmonary artery systolic pressure was calculated using the simplified Bernoulli equation derived from the anvelope of the tricuspid regurgitation jet obtained by continuous spectral Doppler interrogation by adding the right atrial pressure, estimated form the maximal transverse diameter of the inferior vena cava and its inspiratory collapse form the subcostal view4. We measured each of the four cardiac chambers according to the latest guidelines5. End diastolic and end systolic volumes along with left ventricular ejection fraction were measured using the modified Simpson rule5. From long axis parasternal view, we measured the anteroposterior left atrium diameter, while we used the four-chamber view and two-chamber view to assess the left atrial area and volume. Area and volume values were indexed to the body surface area. Besides the maximal indexed left atrial volume (LAvolMax), we also calculated the minimum left atrial volume (right after mitral valve closure – LAvolMin) and, for patients in sinus rhythm, the presystolic left atrial volume (corresponding with the beginning of the P wave on surface ECG – LAvolpreP)6. We used the aforementioned volumes to assess the left atrial phasic functions: LA emptying volume (LAvolMax-LAvolMin) for the reservoir function, LA passive emptying volume (LAvolMax- LAvolpreP) and LA active emptying volume (LAvolpreP-LAvolMin) for booster pump function7.
Left atrial mechanical function assessment
LA phasic function was assessed by using both volumetric and speckle-tracking derived parameters8. The first method is based on using previously measured LA volumes during the cardiac cycle as follows9: for the reservoir function – LA emptying fraction = (LAvolMax- LAvolMin)/LAvolMax, for the conduit function – LA passive emptying fraction = (LAvolMax-LAvolpreP)/LAvolMax and for the booster pump function –LA active emptying fraction = (LAvolpreP-LAvolMin)/ LAvolpreP.
Speckle tracking
For speckle tracking analysis, we used the software provided by EchoPAC, setting as a reference point the P wave on surface ECG (for patients in sinus rhythm). The acquisition was made using the best settings (average frame rate/second between 60-80, proper gain adjustment)10. We traced a line on the left atrial endocardial border to set the region of interest (ROI) and afterwards we adjusted the automatically generated ROI to include the whole LA myocardium11. The strain curves thus obtained allow the assessment of atrial function: the maximum negative peak during atrial systole (GSA-) is a surrogate for booster pump function, LA conduit function is evaluated by the maximum positive peak during early diastole (GSA+), while peak global LA longitudinal strain is useful for assessing the reservoir function12.
Stress echocardiography
Patients performed exercise echocardiography while on medication (including beta-blockers), using an ergometric bicycle, starting with a load of 25 W while gradually increasing the load with 25 W per 3 minutes of exercise or maintaining the same load for patients with decreased exercise tolerance. The test was interrupted if any of the following criteria were met: predicted heart rate or intensely symptomatic patient (dyspnea/angina/uncontrollable arrhythmias/syncope). Blood pressure was measured at rest and during exercise, at 3 minutes intervals, while patients were on continuous 12 lead ECG monitoring. Echographic acquisition was made at rest and during exercise, for each step, including during maximal exercise. Statistical analysis was performed using MS Excel and XL STAT 2014 (AddinSoft Sarl Paris). Measurements are presented as mean ± standard deviation (for numerical variables). Because of the non-Gaussian distribution of the acquired data, we used the Spearman test for establishing correlation between variables, while Mann-Whitney and Kruskal-Wallis tests were used for comparing numerical variables. For interdependence
analysis between categorical data we used the Chi Square test. A two-sided P-value of 0.05 was considered statistically significant.
RESULTS
79 patients with mitral stenosis and preserved left ventricular ejection fraction where initially evaluated clinically and by transthoracic echocardiography – 20 patients were excluded because they needed surgery for severe symptomatic mitral stenosis, 1 patient refused to enroll, 2 patients were unable to perform the exercise echocardiography due to paralysis secondary to embolic stroke and 3 patients had significant coronary artery disease or prior myocardial infarction. From the remaining 53 pts that performed the stress echocardiography, 2 had inducible myocardial ischemia during exercise and were excluded from the study. The demographic characteristics of the final study population (51 patients) were as follows: mean age was 58.8 ± 12.25 years, 82.35% were women and 21 patients (41.17%) had permanent atrial fibrillation. According to the NYHA classification, 10 patients (19.6%) were NYHA class I, 29 pts (54.7%) were NYHA class II and the remaining 12 patients (22%) were NYHA class III.
