Anda Militaru1,2, Marius Militaru1,2, Mircea Iurciuc1, Maria Delamarian2, Petru Matusz1, Daniel Lighezan1,2
1 „Victor Babes” University of Medicine and Pharmacy, Timisoara, Romania
2 Emergency Municipal Hospital, Timisoara, Romania
Abstract: Therapies for different malignancies are associated with an increased risk of cardiotoxicity. Amongst these drugs, anthracyclines and their analogs are most commonly used as chemotherapy agents but, unfortunately, they increase the incidence of cardiovascular side effects. Cardiovascular side effects induced by chemotherapy are highly associated with the cumulative dose of anticancer drugs. Left ventricular disfunction followed by cardiac failure are most frequent side effects of chemotherapy. We evaluated here cardiac disfunction and vascular changes for a young patient without cardio-vascular risk factors before, diagnosed with acute lymphoblastic leukemia (ALL), three months after starting chemotherapy and three months after stem cell transplantation. Occurrence of left ventricular dysfunction, without any clinical signs after chemotherapy was reported. Small changes in vascular remodeling parameters have also been identified, which may suggest a more rapid vascular remodeling in patients receiving chemotherapy and radiotherapy. Keywords: acute lymphoblastic leukemia, chemotherapy, cardiotoxicity, left ventricular dysfunction
INTRODUCTION
Chemotherapy may induce cardiovascular toxicity1. Certified side effects of anthracyclines and other cardiotoxic agents could be reduced by the use of cardioprotective drugs, without any decrease of the antineoplastic efficacy1,2. A complete clinical cardi-ac evaluation of the patient, the identification of the first signs and symptoms of cardiovascular disease, the knowledge of the patient’s comorbidities and the treatment of these, as well as the identification of car-diovascular risk factors and their changes seem to be crucial for the identification of the cardiovascular side effects during and after chemotherapy3,4. Knowing and correctly treating any cardiovascular disease before starting chemotherapy is helpful in preventing possible complications that may occur during such unpleasant treatment. A close collaboration between the oncolo-gist and the cardiologist could prevent the cardiotoxic side effects of chemotherapy and radiotherapy.
CASE PRESENTATION
One year evaluation has been performed for a 23-year-old patient without signifi cant history of cardiovascu-lar or other diseases. This patient with no background of previously certified cardiovascular pathology, was diagnosed with acute lymphoblastic leukemia (ALL) with B precursor, CALLA negative BCR-ABL. The pa-tient was admitted to hospital for an intense pain and swelling of right lower limb, asthenia and fatigue.
The patient had pale tegument, mucous membranes and edema at the level of the right lower limb. Clinical examination revealed normal cardiovascular parame-ters as it follows: SBP/DBP=120/65 mmHg, HR=68 bpm. Electrocardiographic monitoring proved that the patient has a sinusal rhythm, intermediate QRS spindle, without terminal phase changes.
By radiography, it has been shown that the heart was within normal limits but the lungs had a slight and diffuse increase of lung interstitium. Deep veno-us thrombosis has been certifi ed in the right popliteal vein and subcutaneous treatment with low molecular weight heparin has been initiated. Blood count profile, revealed anemia, thrombocytopenia and leukocytosis with high amount of blasts in the peripheral blood. About 92% of bone marrow was infi ltrated with lymphoid B-cell precursor cells with a pro-B CALLA negative lymphoblast and a possible presence of the Philadelphia 1 chromosome has been suspected. Mo-lecular biology emphasized the detection and quanti-fication of Bcr – abl Major (p210) and Bcr – abl minor (p190) both of them being positive. Histopathology certified the diagnosis of acute lymphoblastic leukemia with precursor B cells.
The results described advocate for the diagnosis of acute lymphoblastic leukemia with negative CALLA B precursor, possibly positive Philadelphia chromoso-me. 9 months after the diagnosis was established and chemotherapy was started, allogeneic haematopoie-tic stem cell transplantation was performed from the unrelated donor.
