Sean P. Collins1,Ovidiu Chioncel2, Gregory J. Fermann3, Phillip D. Levy4, Alan B. Storrow1, Peter S. Pang5
1 Department of Emergency Medicine, Vanderbilt University Medical Center, Nashville, TN
2 University of Medicine Carol Davila, Bucharest; Institute of Emergency for Cardiovascular Diseases “Prof. C.C.Iliescu”, Bucharest, Romania
3 Department of Emergency Medicine, University of Cincinnati, Cincinnati, OH
4 Department of Emergency Medicine, Wayne State University, Detroit, OH
5 Department of Emergency Medicine, Indiana University School of Medicine, Indianapolis, IN
Abstract: Objectives – The emergency department (ED) is the starting point of care for the vast majority of patients hospitalized with acute heart failure (AHF). However, the evidence base to guide dispositions decisions and identify patients for early, safe discharge is relatively weak. As a result, the majority of patients are admitted. However, because clinicians are faced with the daily challenge of risk-stratifying patients to help determine who potentially could be sent home, this remains an area of intense investigation. In this review, we outline an expert consensus on how to risk-stratify ED patients with AHF. Methodology – Expert consensus literature review. Results – The evidence to support fi rm conclusions regarding riskstratification to identify a low risk-cohort safe for ED discharge is lacking. Several risk scores have been developed, though all have limitations, suggesting they should not be routinely used in clinical practice. However, several of these scores are currently undergoing external validation. Patients with elevated blood pressure, preserved renal function and a normal cardiac troponin during their ED work-up are lower risk. In combination with good response to ED therapy, close outpatient follow up, and good self-care skills, these patients represent candidates for early, safe ED discharge. Conclusions – Most ED
patients with AHF are admitted, however, a sizable proportion may be safely discharged. Although further work is needed, identification of lower risk patients is currently possible with existing risk markers, such as blood pressure, renal function, and troponin.
Key words: acute heart failure, emergency department, discharge, risk stratification
INTRODUCTION
Emergency department (ED) physicians diagnose and initially manage the vast majority of patients hospitalized with acute heart failure (AHF). Nearly 75% of ED visits for AHF ultimately lead to hospitalization; this high proportion of ED visits with resultant inpatient admission has not changed over the last decade, either in Europe or the US1,2. Hospital length of stay is 4-5 days in the US and 5-10 days or longer in the rest of the world3. The high financial burden and morbidity associated with hospitalization and subsequent rehospitalizations have led to increased scrutiny concerning AHF management and financial penalties for hospitals with excessive readmissions2,4-9. Attempts to reduce hospitalizations and readmissions, as well as improve outcomes have resulted in a myriad of management strategies, including development of novel therapeutics. While no single strategy of care has been proven to work in all clinical settings, HF readmissions are
slowly decreasing10. From a therapeutic standpoint, clinical trials in chronic HF have demonstrated morbidity and mortality improvements11-16. However, similar benefits have yet to be achieved in AHF clinical trials17-19. The burden of inpatient admissions and the failure of acute therapy to defi nitively alter outcomes has led to renewed interest in risk-stratification; namely, determining
who can be discharged home either directly from the ED or after a brief period of observation. As relatively few inpatients receive intensive acute care, mechanical ventilation, circulatory support, or undergo invasive diagnostic or therapeutic interventions, inpatient admissions might be avoided in a sizable proportion of patients20,21. However, it is unclear at present which patients can be safely discharged home.
