Ioana Petre1, Silvia Iancovici2, Sebastian Onciul1, Oana Florentina Tautu1,2, Alexandrina Nastasa2, Maria Dorobantu1,2
1 “Carol Davila”University of Medicine and Pharmacy, Bucharest, Romania
2 Department of Cardiology, Clinical Emergency Hospital, Bucharest, Romania
<strong>Abstract: Objectives – We thought to evaluate left atrium (LA) as an attempt to find an echocardiographic marker of paroxysmal atrial fibrillation (AF) in hypertensive patients without any significant disease. Methods – We prospectively enrolled two groups of hypertensives: forty six patients with hypertension (HTN) without history of arrhythmia (group 1) and twenty three patients with HTN and a recent episode of documented paroxysmal AF (group 2). Left atrial (LA) diameters (anteroposterior, transversal and longitudinal) and LA phasic volumes (maximal, minimal and before atrial contraction) were assessed by 2D echocardiography and left atrial emptying functions (LAEF) (global, active and passive) were calculated; also 3D acquisitions of maximal and minimal volumes of LA were performed. Using the current recommendations of speckle tracking technique, peak longitudinal strain of left atrial walls (PALS) and peak atrial contraction strain (PACS) (mean value from apical four chambers and two chambers view) were analyzed. Results – Age (59.5y+-12 vs 67.7ys+-8, p=0.003) and the mean time of HTN diagnosis (42 mo+-6 vs 80 mo+8, p=0.007) were significantly higher in group 2; the other cardio-vascular risk factors (smoking, dyslipidemia) were similar as prevalence. We found all 2D and 3D LA volumes significantly greater in group 2 (for all p<0.0001) and global, active and passive LA EF significantly lower in the same group, compared with the other (p<0.0001, p<0.0001 and p<0.02). In comparison with 2D values, 3D volumes and emptying fractions were lower, but with no statistical significance. The peak longitudinal strain values were also significantly lower in AF group versus HTN control (26.1+-7.4 vs 15.7+-5.6, p<0.0001) while peak contraction strain was nonsignificant in the two groups. Conclusion – The echocardiographic evaluation of LA function using 2D and 3D volumetric and speckle tracking methods can be successfully used in identifying hypertensive patients at risk of atrial fibrillation. Keywords: hypertension, atrial fibrillation, atrial remodelling, 3D-echocardiography, strain
BACKGROUND
It is well known that hypertension (HTN) increases the pressure in the left atrium (LA), in time modifying the size of this heart chamber that can lead to apprai-sal of atrial fibrillation (AF). HTN remains one of the most important factors for development of AF1,2. In fact, there is a structural and functional atrial remo-deling, with the first changes occurring in the elastic properties of myocardium and refl ecting in LA dys-function, with initial preservation of normal LA size. The left atrial dilatation makes part of the end stage of this process of remodeling. The novel echocardiogra-phic techniques as 3D echocardiography and speckle tracking echocardiography made possible the accura-te assessment of LA function by being able to detect even minor changes3,4. The study of LA myocardial de-formation as two dimensional strain images provides a global but also regional wall function assessment. The clinical importance of this evaluation was established in recent studies that showed its prognostic role in cardiovascular disease5-8.
MATERIAL AND METHODS
Study population
Forty six hypertensive patients without any other cardiac event were prospectively enrolled as the first group. Other twenty four hypertensive patients with a recent episode of atrial fibrillation, while in sinus rhythm were recruited, forming the second group. Interview, physical examination, electrocardiogram and echocardiography were performed in all patients. Subjects with secondary HTN, thyroid dysfunctions, diabetes mellitus or any other significant heart disease (LV ejection fraction <55%, arrhythmias, pacing, signi-ficant valvulopathies, known coronary disease) were excluded. Also, the patients with low quality echocar-diographic views were excluded, because left atrial analysis requires optimal visualization of endocardial borders.
Blood pressure was measured at the brachial artery with the Korotkoff method, in the supine position, by performing a mean value of two measurements in con-formity with guideline recommendations9.
Subjects were followed for twelve months.
Echocardiographic study
All patients were investigated by echocardiography, using a Vingmed Vivid 7 and a Vivid E9 unit with the possibility of offline analysis of the recordings. Pati-ents were studied in the left lateral position and the measurements were done according to the current guidelines recommendations10. During the evaluation all patients were in sinus rhythm.