The patient received anticoagulant treatment with low molecular weight heparin for deep right vein thrombosis and chemotherapy drugs as follows: Dau-norubicin total dose of 877.5 mg/m2, Adriamycin 525 mg/m2, Citarabine in total dose = 188 mg/m2, Metho-trexate total dose = 4612.5 mg/m2, Vincristine total dose = 36 mg/m2, Topoisomerase II-topoisomerase inhibitors -VM26 / VP16 (Teniposide) in the total dose of 2625 mg/m², Alkylating agents -Cyclophosphamide (Imatinib) at a dose of 600 mg/day. The prophylaxis of Cerebral Nervous System with Methotrexate in the total dose of 1635 mg/m2, Cytarabine in a total dose of 180 mg/m2 and corticotherapy with Dexametha-sone in a total dose of 18 mg/m2. During the stem cell transplant, the patient received specific treatment with: Busulfan alkylating agents in the total dose of 864 mg and Corticosteroids with Cyclophosphamide at a total dose of 94g in 28 days. Before bone marrow transplant, the patient followed cranial radiotherapy.
MATERIAL AND METHODS
We used a General Electric Vivid E9 and a 5 MHz transducer for the transthoracic echocardiographic measurements. The patient was monitored by 2D ultrasound, tissue Doppler and Speckle Tracking to assess left ventricular function by calculating LVEF%, S‘, GLS. LVEF% (left ventricular ejection fraction) was determined by planimetric echocardiographic method in 4 chambers apical section, maximum systolic velo-city was measured in the lateral mitral ring (S’), by tissue Doppler imaging (TDI), and by speckle-tracking technique we followed the initial global longitudinal strain (GLS). We also evaluated the following vascular remodeling parameters: Ankle-brachial index (ABI), Intima-Media Thickness (IMT) and pulse wave velocity (PWV) to identify the early appearance of arterial wall damage following chemotherapy.
Pulse wave velocity (PWV) is a method of quan-tifying aortic rigidity. The patient had PWV analysis done using MedExpert Arteriograp ™ TL2. This de-vice enabled us to evaluate arterial dysfunction. The principle of the method consists of recording the sig-nals of the pulse wave at the level of the brachial ar-tery which is occluded for several seconds using an in-flatable cuff. Using a measuring tape, the distance from the pubic symphysis to the sternal notch is determi-ned. This, alongside other information about the pati-ent (age, height, etc.) is introduced into the computer. PWV is calculated using the distance traveled by the pulse wave in the aorta and the time of travel (RT/2). The measurements are wirelessly transmitted to the computer which then processes the information.
To analyze the intima-media thickness (IMT), the same General Electric Vivid E9 was used, the measure-ments being done with a 9 MHz transducer. The IMT was determined at the level of the common carotid artery, 1 cm proximal to the carotid bulb.
The ankle-brachial index (ABI) was calculated with the help of a sphygmomanometer and a SONO TRAX Vascular Doppler device. The brachial systolic blood pressure of both arms was determined, and, using the vascular Doppler device, the systolic pressure of the inferior limbs was recorded. This was equivalent to the pressure in the cuff at the moment of the first Doppler signal being detected in the dorsalis pedis ar-tery. The ratio of systolic pressure of the upper limb to systolic pressure of the lower limb was calculated on both sides, with the greater value being recorded.
These parameters were followed at the beginning of the disease, 3 months after the initiation of che-motherapy and 3 months after stem cell transplanta-tion.
RESULTS
During follow-up, the values of SBP and DBP decrea-sed from the initial recorded value, HR (bpm) incre-ased from the initial recorded value and it was mai-ntained increased also post-transplant, and the body mass index and body surface area decreased during cytostatic therapy, having a slight increase post-trans-plantation of stem cells.
By evaluating 2D echocardiography, it is observed that at 3 months post chemotherapy, left ventricular shortening fraction has been decreased from the ini-tial value, and then returned to its normal values in 3 months after transplantation. This is also noticeable by MAPSE modifi cation that decreases post-chemothera-py but slightly increased at 3 months post-transplant. LVEDD, LVESD, increased compared to the first assessment, as well as LVEDV and LVEVS, which were maintained increased post-transplantation.