Echocardiographic characteristics
At rest, all patients hat preserved left ventricular (LV) ejection fraction (57.07 ±5.2%) and normal LV dimensions, without any statistically significant differences with the control group. The mean mitral valve orifice area was 1.39 cm 2±0.33 calculated by planimetry, 1.36 cm 2±0.29 as determined by the PHT method, with a mean transvalvular gradient of 7.59mmHg ± 3.42, while the mean pulmonary artery pressure at rest was 38.31mmHg (between 28.68 and 47.94mmHg) – Table 1.
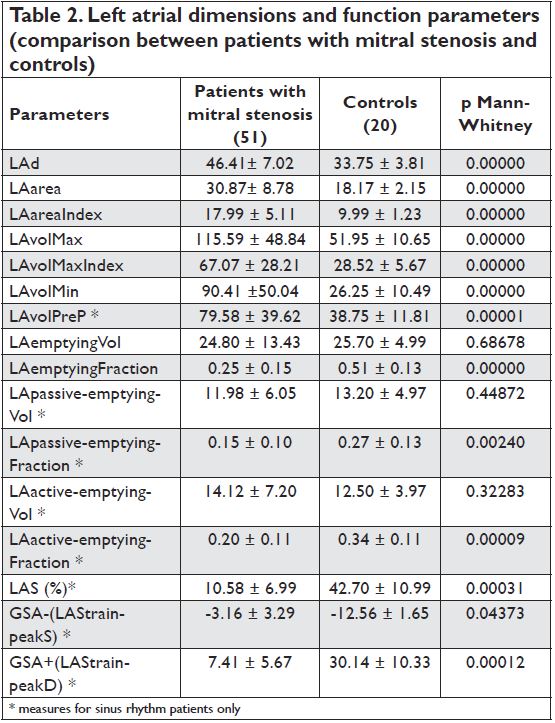
Left atrial dimensions
Compared with the control group, patients with mitral stenosis had significantly larger anterior-posterior LA diameter, area, indexed area and volumes, regardless of the hearth rhythm – Table 2.
Left atrial function in patients with mitral stenosis
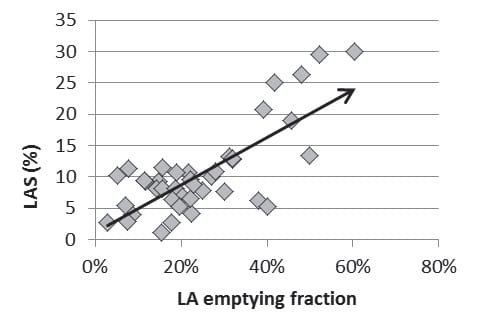
LA phasic function was evaluated by speckle tracking and volumetric methods. There were statistically significant differences between the 2 groups by both methods – all parameters regarding LA function were altered in patients with mitral stenosis compared with the control group (table 2). There was a strong correlation between the 2 methods in evaluating the reservoir function (p= 0.0001, r =0.577) (Figure 1) and conduit function (p=0.0013, r =0.627) but not for the booster pump function (p=0.3039, r = -0.218). Regardless of the mitral valve orifice area (over/under 1.5 cm2), there were no significant differences between LA dimensions (LAarea p=0.357; LAvolMaxIndex p=0.141; LAvolMin p=0,1254) and LA function parameters (LAemptyingFraction p=0.4546; LApassive-emptying Fraction p=0.1535; LAactive-emptying- Fraction p=0.8383; LAS p=0.4984; GSA+ p=0.6555; GSA- p=0.6891), although patients with hemodynamically significant mitral stenosis had higher mean transvalvular gradients.
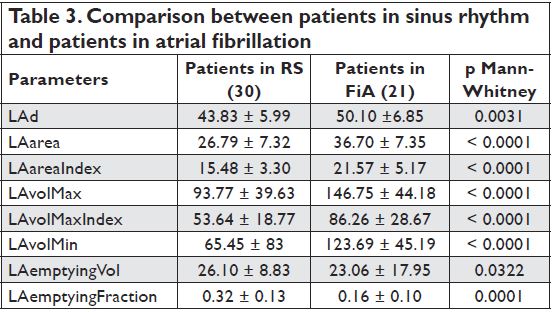
Left atrial function in patients with atrial fibrillation
While there were no significant differences between patients in sinus rhythm and those in atrial fibrillation (AF) regarding the severity of mitral stenosis (evaluated by the mean transvalvular gradient and mitral valve orifice area determined by planimetry and PHT), LA dimensions were significantly higher and the reservoir function was altered (measured by the volumetric method
– LAemptyingFraction p=0,0001) in patients with AF compared with those in sinus rhythm – Table 3.