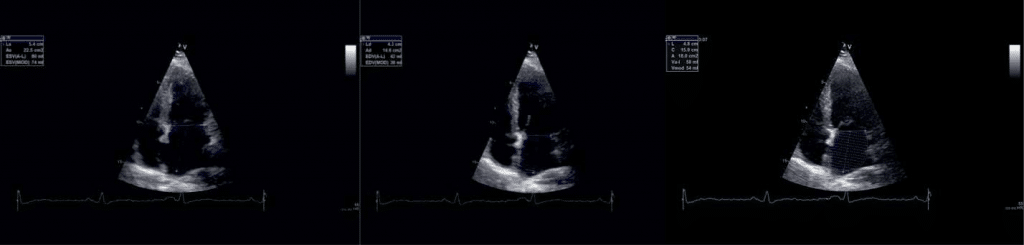
Figure 1. Phasic atrial volumes in 4c apical views: a) LAVmax, b) LAV min, c) LAV preA.
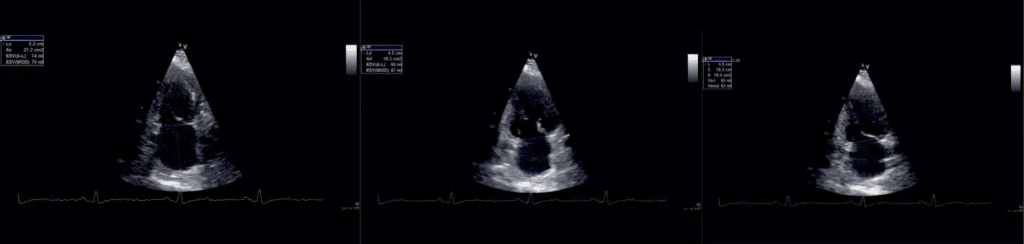
Figure 2. Phasic atrial volumes in 2c apical views: a) LAVmax, b) LAV min, c) LAV preA.
2D left atrial phasic volumes and emptying fractions
LA volumes were evaluated in the apical four and two chamber views, using the Simpson biplane method.
During a cardiac cycle, three volumes were measured:
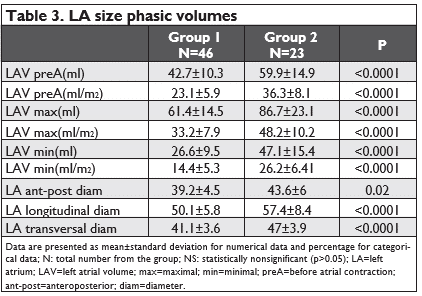
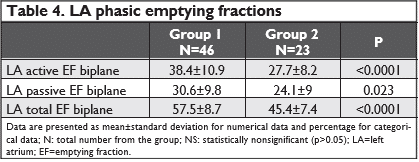
-maximal LA volume (LAVmax) – the volume that occurs at the end of ventricular systole, just be-fore mitral valve opening (the end of the T wave of the ECG) (Figure 1a and Figure 2a).
-minimal LA volume (LAVmin) that occurs at mi-tral valve closure(in enddiastole) (Figure 1b and Figure 2b).
-the volume before active atrial contraction (LAV preA) – timed to the onset of the P wave (Figure3a and Figure 3b).
All the volumes were divided to the body surface area, obtaining LAVmax, LAV min and LAVpreA in-dices. The LA phasic functions were appreciated: the reservoir, the conduit and the booster pump function by calculation total, passive and active emptying frac-tion (Table 1).
3D left atrial assessment
3D echocardiography brings the advantage of mea-suring cardiac cavities with accuracy, without making assumptions, by reconstructing volumes from endo-cardial contours of the entire chamber. The examina-tions were performed using a 3D matrix array transducer with a temporal resolution of 8-22 frames/s. A wide angled full volume acquisition mode was used, in apnea, with six cardiac cycles recording. Offline data was analyzed-the pyramidal volume was displayed in three different cross-sections (two orthogonal apical and one short axis) that could be modified by manu-al shifting of vertical and horizontal lines. Five points were manually placed for left atrium: two points to identify mitral annulus in each apical views and one point to identify the centrum of the posterior wall in either view, than the software automatically iden-tified the endocardial surface. Manual adjustments were made in order to exclude atrial appendage and pulmonary veins from the cavity volumes. The frame with the biggest and smallest atrial dimension were selected and analyzed in this way and atrial maximum (3DLAVmax) and minimum (3DLAVmin) volumes were obtained and atrial EF were derived from the two volumes.
