Anca D. Mateescu1,2, Marinela Serban2, Carmen Ginghina1,2, Bogdan A. Popescu1,2
1 ”Carol Davila” University of Medicine and Pharmacy, Euroecolab, Bucharest
2 Institute of Emergency for Cardiovascular Diseases ”Prof. Dr. C. C. Iliescu” Bucharest
Contact address:
Bogdan A. Popescu
University of Medicine and Pharmacy“Carol Davila”, Euroecolab,
Emergency Institute for Cadiovascular Diseases“ Prof. Dr. C. C. Iliescu”
258 Fundeni Avenue, 2nd District, Zip code: 022328
Bucharest, Romania. Phone/Fax: +40213175227
E-mail: bogdan.a.popescu@gmail.com
Persistent patency of the arterial duct represents one of the most common lesions in the field of congenital heart disease. The most serious potential complication of persistent ductus arteriosus (PDA) is bacterial endocarditis, which isnow a rare occurrence, with a reported incidence of 0.5% per patient per year1. The involved site is usually the pulmonary arterial wall opposite the PDA, butit can also involve, more rarely, the pulmonary valve1,2.
A 33-year-old man who had been diagnosed with PDA in childhood was refferedfor cardiac evaluation for fatigue and progressively worsening dyspnoea on exertion over the past 3 months. Physical examination revealed an afebrile patient, hyperdynamic precordium, a continuous murmur with thrill over the pulmonary area, peripheral oedema and hepatomegaly. The blood pressure was 120/60 mm Hg and the peripheral pulses were prominent. Auscultation of the lungs revealed mild basal crackles bilaterally. There were no clinical stigmata consistent with endocarditis. The ECG showed sinus rhythm at 78 bpm, biatrial abnormality, signs of left ventricular hypertrophy and complete right bundle branch block. There was no inflammatory syndrome or anemia. Transthoracic and transesophageal echocardiography confirmed the presence of a large PDA (defect size 11 mm), with a continuous systolic-diastolic flow signal with high velocities (up to 4.8 m/s) recorded by continuous wave Doppler examination at the level of this turbulent jet from the descending aorta to the pulmonary artery (Figures 1-3). The left ventricle was severely dilated (EDV/ESV=247/124 ml), with mild global systolic dysfunction (ejection fraction of 50%). The assessment of pulmonary artery systolic pressure was carried out by measuring peak tricuspid regurgitation velocity and the estimated value was 52 mm Hg. The TTE exam also revealed mobile vegetations on the pulmonary valve, with severe valve destructions, lack of coaptation and severe, free pulmonary regurgitation on color Doppler examination (Figures 4, 5). No vegetations were seen on the other valves, cardiac chambers or great vessels.
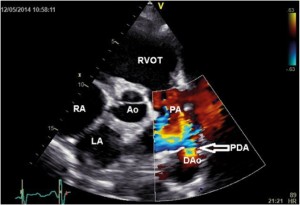
Figure 1. Transthoracic parasternal short axis color Doppler imaging revealed PDA with left to right shunt (arrow); LA-left atrium, RA- right atrium, RVOT- right ventricular outflow tract, Ao- aortic root, PA- pulmonary artery, PDA-patent ductus arteriosus, DAo-descending aorta.
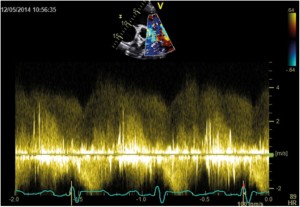
Figure 2. TTE examination, parasternal short-axis view; Spectral Doppler profile of continuous left-to-right ductal flow with high velocities (up to 4.8 m/s).
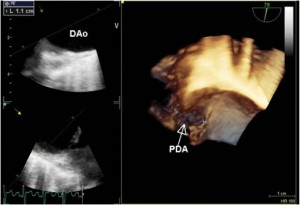
Figure 3. 3D TEE examination confirmed the existence of a large PDA (defect size 11 mm); PDA-patent ductus arteriosus, DAo- descending aorta.
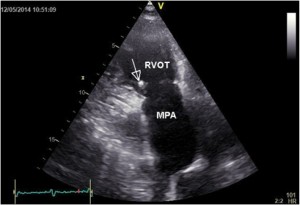
Figure 4. Transthoracic parasternal short axis views vegetations (arrow) on a pulmonary valve with destructions and lack of diastolic coaptation. RVOT- right ventricular outflow tract, MPA- main pulmonary artery.
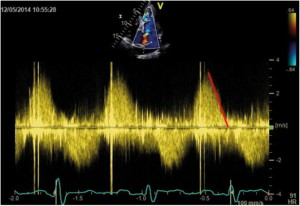
Figure 5. TTE examination, parasternal short-axis view. CW Doppler recording of pulmonary regurgitation showing a rapid flow deceleration during the diastole and increased systolic velocity (flow-related).
There were no clinical or biological signs of acute infective endocarditis. Considering the vegetations on a destroyed pulmonary valve with severe pulmonic regurgitation and the presence of a large PDA with significant left-to-right shunt and severely dilated LV with global systolic dysfunction, surgery is warranted.
Considering the absence of other endocarditis risk factors, isolated pulmonary valve infection could have been initiated by the jet or turbulent flow created by the PDA. Carefully evaluating the pulmonary trunk and the pulmonic valve in echocardiography is very important in patients with PDA, especially when clinical suspicion of infective endocarditis is considered. Echocardiography allows a complete evaluation of lesions’ extension, helpful to determine the best surgical approach. Compared with the left-sided heart valves, the involvement of pulmonic valve infection tends to affect younger patients3. Complete diagnostic assessment of hemodynamics (evaluation of pulmonary vascular resistance, degree of shunting, pressure gradients) is important in patients with PDA and pulmonary arterial pressure which is higher than 50% of systemic pressure4.
Conflict of interests: none declared.
References
1. Sugimura Y, Katoh M, Toyama M. Patent Ductus Arteriosus with Pulmonary Endarteritis. Intern Med 2013; 52:2157-2158.
2. Gokaslan G, Deniz H, Ozcaliskan O, Yasim A, Ustunsoy H. Pericardial monocusp reconstruction for pulmonary valve vegetation secondary to patent ductus arteriosus. J Card Surg. 2011; 26:650-2.
3. Kareem B, Kamarulzaman H, TiongKoh G. Surgical Management of Patent DuctusArteriosusWith Endocarditis. Ann ThoracSurg 2010; 90:1703-5.
4. Baumgartner H, Bonhoeffer P, De Groot N, et al. ESC Guidelines for the management of grown-up congenital heart disease. Eur Heart J 2010;
31:2915-57.
 This work is licensed under a
This work is licensed under a