As in what regards the assessment parameters of the diastolic dysfunction, the E and A waves increased and the E / A ratio decreased from the initial value. By evaluating the tissue Doppler at the level of the mitral lateral ring (TDI-LW), S’ wave decreased from the ini-tial value, E’ wave increased, and the A’ wave and the E/E’ ratio decreased from the initial value.
Speckle tracking shows that global longitudinal stra-in (GLS) decreases at three months post-chemothe-rapy versus the initial value from -25.2% to -16.5%. Three months after transplantation, GLS begins to increase slightly at a value of -17.9%, but remains low compared to the initial value.
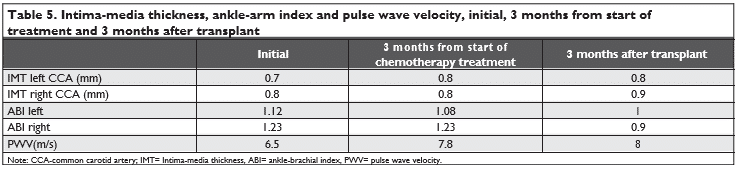
In terms of arterial wall damage, a slight thickening of the IMT in the right carotid artery is observed, the ABI decreased both on the left and on the right com-pared to the initial value and decreased even more post-transplantation. The PWV was initially 6.5 m/s, which corresponds to the 20 to 30-year-old age per-centile, then three months after chemotherapy, the pulse wave velocity increased from the initial value to 7.8 m/s, which corresponds to the 40-50 year old ar-terial age percentiles, this value being also maintained at three months post-transplant.
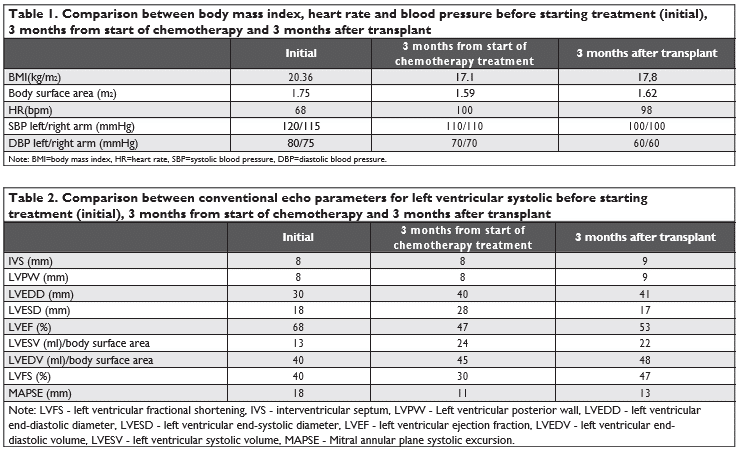
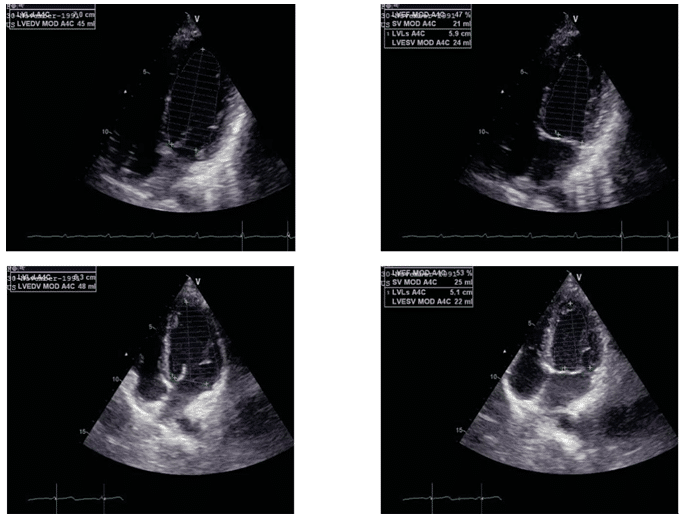
Figure 1. LVEF evaluation in 4 chambers apical section after 3 months from start of chemotherapy and 3 months after transplant.