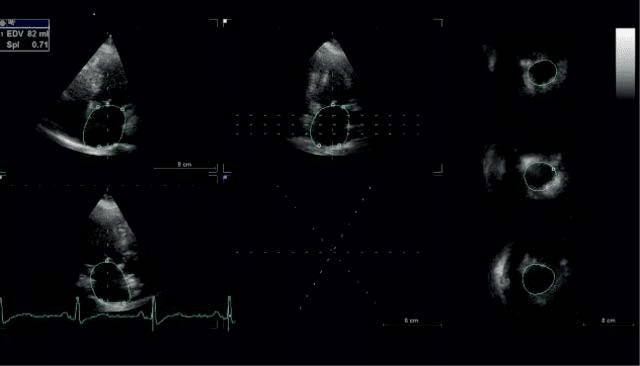
Figure 3. 3D echocardiographic evaluation of left atrium-endocardial border in the 2D cross-sections.
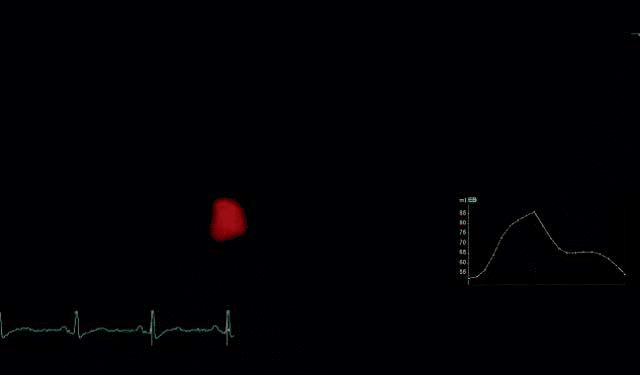
Figure 4. 3D echocardiographic evaluation of left atrium-the 3D LA re-constructed volume.
2D speckle tracking is a new echo technique that analyses myocardium deformation with no angle de-pendency (a nondoppler method)11,12.
Apical four chamber and two chamber views during breath hold were obtained, with a stable ECG recor-ding. Good quality recordings were needed, allowing reliable delineation of myocardial tissue. The settings were made for obtaining an adequate temporal and spatial resolution, with recording of three consecutive heart cycles, using a narrow sector, with optimization of visualizing LA cavity and a frame rate set between 50 and 80 frames per second. LA endocardial border was manually traced in endsystole in both apical views and an epicardial surface tracing was generated by the system, creating the region of interest (ROI) of 6 seg-ments in each apical view. The software also generates strain curves for each atrial segment, throughout the entire cardiac cycle. LA strain has a typical pattern – a high positive wave at the end of ventricular systole (PALS) followed by two distinct descending phases in early diastole and late systole (PACS). The mean measurements for each view was automated generated as a separate white curve. Global PALS and PACS was calculated by averaging the 12 LA segments.
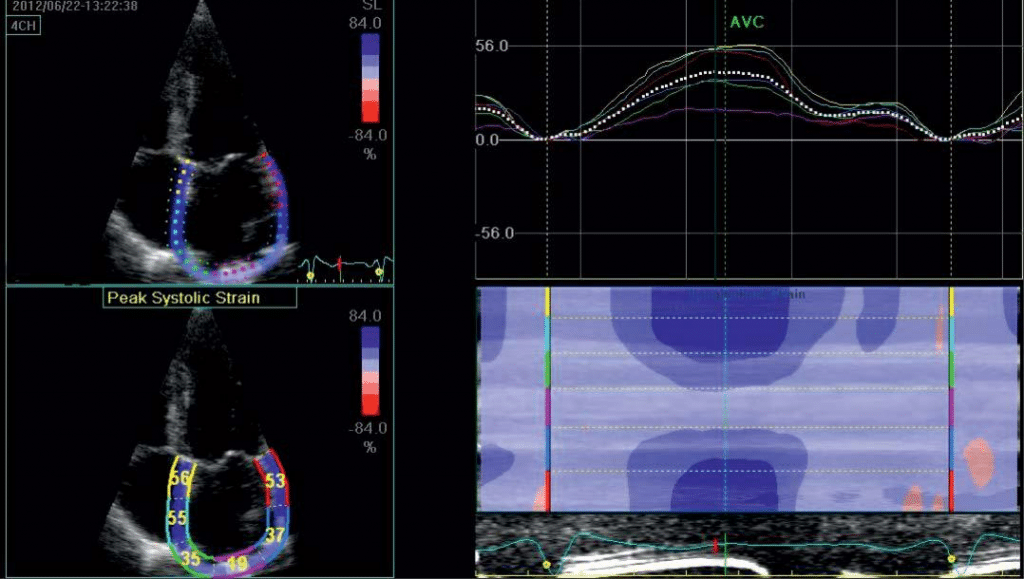
Figure 5. Assesing left atrial strain in apical 4c view.