Figure 1. Graphic representation of the correlation between LAS (%) and LAemptyingFraction.
Exercise echocardiography
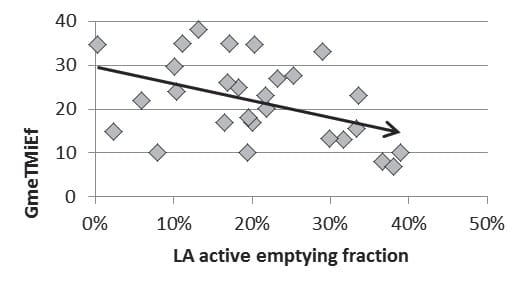
With the exception of LA active emptying fraction measured in patients in sinus rhythm that was inversely correlated with the mean transvalvular gradient measured during peak exercise (p=0.03, r = -0.40) and symptom intensity, there were no correlates between LA dimensions and function and exercise parameters (exercise duration, exercise tolerance, peak systolic pulmonary artery pressure) (Figure 2).
BNP dosing
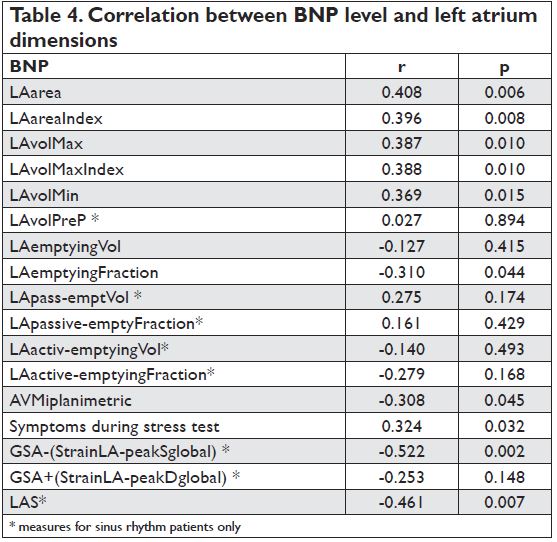
The mean BNP value was 172.7±126.3pg/ml. We found that there was a strong correlation between BNP level and mitral stenosis severity (mitral valve area by planimetry), LA dimensions and reservoir function (higher BNP values were associated with a decrease in the reservoir function) as well as with the booster pump function evaluated by speckle tracking – Table 4.
Figure 2. Graphic representation of the correlation between mean transvalvular gradient measured during peak exercise (GmeTMiEf) and LA active emptying fraction.
Follow-up
Patients were followed up for 1 up to 4 years, with a mean follow up period of 3.2 years. Form the 51 patients that were included in our study, 18 (35.29%) remained in the same NYHA class, while 33 patients (64.7%) saw a worsening in their clinical status. Although patients that deteriorated in time had higher LA dimensions and worse LA function parameters, these differences were not statistically significant. 17 patients were referred for surgery during follow up. Only the parameters evaluating mitral stenosis severity (mean transvalvular gradient, p=0.01, systolic pulmonary artery pressure, p=0.003) and exercise echocardiography parameters (exercise duration, p=0.02, symptom’s presence during exercise, p=0.04, mean transvalvular gradient during peak exercise, p=0.03 and peak systolic pulmonary artery pressure, p=0.02) were useful in predicting the need for surgery, while there was no link with LA echographic parameters. Form the 31 patients in sinus rhythm, 20 had either paroxysmal or permanent atrial fibrillation. Echographic parameters useful in predicting AF occurrence were LA dimensions (area, indexed area, maximal LA volume, indexed maximal LA volume, minimum LA volume and preP LA volume), mean transvalvular gradient and systolic pulmonary artery pressure and peak systolic pulmonary artery pressure during exercise (Table 5).