DISCUSSIONS
By evaluating the periodic echocardiographic of LV cardiac, function in this young patient with ALL, left ventricular ejection fraction was impaired before cli-nical signs occurred. LVEF decreased by >10% from the initial value, from 68% to 47% at three months af-ter chemotherapy. Several papers showed that a 10% decrease of LVEF post-chemotherapy and this may suggest cardiotoxicity in the absence of any signs of heart failure5,6,7. Cardinal end al. studied anthracycline cardiotoxicity on 2.625 patients and reported that only 9% of the patients showed cardiotoxicity immediately after treatment, and 98% of these patients had clinical manifestations only after the first year of treatment. These results suggest that chemotherapy can induce a constant and progressive alteration of LV function 8. The incidence of developing LV dysfunction also de-pends on the chemotherapeutic drug used. Some of them have stronger cardiotoxic effects than others. For the present patient, it has been used anthracycli-nes certified as having a high potential of developing LV dysfunction for doses over 700 mg/m², in about 18 48% of cases. Another drug used was Cyclophospha-mide with a cardiotoxicity risk of 7-28% and Imatinib with a cardiotoxicity risk of 0.2-2.7%5,9.
Another echocardiographic parameter that can help us in early discovery of cardiotoxicity is GLS. A reduction in GLS >15% compared to the initial value may suggest left ventricular dysfunction5,10,11. In our patient’s case, we detected a GLS decrease three months post-chemotherapy of -8.7%. GLS may be a sensitive cardiotoxicity detection parameter, but according to existing data up to now, the chemothe-rapy treatment should not be interrupted or reduced only if a low GLS value is highlighted, but more para-meters are needed5.
Kazuaki end al. studied 159 patients treated with chemotherapeutic agents, including anthracyclines. In 52 of the patients (33%) there was a signifi cant change in GLS, and only 14 out of them had low LVEF with >10%, an aspect that suggests cardiotoxicity12.
Tissue Doppler showed a decrease in S’ wave three months after chemotherapy. As some authors have shown, there is a direct relationship between the maximum velocity of S’ wave and LVEF, especially in pa-tients who do not exhibit kinetic disorders at LV level 13). And in the ESC Position Paper on cancer treat-ment and cardiovascular toxicity, it has been shown that the decrease of S’ wave during the time the pati-ent receives chemotherapy may be an early marker of cytotoxicity5.
Katarzyna et al evaluated 35 women with breast cancer using Doppler tissue, who received anthracycli-nes. They showed that S wave was the only parame-ter that indicated the left ventricular systolic function impairment. This decreased during the chemotherapy treatment14.
In Doppler ultrasound evaluation of carotid arte-ries, a slight increase by almost 1 mm of the intima-media thickness was observed, compared to the initial evaluation. This may suggest a developing of carotid vascular atherosclerotic disease5. Several articles have shown that the risk of developing cerebrovascular di-sease is doubled after cervical or cranial radiotherapy. Mediastinal and cranial radiotherapy can be associa-ted with increased carotid rigidity and intima-media thickness and may lead to accelerated atherosclerosis, and clinical manifestations may occur even 10 years after radiotherapy5,15-19.
Recently, a study including 64 children with ALL treated with antineoplastic drugs revealed the pre-mature appearance of atherosclerotic changes by the increase of IMT for all subjects included in the study. Thus, it has been highlighted that IMT is an important marker of subclinical atherosclerosis identifi cation and a good predictor of cardiovascular and cerebrovascu-lar disease risk for patients previously treated with chemotherapy, radiotherapy or both5,20.
IMT increase observed for our patient may be in-fluenced by the fact that our patient has performed cranial radiotherapy and the risk of cerebrovascular and cardiovascular disease is basically increased due to it and using of chemotherapy treatment.
Other vascular remodeling parameters underwent changes from the initial value, also: the ABI decreased, and PWV increased in the post-transplant evaluation. Alterations of these parameters indicate accelerated atherosclerosis, and may be predictors of possible vas-cular events that may occur in the future. The fact that the changes of these parameters may be markers that can identify possible vascular events is also shown by Katarzyna and others in their studies that highlighted this possibility on his group of patients14. Data from other studies suggests that PWV is a predictor of car-diovascular disease5. PWV represents an increase in arterial rigidity. There are studies that have correla-ted the increase of PWV with strain modifi cations and LVEF decrease, after treatment with anthracyclines21.