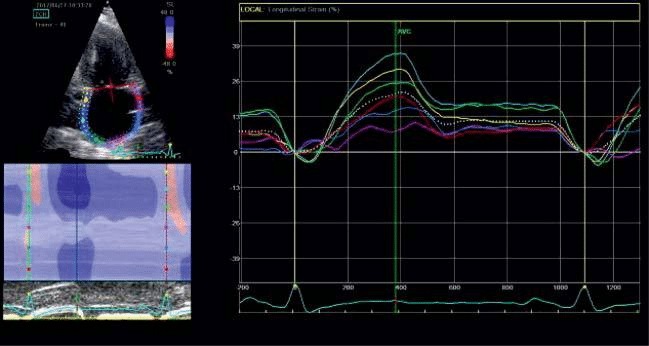
Figure 6. Assesing left atrial strain in apical 2c view.
RESULTS
Baseline characteristics
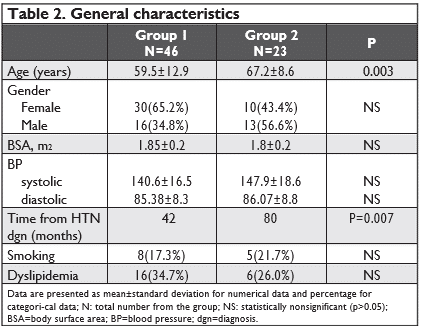
The frequency of female gender was higher in group 1 (65%) comparative to the arrythmia group (43.4%.) Age(59.5y+-12 vs 67.2y+-8, mean time of HTN diagnosis (42 mo+-6 vs 80 mo+8, p=0.007) were significantly higher in group 2; the other cardiovascular risk factors (smoking, dyslipidemia) were similar as prevalence. Table 2 summarizes general characteristics of the two groups.
All the echocardiographic parameters were age and sex-matched.
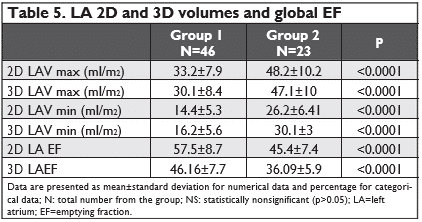
2D LA volumes and emptying fractions
All LA volumes (LAVmax, LAVmin, LAVpreA) and in-dexed values were greater in atrial fibrillation group, with high significance (for all p<0.0001). Also, we found significant lower emptying fractions (active, pas-sive and global) in group 2, proving a more advanced dysfunction in hypertensive patients with atrial fibrillation.
3DLA volumes and emptying fraction
As it was expected, 3D volumes and emptying fractions were also more affected in group 2. In comparison with 2D values, 3D volumes and emptying fractions were lower, but with no statistical significance.
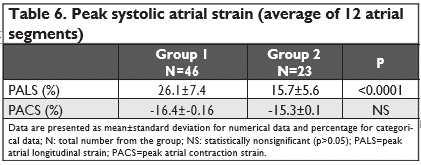
LA strain-PALS and PACS
The positive peak atrial strain values (mean for 12 atrial segments) were significantly higher in the first group (26.1% versus 15.7%, p<0.0001), while the peak contraction strain was lower in the first group, but without any statistical relevance.
FOLLOW-UP
Ten patients has at least an episode of atrial fibrillation in the 12 months of follow up, three patients from group 1 as a first episode of AF (representing 6.7%) and seven from the second group (30% from the group population) had at least a recurrence of AF, from which two of them remained in atrial fibrillation rhythm. None of the subjects had new onset stroke the 12 months follow up.
DISCUSSION
Assessing LA function has a recognized role in progno-sis and risk stratification8,13. LA structural remodeling is a stable indicator of diastolic LV dysfunction and high filling pressures14,15 that we commonly find in hyper-tensive patients. Progressive changes in LA structure leads to fibrosis, which represents a favorable milieu for arrhythmia. So, analysis of LA function can help us to detect the hypertensives at risk of atrial fibrillation. In many studies it was concluded that LA enlargement is an independent factor for atrial fibrillation apprai-sal16-18, but, in the same time, atrial dilatation can be seen as a result of AF. There is an interdependency between the two factors. The increase overload in left atrium (as seen in hypertensives) leads to expansion of the left atrium myocardium, enhancing its reservoir and contractile function to a critical point, after which the emptying fraction starts to decline (Franck Starling law). The structural impairment leads to electrical dis-turbation, that influences the myocardial contraction sequence increasing also the structural alteration.