RISK STRATIFICATION DURING EMERGENCY DEPARTMENT EVALUATION
Risk stratification has typically focused on the prediction of acute inpatient mortality, rather than re-hospitalization. Further, the majority of studies focus on identifying high-risk, rather than low-risk, and have been limited by a retrospective design in hospitalized patients. Identification of patients at low risk is the critical decision threshold for consideration of ED discharge, as most patients are currently admitted. Unfortunately, ED patients have traditionally not been enrolled in risk-stratification studies; most investigations have been hospital based and patients discharged from the ED are rarely included. Despite these limitations, low blood pressure, renal dysfunction, low serum sodium, and elevated cardiac biomarkers (troponin [Tn] or natriuretic peptides [NP]) have been repeatedly shown to be associated with increased morbidity and mortality22. As more studies attempt to delineate high-risk versus low-risk cohorts using simple, rapidly available data
points, such as systolic blood pressure (SBP), heart rate, and oxygen requirement have proven to be important markers for rapid assessment and disposition. In the EHMRG 7-day mortality risk score, mortality risk increased with higher triage heart rate (OR, 1.15 [CI, 1.02 to 1.30]), lower triage SBP (OR, 1.52 [CI, 1.31 to 1.77] per 20 mm Hg), and lower initial oxygen saturation (OR, 1.16 [CI, 1.01 to 1.33] per 5%)23. However, this study was limited by retrospective patient identification, exclusion of early readmission for AHF as an outcome, and a practice environment not reflective of the United States. Stiell and colleagues found both heart rate >110 beats/min and oxygen saturation less than 90% at ED arrival were independent predictors of serious adverse events (SAEs)24. In AHF patients who are ultimately admitted, those with SBP of less than 120 mmHg had threefold higher inpatient mortality than those with SBP greater than 140 mmHg (7.2%
vs 2.5%, p <0.001)25. In the HF patient who presents in acute distress, a lower initial SBP may reflect reduced left ventricular contractile reserve while a higher initial heart rate suggests the need for increased chronotropy to maintain cardiac output and increased sympathoadrenergic response. A lower initial oxygen saturation demonstrates increased pulmonary congestion and underlying respiratory compromise and therefore places the patient at increased risk for mortality23. Similar to the Lee et al. study, the practice environment in this Canadian study is markedly different than the US, as evidenced by the majority of ED patients being discharged. Auble and colleagues derived a prediction rule using administrative data from over 33,000 patients. Their goal was to utilize variables readily available during an ED evaluation to identify a patient cohort whose risk of death or serious inpatient complications was less than 2%26. Secondary outcomes included death from any cause within 30 days of the index ED admission and the first hospital readmission during this interval with a primary discharge diagnosis of heart failure. Their complex model used 21 predictor variables to identify 17% of patients as low-risk. They subsequently externally tested their 21-variable model in an administrative cohort of over 8300 patients 27 and identified 19.2% of patients as being low risk (<2% inpatient death or complications and <1% inpatient death). Within this group of low-risk patients, 2.9% died within 30 days. Their model is robust, but is based
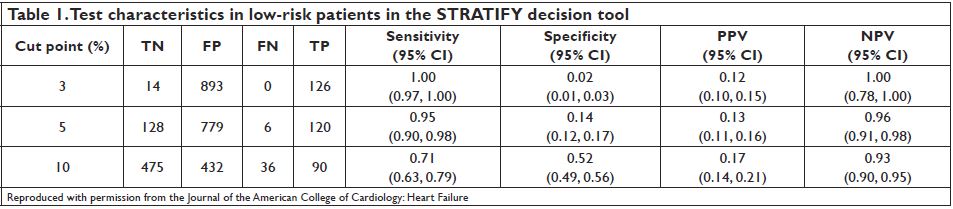
on administrative data and does not account for other important events during the 30-day outpatient period such as ACS, unstable arrhythmias or readmission. STRATIFY was a recent multi-center, prospective cohort study that enrolled 1,033 ED patients with AHF, including 7.7% that were discharged from the ED, to evaluate the incidence of SAEs within 30 days of ED evaluation (ACS, coronary revascularization, emergent dialysis, intubation, mechanical cardiac support, CPR, and death)28. The study assessed readily identifiable ED variables to select a patient cohort who may be eligible for ED discharge. The resultant decision tool was highly sensitive for 30-day mortality and SAE. Those patients with less than 3% and 5% risk for 30-day events were detected with 100% and 95% sensitivity, respectively (Table 1). Importantly, there were no deaths in the group at less than 3% risk and only one in the group with less than 5% risk of events. The rule did not consider HF readmission as an endpoint.
Organ Injury as a Marker of Risk: Do all patients with stable, elevated Troponin need admission?
Evidence of ongoing ischemia or myocardial injury, as demonstrated by ECG changes and elevated troponin, continues to be strongly associated with increased inpatient and post-discharge mortality and increased readmission rates. The presence of ST-depression on the ECG provided improved recognition of those patients with AHF at higher risk of 30-day mortality29. Peacock et al. illustrated that AHF patients with elevated cTn (and SCr <2.0) had higher in-hospital mortality compared to those without elevated cTn. However, this study utilized fi rst generation troponin
assays; many patients who may have elevated troponin levels as measured by the contemporary assays may have been in the “normal troponin” group in this study. Diercks et.al. showed a small OU cohort with a SBP >160 mmHg and a normal cTn suffered no 30-day adverse events (death, readmission, myocardial infarction, or arrhythmias)30. The recently derived risk prediction
tools outlined above have likewise identifi ed an elevated cTn level as an independent predictor of both SAE and mortality23,24,28. Despite these fi ndings identifying a higher-risk cohort, patients with minimally elevated cTn levels may still be candidates for observation management, especially if serial troponin measurements are followed to exclude acute coronary syndrome (ACS). Troponin elevation in patients with AHF is not uncommon, though the majority are not due to ACS31. Many patients have low cTn levels above the 99%ile cutoff that may not confer an elevated risk of cardiac events when compared to those with ACS or significant cTn elevation. In the STRATIFY study cTn elevation did not confer increased risk until it was above 0.13 ng/ml (99%ile cutoff <0.04 ng/ml). Further study is needed however before recommended use in clinical practice. With the anticipated introduction of highsensitive cTn assays in the US, identifying a level of cTn
elevation that differentiates low-risk from non-lowrisk patients with AHF is critical. Pang et.al. recently found when hsTnT was not above the 99th%ile patients were at very low risk for 180 day CV mortality32. In the past, absence of high risk features did not necessarily translate to sufficiently low risk for discharge. However, a pilot trial to prospectively test both the utility of a nondetectable or very low hsTnT release is ongoing. Additionally, this trial will prospectively collect STRATIFY variables to allow for external validation of that decision rule33.
