Gabriela Silvia Gheorghe1,2, Andreea Simona Hodorogea1,2, Irina Parvu2, Adriana Mihaela Iliesiu1,2, Ana Ciobanu1,2, Ioan Tiberiu Nanea1,2
1 “Carol Davila” University of Medicine and Pharmacy, Bucharest, Romania
2 Department of Internal Medicine and Cardiology, “Theodor Burghele” Clinical Hospital, Bucharest, Romania
Abstract: Purpose – The objective of this study was to evaluate the prevalence of subclinical thyroid dysfunction in patients(pts) aged over 60 admitted to a cardiology department for persistent atrial fi brillation (AF). Methods – Inclusion criteria were: age over 60, persistent nonvalvular AF, hospitalized within 6 months, beta blocker therapy at maximum dose. Exclusion criteria were: acute coronary syndromes, known thyroid disease, treatment with amiodarone, thyroid changes on ultrasound, congestive heart failure class III/IV, left ventricular ejection fraction (LVEF)<40%, severe comorbidities. In all patients cardiovascular history was noted, TSH, freeT4 (FT4) were determined and thyroid ultrasound was performed. Patients with TSH and FT4 within normal ranges were considered euthyroid, those with TSH below 0.1 mU/L and normal FT4 were diagnosed with clinically latent hyperthyroidism and those with TSH over 6.5 mU/L and normal FT4 with clinically latent hypothyroidism. Results – 290 patients were included, 52.2% men, mean age 72.14±12, mean LVEF 48.4±4%. 274 patients were euthyroid, 6(2%) had subclinical hypothyroidism and 10(3.44%) subclinical hyperthyroidism, with mean resting heart rate (HR)10 beats/min more than euthyroid pts. Conclusions – Clinically latent hyperthyroidism was found in 3.4% of patients with persistent AF aged over 60, with higher prevalence than in the general population and higher rest HR than in euthyroid patients. Latent hypothyroidism was found in 2% of patients with persistent AF, to a lesser extent than in the general population.
Keywords: subclinical hyperthyroidism, subclinical hypothyroidism, atrial fibrillation, heart failure
BACKGROUND
Subclinical thyroid dysfunction is defined as asymptomatic thyroid tests abnormalities in people with no history of thyroid disease or treatment with thyroid hormones. Subclinical hypothyroidism is defined by increased TSH levels over 6.5 mU/l concurrently with normal T4 and/or T3. Subclinical hyperthyroidism is defined by low TSH levels, generally below 0.1 mU/l, with normal T4 and/or T31. TSH determination as a screening test in the general population has low positive predictive value1 and test interpretation is infl uenced by concomitant diseases that alter thyroid function. Subclinical hyperthyroidism is associated with atrial fi brillation, dementia, osteoporosis but not with progression towards overt hyperthyroidism1. The association of subclinical hypothyroidism with faster development of atherosclerosis in adults is not clearly established1. In the general population the prevalence of subclinical hypothyroidism is of 5% in women and 3% in men, increasing with age, whilst the prevalence of subclinical hyperthyroidism is of 1% in men and 1.5% in women over 60 years1. Atrial fibrillation is the most common arrhythmia in the general population, with a prevalence of 1.5-2%, which increases with age2. In the US, 12% of pts with atrial fibrillation are between 75 and 84 years old. Over 30% of pts with atrial fi brillation are over 80 years old3. Myocardial ischemia, atrial dilation, heart failure, overt hyperthyroidism are important factors involved in the occurrence of atrial fibrillation. It is possible that thyroid dysfunction may intervene in the occurrence of atrial fibrillation. Revealing subclinical thyroid dysfunction could have therapeutic consequences.
AIM
The objective of this study was to evaluate the prevalence of subclinical thyroid dysfunction in pts aged over 60 admitted to a cardiology department for persistent atrial fibrillation.