DISCUSSIONS
The most important findings of this study can be summarized as follows: a) LA dimensions are higher in patients with mitral stenosis, regardless of the severity of mitral stenosis; b) there is a significant decrease in LA phasic function, with LA booster pump function being the most affected; c) alterations in LA contractility are associated with symptoms occurrence and an increase in the mean transvalvular gradient during peak exercise; d) both the increase in LA size and the alteration of the LA reservoir and pump function are correlated with increased BNP serum levels; e) LA dilation is a predictor for AF occurrence. Stressors like LA volume and pressure overload are triggers for LA remodeling. The extent of LA remodeling is dependent on the duration and the intensity of the aggressive factors13. While at first these changes are reversible, as the time of exposure increases, irreversible morphologic and functional alterations may occur13. LA pressure overload secondary to mitral stenosis leads to LA dilation, as seen in our study, patients with mitral stenosis having increased LA dimensions regardless of the method used for measuring LA size
and regardless of the severity of mitral stenosis, which in turn suggests that there are other factors beside the valvular obstacle that may influence LA dimensions, like intraatriale pressure variability14 and valvular compliance15,16 and resistance17. Data derived from histopathological findings in atrial specimens obtained during surgery suggest there is an extensive interstitial fibrosis18 in both atria, associated with myocyte hypertrophy especially seen in patients in sinus rhythm, compared with patients with atrial fibrillation, where myocytolysis is the predominant histological change19. Myocyte hypertrophy is a marker for cellular degeneration, associated with significant ultrastructural changes, such as myofibrillar destruction, a process that leads in time to a decrease and even to a loss of contractile function18. During ventricular systole, the left atrium has a reservoir function, collecting blood drained by the pulmonary veins that leads to LA filling and distension8. In patients with mitral stenosis, elevated LA filling pressures lead to a decrease in pulmonary vein blood flow and, on the other side, to an increase in LA parietal tension, which in turn determines LA dilation and an alteration of the reservoir function20, 21, a fact observed in our study, where both LA emptying faction and LA global longitudinal strain were significantly lower in patients with mitral stenosis. During early diastole, once the mitral valve opens, the LA acts as a conduit, allowing passive blood flow from the atrium to the left ventricle, this function being influenced by both LV diastolic relaxation and by mitral valvular resistance23. In patients with mitral stenosis, although there is an increase in intra-atrial pressure and a decrease in LV intraventricular pressure, leading to a significant atrioventricular diastolic gradient, the passive blood fl ow during early diastole is restricted because of important valvular resistance. Thus, the LA conduit function is altered, a fact proven by the statistically significant decrease in patients with mitral stenosis in sinus rhythm of both LA passive emptying fraction and positive global longitudinal strain (GSA+), as seen in other studies that used both the speckle tracking20 and tissue Doppler methods24. The current study proved the existence of a negative correlation between GSA+ and mitral valvular resistance (p=0.004, r=-0.396). LA booster pump function plays a very important role in maintaining an adequate cardiac output in patients with mitral stenosis, despite the valvular obstacle, therefore a loss of this function may lead to a worsening of heart failure25. Compared with the control group, in patients with mitral stenosis both LA active emptying fraction and GSA- were significantly impaired, suggesting a failure of the LA pump secondary to LA dilation, a negative correlation between the two aforementioned parameters and LA maximal volume exists, similar to data form other studies26,27,28. Therefore, adaptative LA dilation as a response to LA pressure overload due to the valvular obstacle is correlated with a loss in LA contractility29.
Moreover, our study has shown a relationship between LA active emptying fraction and mitral stenosis severity (evaluated by the mean transvalvular gradient and mitral valve orifice area), probably explained by the increase in atrial pressures along with the progression of mitral stenosis, which in turn leads to a decrease in LA booster pump function, or during exercise, where symptom occurrence and the increase in transvalvular mean gradient were inversely correlated with LA booster pump function. Unlike a similar study performed by Ancona et al30, were systolic LA strain was a predictor for atrial fibrillation, the current study failed to establish a prognostic value for this parameter. Besides the aforementioned changes, the LA remodeling process has also functional consequences, leading to neurohormonal changes31, a fact confirmed in our study, where the increase in BNP was positively correlated with LA dimensions, and negatively correlated with mitral stenosis severity and LA reservoir and booster pump function. These data are similar to those found in other studies32 and the correlation between LA booster pump function assessed by GSA- and BNP was also described in other heart diseases. Interstitial fibrosis and cellular dissociation in patients with atrial dilation lead to electrical dispersion, a favorable substrate for initiating and maintaining reentry circuits which in turn are the main determinants for atrial fibrillation18. Atrial fibrillation is the most common arrhythmia in patients with mitral stenosis, and data from different studies34,35 suggest that atrial dilation is its main predictor36, as seen in the current study, where an increase in LA dimensions is associated with the risk for developing atrial fibrillation.