Natriuretic Peptides
The natriuretic peptides, B-type natriuretic peptide (BNP) and its N-terminal precursor fragment (NTproBNP,) are the most established AHF biomarkers for evaluating undifferentiated dyspnea and assessing for worsening HF34,35. Patients with a BNP level less than 100 pg/mL are unlikely to have AHF and multiple studies have shown that rising BNP and NT-proBNP levels are associated with increased disease severity as well as an increased risk for mortality in AHF36-38. Nonetheless, there remains no absolute cutoff for these markers in regards to evaluating safe ED discharge. Clinical trials have shown limited effect of routine BNP measurement in predicting patient outcomes, and following BNP levels to gauge response to therapy or suitability for “safe” discharge have revealed mixed results39-42.
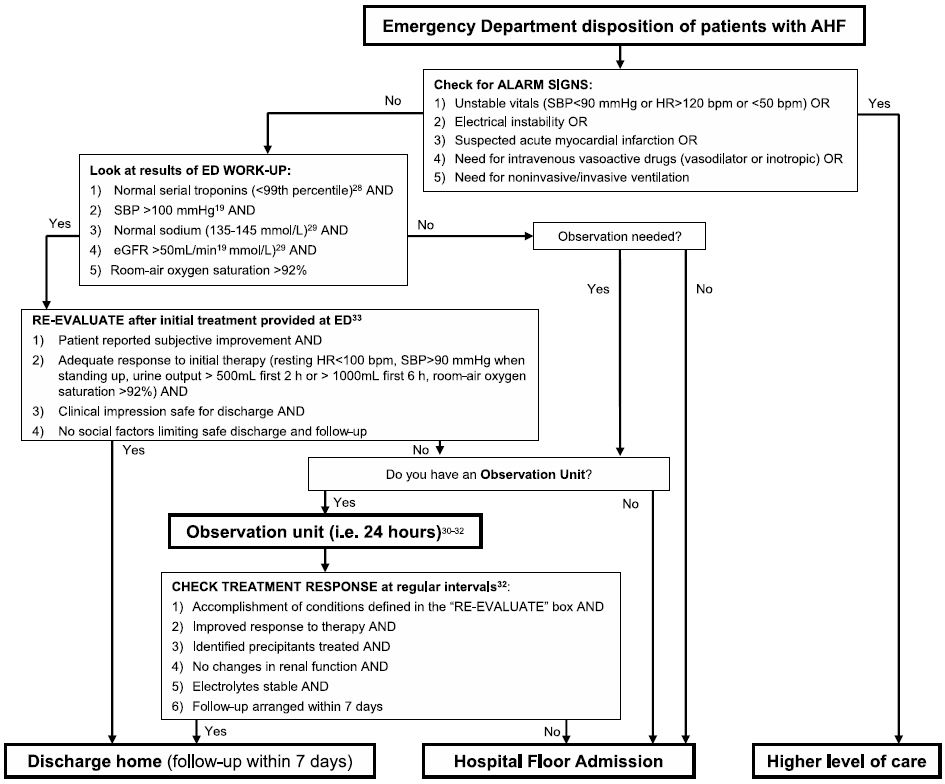
Figure 1. Consensus algorithm identifying patients who may be discharged directly from the ED or after a brief period of observation.
International Consensus
A recent consensus paper from an international group of physicians and nurses, comprised primarily of ED caregivers, has further highlighted this issue of identifying ED patents for early, safe discharge from the ED43. A significant knowledge gap was highlighted by the group: what are the current event rates for discharged ED patients with AHF, and what is acceptable? Despite an extensive literature search, no universal evidence based consensus recommendation was provided, highlighting the need for continued work in this area. However, the group did propose event thresholds to advance research in this area, and suggested stratifying event rates by the ability of the ED to provide observation care (<48 hours). In addition, a consensus algorithm was proposed (Figure 1). While not intended for immediate clinical use, the consensus algorithm was put forth for local institutions to consider for both quality improvement and research efforts until it
can be prospectively tested.