METHODS
Inclusion criteria were: age over 60 years, persistent nonvalvular atrial fibrillation- defined by atrial fibrillation lasting for more than 7 days, hospitalized within 6 months, beta blocker therapy for ventricular rate control. Exclusion criteria were atrial fi brillation in acute coronary syndromes, personal history of thyroid disease or thyroid hormone therapy, treatment with Amiodarone during the last six months, thyroid changes on ultrasound examination (appearance of thyroiditis, thyroid nodules over 2 cm), congestive heart failure class III and IV, left ventricular ejection fraction
(LVEF) below 40%, severe comorbidities. We noted: demographics, history of arterial hypertension (AH), stable coronary artery disease (CAD), old myocardial infarction (OMI), diabetes mellitus (DM), echocardiographic data: left ventricular ejection fraction (LVEF), left ventricular mass index (LVMI)- normal values less than 115 g/m2 in men and less than 95 g/m2 in women, serum cholesterol, ventricular rate (HR) at discharge and also the drug therapy. In all pts TSH, free T4 (FT4) were determined and thyroid ultrasound was performed. Normal laboratory values were between 0.34-5.6 mU/ml for TSH and between 0.58-1.64 ng/dL for FT4. Patients with TSH and FT4 within normal ranges were considered euthyroid, those with TSH below 0.1 mU/L and normal FT4 were diagnosed with clinically latent hyperthyroidism and those with TSH over 6.5 mU/L and normal FT4 with clinically latent hypothyroidism. Patients were divided into 3 groups according to TSH levels: G1, euthyroid (EuT), G2 clinically latent hypothyroidism (HipoT), G3 clinically latent hyperthyroidism (HiperT). The 3 groups were compared in terms of resting ventricular rate, of beta blockers doses needed to control ventricular rate, of LVEF. Statistical analysis was performed using Statistica8.
RESULTS
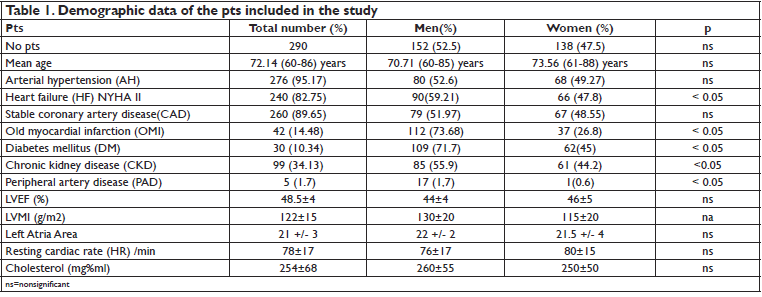
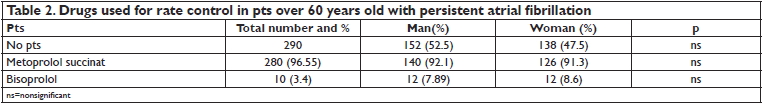
290 consecutive pts admitted within 6 month to the cardiology department for persistent nonvalvular atrial fibrillation were included in the study. Demographic data are shown in Table 1. 152 of the 290 pts (52.2%) were men. The mean age of pts was 72.14 ± 12 years, with no significant differences between women and men. 95.17% of pts had AH, 82.75% HF, 14.48% OMI, 89.65% CAD, 10.34% DM, 34.13% CKD. There were no significant differences between men and women regarding the age and history of AH, CAD. Male pts had significantly more often HF (59.21% in men versus 49.27% in woman, p<0.05), OMI (73.68% in men versus 26.8% in women, p<0.05), peripheral artery disease (PAD) (1.7% in men versus 0.6% in women, p<0.05), DM (71.7% in men versus 45% in women, p<0.05), CKD (55.9% in men versus 44.2% in women, p<0.05). Pts had increased LVMI. The mean resting heart rate at discharge was 78 ± 17/min, with no signifi cant differences between men and women. Total serum cholesterol level was 254 ± 68 mg% ml, with no significant differences between men and women. All pts received treatment with beta blockers (metoprolol succinate, bisoprolol), ACEI or ARBs, statins and anticoagulants (Table 2). 95% of pts reached the maximum dose of the beta blocker therapy: metoprolol succinate 200 mg/ day, bisoprolol 10 mg / day. 3.4% of pts received also digoxin for ventricular rate control. Group 1 (EuT), included 274 pts. Group 2 (HipoT) consisted of 6 pts. Group 3 (HiperT) consisted of 10
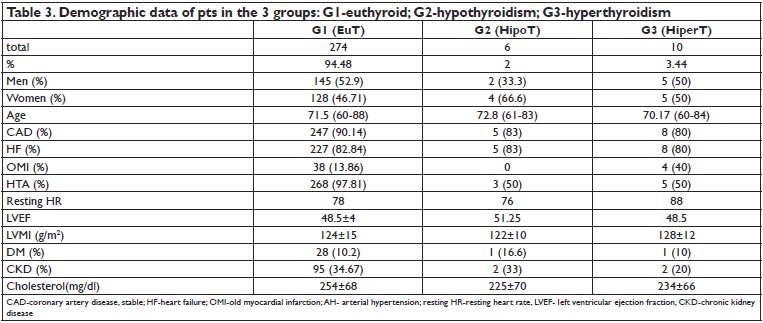
pts. Patients with HipoT represent 2% of all pts and those with HiperT, 3.44%. Demographic data of the 3 groups are in Table 3. There were no significant differences between the 3 groups regarding demographic data and medical history of cardiovascular disease. A higher proportion of pts in group G2 (HipoT), but not statistically signifi cant, had DM and CKD. Serum cholesterol
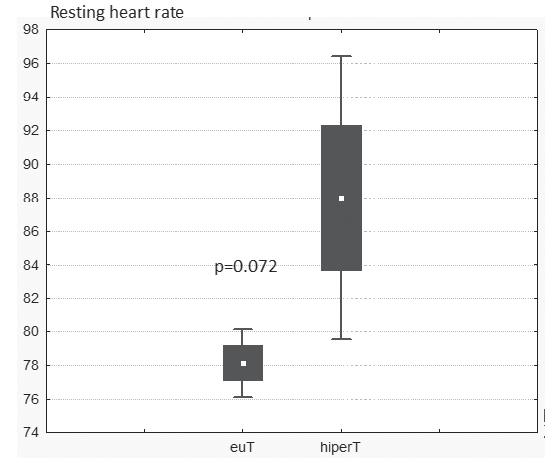