Guidelines strongly recommend the echocardiogra-phic measurement of LA maximal volume(measured in apical four chamber view, indexed for BSA). LAVI has proved its value in clinical practice, being an esta-blished marker for assessing prognosis in general po-pulation19. Though most studies investigated LAVmax, lately also LAV min proved to be an accurate para-meter for adverse outcomes, as for paroxysmal atrial fibrillation20. Several studies have showed reproducibi-lity and feasibility of 3D echocardiography in detecting structural and functional changes in LA, adding a low interobserver variability in comparison with traditio-nal echocardiography21,22. Though it is not a method used on a large scale, normal ranges for 3D maximal and minimal volume and LAEF were defined, with a LAEF low value limit at 45%23. Our results were si-milar with those presented in literature and we also found a good correlation with 2D results.
Atrial fibrillation aggravates all LA function: reservo-ir, conduit and contractile function24,1. In some studies, LA passive emptying function was found increased in patients with hypertension and atrial fibrillation com-parative to hypertensive in sinus rhythm25. The expla-nation was that the recoil function of left atrium is altered in atrial fibrillation, creating increased LA pre-ssure and an early increase in passive emptying function. In our study the passive LA emptying function was lower in atrial fibrillation group, but with lower statistical relevance compared to the differences of the other LA functions in the two groups.
The contractile function of LA was disturbed in our study in both hypertensives groups, with a higher al-teration in atrial fibrillation group. At first, LA tries to compensate decreased LV filling by increasing its contractile function and in some studies the contracti-le function of LA was enhanced compared to normo-tensives. With progression of the hypertension and enlargement of LA, contractile function tends to dete-riorate; also the electrical disorder, as FA, affects the contractile sequence resulting in more reduced LA ac-tive emptying18. This was proven not only by volume-tric measurements, but also through speckle tracking appreciation of atrial myocardial deformation26,27. LA booster pump function appreciated by PACS was de-creased in hypertensives with atrial fibrillation versus without AF, as expected, because of a more advan-ced remodeling atrial process. Some studies have used the echocardiographic assessment of LA function to predict recurrence of atrial fibrillation. The best pre-dictors were peak atrial strain, as a measure of atrial reservoir capacity28,29.
In our study we found also that the hypertension period duration is important and can be considered an underlying precipitating factor for atrial fibrillation. On the other hand, the long term effects of hypertension (and not only the atrial fibrillation) could explain the differences in LA volumes and function.
LIMITATIONS
The small number of patients in both groups and the heterogeneity of the hypertensives(all classes of hyper-tension, that were treated with different drug combi-nations) represent some limitations of this study.
Between the two groups there was a statistically significant difference of the age of the population. Even we tried to compensate this finding by age-matching all the parameters, the increased age in the AF group comparing to the other group might have influenced the results.
CONCLUSIONS
Deterioration of left atrial function is frequently seen in HTN, but dysfunction is higher when atrial fibrilla-tion occurs, suggesting a more advanced process of atrial remodeling and fibrosis. Hypertensives should be closely monitored and evaluated for detection of left atrial dysfunction. Echocardiography-2d assessment and the novel methods as speckle tracking and 3D echocardiography proved to be a reliable method for evaluation of LA remodeling.
Acknowledgement: This work was supported by CREDO Project – ID: 49182, fi nanced by the Natio-nal Authority of Scientifi c Research and Innovation, on behalf of the Romanian Ministry of European Funds-through the Sectoral Operational Programme “Increa-sing of Economic Competitiveness”, Priority Axis 2, Operation 2.2.1 (SOP IEC -A2-0.2.2.1-2013-1) cofi-nanced by the European Regional Development Fund. Conflict of interest: none declared.
References:
1. E. L. Simpson, M. D. Stevenson, a. Scope, E. Poku, J. Minton, and P. Evans, “Echocardiography in newly diagnosed atrial fi brillation pa-tients: A systematic review and economic evaluation,” Health Tech-nol. Assess. (Rockv)., vol. 17, no. 36, pp. 1–263, 2013.