Other chemotherapy agents like small molecule ty-rosine kinase inhibitors may induce cardiotoxicity by left ventricular dysfunction but also by vascular para-meters changes, especially ABI22. There were some studies reporting that ABI changed during and after small molecule tyrosine kinase inhibitors treatment. Kim et al described an increase of atherosclerotic de-velopment especially by the development of periphe-ral arterial disease for patients with chronic myeloid leukemia treated with small molecule tyrosine kinase inhibitors. About 6.3% out of 129 treated patients de-veloped peripheric arterial disease23. Levato et al also reported that 14.8% out of 82 patients with chronic leukemia developed peripheral arterial disease24. This may support our observation regarding the increase of ABI for our patient, also treated with small molecu-le tyrosine kinase inhibitors (imatinib).
Anthracyclines used for the treatment of our pa-tient were responsible for the appearance of cardiac disfunction observed 3 month after the beginning of the therapy. Present therapeutic guides highlight that anthracyclines may induce irreversible cardiac dys-function affecting patients prognosis5. In our case, me-dullary transplant was followed by a complete remis-sion of the disease and also, followed by the recovery of echocardiographic parameters and vascular remo-deling most probably due to the lack of chemotherapy after transplant. All together these may be considered a good prognostic factor for the long term follow up
Present study has as the main limit the lack of high-sensitivity troponin I evaluation, this parameter being an important marker for the early detection of post chemotherapy myocardial changes.
Post-treatment assessment should be for as long as possible. In the case presented, the patient will be evaluated at six months, one year, and then annually after stem cell transplantation, to detect any manifestations of heart failure, valvular, rhythm disorders or vascular manifestations.
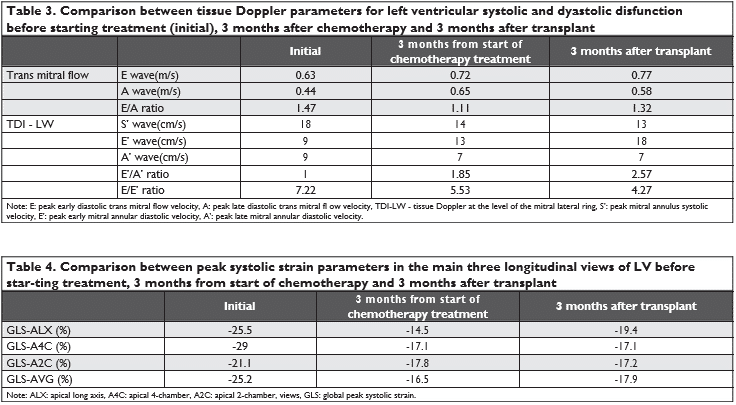
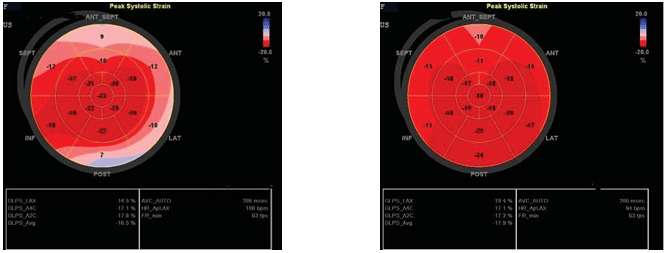
Figure 2. Speckle tracking: GLS 3 months from start of chemotherapy and 3 months after transplant.
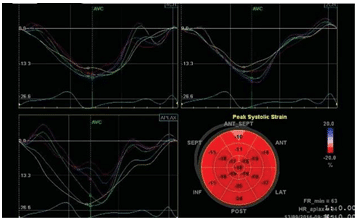
Figure 3. Speckle tracking: GLS 3 months after transplant.