level was not statistically different between the 3 groups. LVEF was similar between the 3 groups. LVMI was increased in all 3 groups. Left atrial area was 21+/- 3 cm2 in G1, 22 +/- 2 cm2 in G2 and 21,5 +/- 4 cm2 in G3 (p=ns). Pts in G3 had a mean resting HR of 10 beats/min higher than pts in G1 pts, on maximum doses of beta blockers (Figure 1).
DISCUSSION
Cardiovascular effects of thyroid hormones acting through genomic and non-genomic changes are well known4. Thyroid gland synthesize thyroxin (tetraiodothyronine -T4), which is transformed into triiodothyronine (T3), the active form, within the peripheral cells by deiodination with 5 deiodinase. Cardiac myocytes cannot perform this conversion but T3 can enter the myocyte as it enters other cells. Genomic effects are achieved by activation of transcription or by repression of genes encoding structural and functional proteins. T3 enters the myocardial cells through
specifi c membrane transporters, enters the nucleus, acting on nuclear receptor 1 (activator) and 2 (repressor). T3 – receptor complex engages or disengages from certain sequences of DNA. Expression of myosin heavy chains, sarcoplasmatic reticulum protein involved in the regulation of intracellular calcium handling and phospholamban, its inhibitor are modifi ed5,6. There is an increase of the transcription of the myosin heavy chain (MHC) gene and a decrease of the transcription of the MHC gene. There is an increased synthesis of myosin V1 isoform consisting of two MHC having enhanced ATPase activity.
Figure 1. Resting heart rate compared in G1 (euT) and G3 (HiperT).
There is a decrease in the synthesis of myosin V3 isoform consisting of two MHC with low ATPase activity6. Calcium (Ca2+) reuptake within the smooth endoplasmic reticulum is faster. T3 accelerates the speed of myocardial fi ber contraction and relaxation and increase the consumption of ATP within the heart6. Nongenomic mechanisms are responsible for the faster actions of T3. They induce rapid phosphorylation of phospholamban and prevent its inhibitory action on sarcoplasmatic reticulum ATPase activated by Ca2+. This is done by intracellular kinases system, also involved in adrenergic activity, which explain the similarity of thyroid hormone effects with those of the adrenergic system4. Evaluation of subclinical thyroid dysfunction is difficult because old age, stress, alcohol consumption, chronic inflammatory diseases, heart failure reduce T4 conversion to T3. Non-thyroid severe disease can cause false positivity for TSH tests1. It was also found that TSH level in the elderly with subclinical hypothyroidism or hyperthyroidism is normalized by itself in a few months1,7, without being clear whether there are pathological implications. However, studies have shown that subclinical persistent changes of thyroid function induced myocardial pathological changes4. Subclinical hyperthyroidism is associated most commonly with the appearance of atrial fibrillation and left ventricular hypertrophy1,4. Subclinical hypothyroidism is associated with dyslipidemia, atherosclerosis acceleration, slight increase in long-term myocardial infarction but statistically insignificant8. However, some studies have shown that in women with subclinical hypothyroidism serum levels of cholesterol are lower than in the euthyroid, which would imply the existence of other risk factors in a rapidly evolution of atherosclerosis, such as lipoprotein a (Lp (a)), homocysteine8. We studied the prevalence of subclinical thyroid dysfunction, defi ned by TSH changes concurrently with normal FT4 in pts aged over 60 years and persistent atrial fibrillation. All pts had personal history of cardiovascular