2. J.-H. Lee and J.-H. Park, “Role of echocardiography in clinical hyper-tension,” Clin. Hypertens., vol. 21, p. 9, 2015.
3. C.-T. Tsai, C.-L. Hung, C. J.-Y. Hou, T.-C. Hung, H.-I. Yeh, and C.-H. Tsai, “Real-Time Three-Dimensional Echocardiography in the Evalu-ation of Left Atrial Structure and Function in Normal, Aging, Hyper-tensive and Heart Failure Patients: New Insights into Left Atrial Ad-aptation and Remodeling,” Int. J. Gerontol., vol. 3, no. 1, pp. 53–65, Mar. 2009.
4. M. C. Todaro, I. Choudhuri, M. Belohlavek, A. Jahangir, S. Carerj, L. Oreto, and B. K. Khandheria, “New echocardiographic techniques for evaluation of left atrial mechanics,” Eur. Hear. J. – Cardiovasc. Imaging , vol. 13 , no. 12 , pp. 973–984, Dec. 2012.
5. H. Tamura, T. Watanabe, S. Nishiyama, S. Sasaki, T. Arimoto, H. Takahashi, T. Shishido, T. Miyashita, T. Miyamoto, J. Nitobe, O. Hi-rono, and I. Kubota, “Increased Left Atrial Volume Index Predicts a Poor Prognosis in Patients With Heart Failure,” J. Card. Fail., vol. 17, no. 3, pp. 210–216, Sep. 2014.
6. Y. Takemoto, M. E. Barnes, J. B. Seward, S. J. Lester, C. A. Appleton,
B. J. Gersh, K. R. Bailey, and T. S. M. Tsang, “Usefulness of Left Atrial Volume in Predicting First Congestive Heart Failure in Patients ≥65 Years of Age With Well-Preserved Left Ventricular Systolic Func-tion,” Am. J. Cardiol., vol. 96, no. 6, pp. 832–836, Sep. 2014.
7. G. G. Blume, C. J. Mcleod, M. E. Barnes, J. B. Seward, P. A. Pellikka, P.M. Bastiansen, and T. S. M. Tsang, “Left atrial function: physiology, assessment, and clinical implications,” Eur. J. Echocardiogr., vol. 12, no. 6 , pp. 421–430, Jun. 2011.
8. B. D. Hoit, “Left atrial size and function: role in prognosis.,” J. Am. Coll. Cardiol., vol. 63, no. 6, pp. 493–505, Feb. 2014.
9. G. Mancia, R. Fagard, K. Narkiewicz, J. Redon, A. Zanchetti, M. Böhm, T. Christiaens, R. Cifkova, G. De Backer, A. Dominiczak, M. Galderisi, D. E. Grobbee, T. Jaarsma, P. Kirchhof, S. E. Kjeldsen, S. Laurent, A. J. Manolis, P. M. Nilsson, L. M. Ruilope, R. E. Schmieder, P. A. Sirnes, P. Sleight, M. Viigimaa, B. Waeber, F. Zannad, M. Burni-er, E. Ambrosioni, M. Caufield, A. Coca, M. H. Olsen, C. Tsioufis, P. Van De Borne, J. L. Zamorano, S. Achenbach, H. Baumgartner, J. J. Bax, H. Bueno, V. Dean, C. Deaton, C. Erol, R. Ferrari, D. Hasdai, A.W. Hoes, J. Knuuti, P. Kolh, P. Lancellotti, A. Linhart, P. Nihoyan-nopoulos, M. F. Piepoli, P. Ponikowski, J. L. Tamargo, M. Tendera, A. Torbicki, W. Wijns, S. Windecker, D. L. Clement, T. C. Gillebert, E.A. Rosei, S. D. Anker, J. Bauersachs, J. B. Hitij, M. Caulfield, M. De Buyzere, S. De Geest, G. A. Derumeaux, S. Erdine, C. Farsang, C. Funck-Brentano, V. Gerc, G. Germano, S. Gielen, H. Haller, J. Jor-dan, T. Kahan, M. Komajda, D. Lovic, H. Mahrholdt, J. Ostergren, G. Parati, J. Perk, J. Polonia, B. a. Popescu, Ž. Reiner, L. Rydén, Y. Sirenko, A. Stanton, H. Struijker-Boudier, C. Vlachopoulos, M. Volpe, and D. a. Wood, “2013 ESH/ESC guidelines for the management of arterial hypertension: The Task Force for the management of arte-rial hypertension of the European Society of Hypertension (ESH) and of the European Society of Cardiology (ESC),” Eur. Heart J., vol. 34, no. May, pp. 2159–2219, 2013.