CONCLUSIONS
Hematological patients that are about to receive che-motherapy or radiotherapy have to be cardiologically assessed. The better we know the cardiovascular risk factors or the history of the patient’s cardiac disease, the faster we will be able to prevent the occurrence of signs of cardiac failure secondary to chemotherapy or radiotherapy.
By tracking echocardiographic parameters, we can detect signs of LV dysfunction long before clinical signs appear, and the quantification of vascular remodeling parameters may provide indications of early athe-rosclerosis and thus prevent potential cardiovascular events.
Conflict of interest: none declared.
References
1. Ruxandra Jurcut, Cardio-oncologia – o nouă specializare pentru car-diologi?, Revista Stetoscop, 2014, 3: 1-4.
2. Jurcut R, Wildiers H, Ganame J, D’hooge J, Paridaens R, Voigt JU, Detection and Monitoring of Cardiotoxicity – What Does Modern Cardiology Offer?, Supportive Care in Cancer, 2008,16(5):437-45.
3. Carmen Ginghina, Compendiu de terapie a bolilor cardiovasculare, Editura Medicala, Bucuresti , 2016, 365-366.
4. ESMO Clinical Practice Guidelines: Cardiovascular toxicity inducedby chemotherapy, targeted agents and radiotherapy: Annals of Oncology 23 (Supplement 7): vii155–vii166, 2012. doi:10.1093/annonc/ mds293, Journal of Radiotheraphy & Medical Oncology, On-Line supplement Nr 1, 2014.
5. ESC Committee for Practice Guidelines (CPG), 2016 ESC Position Paper on cancer treatmentsand cardiovascular toxicity developed under the auspices of the ESC Committee for Practice Guidelines, European Heart Journal, 2016, 37, 2768–2801 doi:10.1093/eur-heartj/ehw211.
6. Plana JC, Galderisi M, Barac A, Ewer MS, Ky B, Scherrer-Crosbie M, Ganame J,Sebag IA, Agler DA,Badano LP, Banchs J, Cardinale D, Carver J, Cerqueira M,DeCara JM, Edvardsen T, Flamm SD, Force T, Griffin BP, Jerusalem G, Liu JE,Magalhaes A, Marwick T, Sanchez LY, Sicari R, Villarraga HR, Lancellotti P. Expert consensus for multi-modality imaging evaluation of adult patients during and after cancer therapy: a report from the American Society of Echocardiography and the European Association of Cardiovascular Imaging, Eur Heart J Cardiovasc Imaging, 2014;15:1063–1093.
7. Lang RM, Badano LP, Mor-Avi V, Afilalo J, Armstrong A, Ernande L,Flachskampf FA, Foster E, Goldstein SA, Kuznetsova T, Lancel-lotti P, Muraru D,Picard MH, Rietzschel ER, Rudski L, Spencer KT, TsangW, Voigt JU. Recommendations for cardiac chamber quantifi-cation by echocardiography in adults:an update from the American Society of Echocardiography and the European Association of Car-diovascular Imaging. J Am Soc Echocardiogr 2015;28:1–39.e14.
8. Cardinale D, Colombo A, Bacchiani G, Tedeschi I, Meroni CA, Veg-lia F, Civelli M,Lamantia G, Colombo N, Curigliano G, Fiorentini C, Cipolla CM. Early detection of anthracycline cardiotoxicity and improvement with heart failure therapy. Circulation 2015;131:1981– 1988.
9. Swain SM, Whaley FS, Ewer MS. Congestive heart failure in patients treated with doxorubicin: a retrospective analysis of three trials. Cancer 2003;97:2869–2879.
10. Sawaya H, Sebag IA, Plana JC, Januzzi JL, Ky B, Tan TC, Cohen V, Banchs J, Carver JR, Wiegers SE, Martin RP, Picard MH, Gerszten RE, Halpern EF, Passeri J, Kuter I, Scherrer-Crosbie M. Assessment of echocardiography and biomarkers for the extended prediction of cardiotoxicity in patients treated with anthracyclines,taxanes, and trastuzumab. Circ Cardiovasc Imaging 2012;5:596–603.