disease that may be associated with atrial fibrillation but we searched if there was any impact of possible thyroid dysfunction. We excluded pts with severe comorbidities, NYHA class III and IV heart failure, acute coronary syndromes, situations in which unexpected changes in TSH may arise. Most pts were euthyroid. 3.4% of pts over 60 years old with persistent atrial fibrillation had subclinical hyperthyroidism. This prevalence exceeds the prevalence of latent hyperthyroidism in the general population aged over 60, which is 1.5% in women and 1% in men1. This is explained by selecting the population with atrial fi brillation. It was also noticed that latent hyperthyroidism was present both in women and in men. There were no demographic differences between the 3 groups of pts. Patients with latent hyperthyroidism had a mean resting heart rate of 10 beats/min higher than euthyroid pts, with the same doses of beta blockers. LVMI was increased, but with no differences between pts with different thyroid functional status. This is contrary to literature data8 describing the increase of the left ventricle muscle mass in pts with latent hyperthyroidism. One explanation could be the small number of pts with latent hyperthyroidism and also the associated cardiovascular diseases that induce left ventricular hypertrophy independent of thyroid function. Clinically latent hypothyroidism was present in 2% of pts with atrial fi brillation. The prevalence is lower than that of the general population1 but the small number of pts does not allow us to
interpret this observation. Moderately elevated cholesterol values were similar in the 3 groups, infl uenced by statin therapy. There is no consensus in the literature whether clinically latent thyroid dysfunction should be treated.
CONCLUSIONS
Clinically latent hyperthyroidism was present in 3.4% pts over 60 years old with persistent atrial fibrillation. Its prevalence in pts with atrial f brillation is greater than in the general population. Ventricular rate at rest was higher in the group with latent hyperthyroidism than in euthyroid pts, at the same doses of beta blockers. Latent hypothyroidism was present in 2% of pts with persistent atrial fi brillation, to a lesser extent than in the general population.
THE LIMITS OF THE STUDY
The low prevalence of subclinical thyroid dysfunction in our group of 290 pts makes interpretation of data difficult and we could not determine the appropriateness of initiating the hormonal treatment. We did not perform echographic assessment of the left atrial strain and of the left atrial functions (conduit, reservoir and pump).
Conflict of interest: none declared.
References
1. U.S. Preventive Services Task Force. Screening for Thyroid Disease, Recommendation Statement. Ann Intern. Med, 2004; 140:125-127
2. Camm J, Lip GYH, De Caterina R, Savelieva I, Atar D, Hohnloser SH, Hindricks G, Kirchhof P. 2012 focused update of the ESC Guidelines, for the management of atrial fi brillation An update of the 2010 ESC Guidelines for the management of atrial fi brillation Developed with the special contribution of the European Heart Rhythm Association, European Heart Journal, 2012; 33: 2719–2747
3. Craig T, January L, Wann S, Alpert JS, Calkins H, Cigarroa JE,. Cleveland Jr JC, Conti JB, Ellinor PT, Ezekowitz MD, Field ME, Murray KT, Sacco RL, Stevenson WG, Tchou PJ, Tracy CM, Yancy CW. 2014 AHA/ACC/ HRS Guideline for the Management of Pts With Atrial Fibrillation A Report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines and the Heart Rhythm Society Circulation 2014;130:e199-e267.
4. Fazio S, Palmieri EA, Lombardi G, Biondi B. Effects of Thyroid Hormone on the Cardiovascular System, The Endocrine Society, 2004: 31-44 5. Kenessey A, Ojamaa K. Thyroid hormone stimulates protein synthesis in the cardiomyocyte by activating the Akt-mTOR and p70S6K pathways. J Biol Chem. 2006; 281(30):20666-72.
6. Dillmann WH, Biochemical basis of thyroid hormone action in the heart Am J Med 1990, 88 (6): 626–630.
7. Aggarwal N, Razvi S. Thyroid and Aging or the Aging Thyroid? An Evidence- Based Analysis of the Literature J. of Thyroid Research, 2013, article ID 481287, pag 1-8.
8. Cooper DS. Subclinical Hypothyroidism, N Engl J Med, 2001, 345 (4): 260-264.
 This work is licensed under a
This work is licensed under a