10. R. M. Lang, L. P. Badano, V. Mor-Avi, J. Afilalo, A. Armstrong, L. Er-nande, F. a Flachskampf, E. Foster, S. a Goldstein, T. Kuznetsova, P. Lancellotti, D. Muraru, M. H. Picard, E. R. Rietzschel, L. Rudski, K. T. Spencer, W. Tsang, and J.-U. Voigt, “Afbeelding 5,” J. Am. Soc. Echo-cardiogr., vol. 28, no. 1, pp. 1–39.e14, 2015.
11. M. Cameli, M. Caputo, S. Mondillo, P. Ballo, E. Palmerini, M. Lisi, E. Marino, and M. Galderisi, “Feasibility and reference values of left atri-al longitudinal strain imaging by two-dimensional speckle tracking.,” Cardiovasc. Ultrasound, vol. 7, p. 6, Jan. 2009.
12. S. Mondillo, M. Cameli, M. L. Caputo, M. Lisi, E. Palmerini, M. Pade-letti, and P. Ballo, “Early detection of left atrial strain abnormalities by speckle-tracking in hypertensive and diabetic patients with normal left atrial size.,” J. Am. Soc. Echocardiogr., vol. 24, no. 8, pp. 898– 908, Aug. 2011.
13. G. G. Blume, C. J. McLeod, M. E. Barnes, J. B. Seward, P. a. Pellikka,P. M. Bastiansen, and T. S. M. Tsang, “Left atrial function: Physiology, assessment, and clinical implications,” Eur. J. Echocardiogr., vol. 12, no. May, pp. 421–430, 2011.
14. C. P. Appleton and S. J. Kovács, “The Role of Left Atrial Function in Diastolic Heart Failure,” Circ. Cardiovasc. Imaging , vol. 2 , no. 1 , pp. 6–9, Jan. 2009.
15. S. Arques, M.-P. Jaubert, L. Bonello, P. Sbragia, A. Nicoud, and F. Paganelli, “Usefulness of left atrial volume for the diagnosis of dia-stolic heart failure: an echocardiographic-catheterization study.,” Int.J. Cardiol., vol. 144, no. 2, pp. 317–9, Oct. 2010.
16. G. Casaclang-Verzosa, B. J. Gersh, and T. S. M. Tsang, “Structural and Functional Remodeling of the Left Atrium. Clinical and Therapeutic Implications for Atrial Fibrillation,” J. Am. Coll. Cardiol., vol. 51, no. 1, pp. 1–11, 2008.
17. W. P. Abhayaratna, J. B. Seward, C. P. Appleton, P. S. Douglas, J. K. Oh, A. J. Tajik, and T. S. M. Tsang, “Left atrial size: physiologic deter-minants and clinical applications.,” J. Am. Coll. Cardiol., vol. 47, no. 12, pp. 2357–2363, Jun. 2006.
18. E. Tenekecioglu, F. V. Agca, O. A. Ozluk, K. Karaagac, S. Demir, T. Peker, M. Kuzeytemiz, M. Senturk, and M. Yilmaz, “Disturbed Left Atrial Function is Associated with Paroxysmal Atrial Fibrillation in Hypertension.,” Arq. Bras. Cardiol., vol. 102, pp. 253–62, 2014.
19. S. Gupta, S. A. Matulevicius, C. R. Ayers, J. D. Berry, P. C. Patel, D.W.Markham, B. D. Levine, K. M. Chin, J. A. de Lemos, R. M. Pe-shock, and M. H. Drazner, “Left atrial structure and function and clinical outcomes in the general population,” Eur. Hear. J. , vol. 34 , no. 4, pp. 278–285, Jan. 2013.
20. M. Schaaf, P. Andre, M. Altman, D. Maucort-Boulch, J. Placide, P. Chevalier, C. Bergerot, and H. Thibault, “Left atrial remodelling as-sessed by 2D and 3D echocardiography identifies paroxysmal atrial fibrillation,” Eur. Heart J. Cardiovasc. Imaging, vol. 18, no. May, pp. 46–53, 2017.
21. A. M. Anwar, O. I. I. Soliman, M. L. Geleijnse, A. Nemes, W. B. Vlet-ter, and F. J. ten Cate, “Assessment of left atrial volume and function by real-time three-dimensional echocardiography.,” Int. J. Cardiol., vol. 123, no. 2, pp. 155–61, Jan. 2008.