11. Negishi K, Negishi T, Hare JL, Haluska BA, Plana JC, Marwick TH. In-dependent and incremental value of deformation indices for predic-tion of trastuzumab-induced cardiotoxicity. J Am Soc Echocardiogr 2013;26:493–498.
12. Kazuaki Negishi, Tomoko Negishi, Brian A. Haluska, James L. Hare,Juan Carlos Plana, and Thomas H. Marwick, Use of speckle strain to assess left ventricular responses to cardiotoxic chemother-apy and cardioprotection, European Heart Journal – Cardiovascular Imaging (2014) 15, 324–331doi:10.1093/ehjci/jet159.
13. Bogdan A.Popescu, Carmen Ginghina,Ecografia Doppler ,Editura Medicala Bucuresti 2011, 76.
14. Katarzyna Mizia-Stec, Adrianna Gościńska, Magdalena Mizia, Ma-ciej Haberka, Artur Chmiel, Wojciech Poborski, Zbigniew Gąsior. Anthracycline chemotherapy impairs the structure and diastol-ic function of the left ventricle and induces negative arterial re-modeling. Kardiologia Polska 2013; 71, 7: 681–690; DOI: 10.5603/ KP.2013.0154.
15. De Bruin ML, Dorresteijn LD, van’t Veer MB, Krol AD, van der Pal HJ,Kappelle AC, BoogerdW, Aleman BM, van Leeuwen FE. Increased risk of stroke and transient ischemic attack in 5-year survivors of Hodgkin lymphoma. J Natl Cancer Inst 2009; 101: 928–937.
16. Yuan H, Gaber MW, Boyd K, Wilson CM, Kiani MF, Merchant TE. Effects of fractionated radiation on the brain vasculature in a murine model: blood-brain barrier permeability, astrocyte proliferation, and ultrastructural changes. Int J Radiat Oncol Biol Phys 2006;66:860– 866..
17. Louis EL, McLoughlin MJ, Wortzman G. Chronic damage to medium and large arteries following irradiation. J Can Assoc Radiol 1974;25: 94–104.
18. Fajardo LF. The pathology of ionizing radiation as defined by mor-phologic patterns . Acta Oncol 2005; 44: 13–22.
19. Yeh ET, Bickford CL. Cardiovascular complications of cancer ther-apy: incidence, pathogenesis, diagnosis, and management. J Am Coll Cardiol 2009; 53: 2231–2247.
20. Elżbieta Sadurska, Agnieszka Zaucha-Prażmo, Agnieszka Brodzisz, Jerzy Kowalczyk, Iwona Beń-Skowronek, Premature atherosclero-sis after treatment for acute lymphoblastic leukemia in childhood, Annals of Agricultural and Environmental Medicine, https://doi. org/10.5604/12321966.1230680.
21. Drafts BC, Twomley KM, D’Agostino R Jr, Lawrence J, Avis N, Ellis LR, Thohan V, Jordan J, Melin SA, Torti FM, Little WC, Hamilton CA, Hundley WG. Low to moderate dose anthracycline-based chemo-therapy is associated with early noninvasive imaging evidence of sub-clinical cardiovascular disease. JAAC Cardiovascular Imaging.2013;6
(8):877-85.
22. A., Tefferi, Nilotinib treatment-associated accelerated atheroscle-rosis: when is the risk justifi ed? Leukemia (2013) 27, 1939–1940; doi:10.1038/leu.2013.112.
23. Kim TD, Rea D, Schwarz M, Grille P, Nicolini FE, Rosti G et al. Pe-ripheral artery occlusive disease in chronic phase chronic myeloid leukemia patients treated with nilotinib or Imatinib. Leukemia (e-pub ahead of print 5 March 2013; doi: 101038/leu2013.70).
24. Levato L, Cantaffa R, Kropp MG, Magro D, Piro E, Molica S. Progres-sive peripheral arterial occlusive disease and other vascular events during nilotinib therapy in chronic myeloid leukemia: a single institu-tion study. Eur J Haematol 2013; 90:531–532.
 This work is licensed under a
This work is licensed under a