CONCLUSIONS
Mitral stenosis leads to extensive LA remodeling, having important structural and functional consequences with implications over LA mechanical, electrical (the occurrence of atrial fibrillation) and neurohormonal function. The assessment of LA dimension and function can therefore provide further insights in patients with mitral stenosis, independent of its severity, adding prognostic
and clinical value.
Conflict of interest: none declared.
References
1. Chandrashekhar Y, Westaby S, Narula J. Mitral stenosis. Lancet 2009; 374: 1271–1283
2. Abhayaratna W.P., Seward J.B., Appleton C.P., et al. Left atrial size: physiologic determinants and clinical applications. J Am Coll Cardiol 2006; 47: 2357–2363
3. Baumgartner H., Hung J., Bermejo J., et al. Echocardiographic assessment of valve stenosis: EAE/ASE recommendations for clinical practice European Journal of Echocardiography 2009; 10: 1 –25
4. Lancellotti P., Budts W., De Wolf D., et al. Practical recommendations on the use of echocardiography to assess pulmonary arterial hypertension–a Belgian expert consensus endorsed by the Working Group on Non-Invasive Cardiac Imaging. Acta Cardiol. 2013;68:59-69.
5. Lang RM, Badano LP, Mor-Avi V et al. Recommendations for cardiac chamber quantification by echocardiography in adults: an update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J Am Soc Echocardiogr. 2015; 28:1-39.e14.
6. Todaro M.C., Choudhuri I., Belohlavek M. et al. New echocardiographic techniques for evaluation of left atrial mechanics Eur Heart J. – Cardiovascular Imaging 2012; 13: 973–984
7. Nikitin N.P., Witte K.K., Thackray S.D., Goodge L.J., Clark A.L., Cleland J.G. Effect of age and sex on left atrial morphology and function. Eur J Echocardiogr, 2003;4: 36–42
8. Roşca M., Lancellotti P., Popescu B.A. et al., Left atrial function: pathophysiology, echocardiographic assessment, and clinical applications Heart 2011;97:1982-1989
9. Blume G.G., Mcleod C.J., Barnes M.E., et al. Left atrial function, physiology, assessment and clinical implications. Eur J Echocardiogr. 2011; 12:421-430
10. Mor-Avi V., Lang R.M., Badano L.P. et al. Current and Evolving Echocardiographic Techniques for the Quantitative Evaluation of Cardiac Mechanics: ASE/EAE Consensus Statement on Methodology and Indications Endorsed by the Japanese Society of Echocardiography. Eur J Echocardiogr .2011;12:167–205
11. Vianna-Pinton R, Moreno CA, Baxter CM. et al Two-dimensional speckle tracking echocardiography of the left atrium: feasibility and regional contraction and relaxation differences in normal subjects. J Am Soc Echocardiogr 2009; 22: 299–305
12. Kim D.G., Lee K.J. Lee S. et al. Feasibility of two-dimensional global longitudinal strain and strain rate imaging for the assessment of left atrial function: a study in subjects with a low probability of cardiovascular disease and normal exercise capacity,” Echocardiography, 2009; 26:1179–1187.
13. Casaclang-Verzosa G., Gersch B.J., Tsang T.S.M. et al. Structural and Functional Remodeling of the Left Atrium. Clinical and Therapeutical Implications for Atrial Fibrillation. J Am Coll Cardiol 2008;51:1-11.
14. Ko YG, Ha JW. Chung N. et al. Effects of Left Atrial Compliance on Left Atrial Pressure in Pure Mitral Stenosis Cathet Cardiovasc Intervent 2001;52:328–333
15. Nunes MCP, Hung J, Barbosa MM et al. Impact of Net Atrioventricular Compliance on Clinical Outcome in Mitral Stenosis Circ Cardiovasc Imaging. 2013; 6: 1001–1008
16. Kim HK, Kim YJ, Hwang SJ, et al. Hemodynamic and prognostic implications of net atrioventricular compliance in patients with mitral stenosis. J Am Soc Echocardiogr. 2008; 21: 482–486
17. Izgi C, Ozdemir N, Cevik C, et al. Mitral valve resistance as a determinant of resting and stress pulmonary artery pressure in patients with mitral stenosis: a dobutamine stress study. J Am Soc Echocardiogr 2007; 20:1160–1166.