22. C. Jenkins, K. Bricknell, and T. H. Marwick, “Use of real-time three-dimensional echocardiography to measure left atrial volume: com-parison with other echocardiographic techniques.,” J. Am. Soc. Echocardiogr., vol. 18, no. 9, pp. 991–997, Sep. 2005.
23. E. Aune, M. Baekkevar, J. Roislien, O. Rodevand, and J. E. Otterstad, “Normal reference ranges for left and right atrial volume indexes and ejection fractions obtained with real-time three-dimensional echocardiography,” Eur. J. Echocardiogr., vol. 10, no. January, pp. 738–744, 2009.
24. K. Sakabe, N. Fukuda, Y. Fukuda, S. Morishita, H. Shinohara, and Y. Tamura, “Prediction of transition to chronic atrial fibrillation in el-derly patients with nonvalvular paroxysmal atrial fibrillation by transthoracic Doppler echocardiography,” Clin. Cardiol., vol. 32, no. De-cember 2004, pp. 23–28, 2009.
25. Q. Cui, H. Wang, W. Zhang, H. Wang, X. Sun, Y. Zhang, and H. Yang, “Enhanced left atrial reservoir, increased conduit, and weak-ened booster pump function in hypertensive patients with paroxys-mal atrial fibrillation.,” Hypertens. Res., vol. 31, no. 3, pp. 395–400, Mar. 2008.
26. M. Henein, Y. Zhao, M. Y. Henein, and P. Lindqvist, “Disturbed left atrial mechanical function in paroxysmal atrial fi brillation: a speckle tracking study.,” Int. J. Cardiol., vol. 155, no. 3, pp. 437–441, Mar. 2012.
27. M. Sahebjam, A. Mazareei, M. Lotfi-Tokaldany, N. Ghaffari, A. Zo-roufian, and M. Sheikhfatollahi, “Comparison of Left Atrial Function between Hypertensive Patients with Normal Atrial Size and Normo-tensive Subjects Using Strain Rate Imaging Technique,” Arch. Car-diovasc. Imaging, vol. 2, no. 1, pp. 1–6, 2014.
28. G. Di Salvo, P. Caso, R. Lo Piccolo, A. Fusco, A. R. Martiniello, M. G. Russo, A. D’Onofrio, S. Severino, P. Calabro, G. Pacileo, N. Mininni, and R. Calabro, “Atrial myocardial deformation properties predict maintenance of sinus rhythm after external cardioversion of recent onset lone atrial fibrillation: a color Doppler myocardial imaging and transthoracic and transesophageal echocardiographic study.,” Circu-lation, vol. 112, no. 3, pp. 387–395, Jul. 2005.
29. A. En, P. Sometidos, a C. Valvular, N. L. Muñoz, I. M. Fernández, R. M. De Oca, G. M. Mercado, L. a Flashman, K. Paulsen, R. M. Green-wald, L. Gabrielli, P. M. Nab, H. Verdejo, D. K. Gupta, A. M. Shah, R. P. Giugliano, C. T. Ruff, E. M. Antman, L. T. Grip, N. Deenadayalu, E. Hoffman, I. Patel, M. Shi, M. Mercuri, V. Mitrovic, E. Braunwald, S. D. Solomon, C. Gutierrez, D. G. Blanchard, G. Y. H. Lip, R. Unido, U. Schotten, P. Bajos, I. S. Reino, S. Ernst, R. Unido, I. C. Van Gelder, P. Bajos, N. A. Francia, G. H. Alemania, B. Prendergast, R. Unido, H. H. Bélgica, J. D. S. Bélgica, A. G. Alemania, B. G. Turquía, P. Ponikowski, F. H. Rutten, P. Bajos, R. N. Melgarejo, A. Y. Shaikh, A. Maan, U. a Khan, G. P. Aurigemma, J. C. Hill, J. L. Kane, D. a Tighe, E. Mick, D. D. McManus, J. Tazar, M. E. De Haro, N. Espínola-zavaleta, P. Haurigot, W. C. Tsai, C. H. Lee, C. C. Lin, Y. W. Liu, Y. Y. Huang, W. T. Li, J. Y. Chen, and L. J. Lin, “Speckle echocardiographic left atrial strain and stiffness index as predictors of maintenance of si-nus rhythm after cardioversion for atrial fi brillation: a prospective study.,” Rev. Colomb. Cardiol., vol. 10, p. 48, 2014.
 This work is licensed under a
This work is licensed under a