18. Thiedemann K.U., Ferrans V.J. Left Atrial Ultrastructure in Mitral Valvular Disease American Journal of Pathology 1977; 89:575-594
19. Shenthar J., Kalpana S.R., Prabhu M.A. et al. Histopathological Study of Left and Right Atria in Isolated Rheumatic Mitral Stenosis With and Without Atrial Fibrillation. J Cardiovasc Electrophysiol. 2016; 27:1047-1054
20. Demirkol S., Kucuk U., Baysan O. et al. The Impact of Mitral Stenosis on Left Atrial Function Assessed by Two-Dimensional Speckle Tracking Echocardiography. Echocardiography 2012;29: 1064–1070
21. Caso P., Ancona R., Di Salvo G. et al., Atrial reservoir function by strain rate imaging in asymptomatic mitral stenosis: prognostic value at 3 year follow-up European Journal of Echocardiography, 2009; 10:753–759
22. Ancona R, Pinto C.S, Caso P. et al. Left atrium by echocardiography in clinical practice: from conventional methods to new echocardiographic techniques. ScientificWorldJournal. 2014; http://dx.doi.org/10.1155/2014/451042
23. Meisner JS, Keren G, Pajaro OE et al. Atrial Contribution to Ventricular Filling in Mitral Stenosis Circulation 1991;84:1469-1480
24. Nikdoust F, Sadeghian H, Lotfi -Tokaldany M. Regional quantifi cation of left atrial early diastolic strain in two groups of patients with mitral stenosis: normal sinus rhythm vs atrial fibrillation Echocardiography 2016; 33:1818-1822
25. Parris TM, Mintz GS, Ross J, et al. Importance of atrial contraction to left ventricular fi lling in mitral stenosis. Am J Cardiol 1988; 61:1135–36.
26. Triposkiadis F, Wooley CF, Boudoulas H. Mitral stenosis: left atrial dynamics reflect altered passive and active emptying. Am Heart J. 1990; 120(1):124-132.
27. Shin MS, Kim BR, Oh KJ, et al. Echocardiographic Assessments of Left Atrial Strain and Volume in Healthy Patients and Patients With Mitral Valvular Heart Disease by Tissue Doppler Imaging and 3-Dimensional Echocardiography Korean Circ J 2009; 39:280-287
28. Stefanadis C, Dernellis J, Stratos C, et al: Effects of balloon mitral valvuloplasty on left atrial function in mitral stenosis as assessed by pressure–area relation. J Am Coll Cardiol 1998;32:159–168.
29. Kono T, Sabbah HN, Rosman H, et al. Left atrial contribution to ventricular filling during the course of evolving heart failure. Circulation 1992;86:1317–22.
30. Ancona R., Pinto S.C., Caso P. et al. Two-Dimensional atrial systolic strain imaging predicts atrial fi brillation at 4-year follow-up in asymptomatic rheumatic mitral stenosis. J Am Soc Echocardiogr, 2013; 26:270–277.
31. Uçar O, Bayar N, Karagöz A et al. Valvular heart disease: plasma Btype natriuretic peptide levels in patients with pure rheumatic mitral stenosis. Acta Cardiol. 2012 ;67:59-64.
32. See comment in PubMed Commons belowGölbaşý Z., Uçar O., Yüksel A.G., et al. Plasma brain natriuretic peptide levels in patients with rheumatic heart disease. Eur J Heart Fail. 2004 ;6(6):757-60
33. Dogan C, Ozdemir N, Hatipoglu S et al. Relation of left atrial peak systolic strain with left ventricular diastolic dysfunction and brain natriuretic peptide level in patients presenting with ST-elevation myocardial infarction. Cardiovasc Ultrasound. 2013;11:24. doi:10.1186/1476-7120-11-24.
34. Diker E, Aydogdu S, Ozdemir M, et al. Prevalence and predictors of atrial fibrillation in rheumatic valvular heart disease Am J Cardiol, 1996; 77(1): 96-98
35. Kim H.J., Cho G.Y., Kim Y.J. ,et al. Development of atrial fibrillation in patients with rheumatic mitral valve disease in sinus rhythm. Int J Cardiovasc Imaging. 2015; 31(4):735-742
36. Hoit B.D. Left Atrial Size and Function. Role in Prognosis. J Am Coll Cardiol 2014; 63(6): 493-505.


 This work is licensed under a
This work is licensed under a