WHAT OTHER FACTORS SHOULD BE CONSIDERED IN PATIENTS WHO CAN BE DISCHARGED?
While the formal ED clinical evaluation is a crucial component of identifying patients safe for discharge, evaluating the patient’s ability to provide self-care as well as the availability and degree of caregiver support may be equally important44. This includes socioeconomic considerations, such as the ability to afford medications, as well as transportation to follow-up appointments.
Patients who have poor disease insight or lack access to medications or close outpatient follow-up may be poor candidates for direct ED discharge45. They may require an extended time period of ED-based observation or inpatient admission while an outpatient plan of care is established. While this may introduce additional cost, the potential subsequent healthcare costs and quality of life should also be considered. Finally, an improvement in symptoms due to ED-based AHF therapy is equally important. Often patients who have a low-risk ED-based evaluation and good social
support may still require admission because of the inability of ED-delivered therapy to improve symptoms sufficiently to allow ED discharge.
CONCLUSION
Summarizing the available data to date suggests patients with elevated blood pressure, normal cTn, serum sodium and renal function, as well as an adequate response to ED therapy and good outpatient support are candidates for ED discharge. However, with external testing of the two prospectively derived risk-stratification studies this may change subsequent recommendations. The availability of a HF score utilizing readily available ED data would significantly impact current disposition strategies. The STRATIFY rule is currently being externally tested in a prospective multicenter study with anticipated completion of patient enrollment in 201833. Until the results of these studies are available we suggest using a focused ED-based evaluation to safely transition a subset of ED patients with AHF away from hospitalization.
Teaching points:
1. The ED is the focal point for the initial diagnosis and management of the majority of patients who are admitted to the hospital with AHF.
2. The identification of low risk patients in ED remains challenging and is more complex than many other disease processes that present to the ED.
3. The vast majority of AHF studies have focused on identification of high-risk features in the ED patients.
4. Just because a patient does not have high-risk features does not mean they are low-risk and able to be discharged from the ED.
5. Clinical variables have been identifi ed that may delineate low-risk from high-risk patients (SBP, cTn, BNP, renal function, serum sodium) but the absence of prospective validation limits their implementation in clinical practice.
6. In addition to clinical variables, two important components of ED evaluation include response to initial therapy and the patient’s self-care ability, which may be influenced by socio-economic and caregiver factors.
Conflicts of in terest:
Collins: Research Support: NIH/NHLBI, PCORI, Cardiorentis, Novartis, Trinity; Consulting: Trevena, Novartis, Siemens
Chioncel: Research Support: Servier, Vifor, Novartis, Philips
Fermann: Research Support: PCORI, Novartis, Siemens, Nanodetection, Cardiorentis, Trevena, Pfizer, Portola. Consulting: Janssen. Speakers Bureau-Janssen
Levy: Research Support: NIH/NIMHD, NIH/NHLBI, PCORI, Cardiorentis; Consulting: Cardiorentis, Trevena, Novartis, Siemens; Roche Diagnostics, ZS Pharma
Storrow: Current or Recent Research Support: Centers for Medicaid and Medicare Services (CMS), NIH / NHLBI, National Center for Advancing Translational Sciences/NIH, Beckman Coulter, PCORI. Current or Recent Consultant: Trevena, Beckman Coulter, Siemens, MCM Education
Pang: Consultant for: BMS, Medtronic, the Medicines Company, Novartis, Trevena, scPharmaceuticals, Cardioxyl, Roche Diagnostics, Relypsa; Research Support: Roche, Novartis, PCORI, IUSM
References
1 . Storrow AB, Jenkins CA, Self WH, et al. The burden of acute heart failure on U.S. Emergency departments. JACC Heart failure 2014;2:269- 77.
2 . Mozaffarian D, Benjamin EJ, Go AS, et al. Heart Disease and Stroke Statistics-2016 Update: A Report From the American Heart Association. Circulation 2016;133:e38-e360.
3 . Filippatos G, Khan SS, Ambrosy AP, et al. International REgistry to assess medical Practice with lOngitudinal obseRvation for Treatment of Heart Failure (REPORT-HF): rationale for and design of a global registry. European journal of heart failure 2015;17:527-33.
4 . Abraham WT, Fonarow GC, Albert NM, et al. Predictors of in-hospital mortality in patients hospitalized for heart failure: insights from the Organized Program to Initiate Lifesaving Treatment in Hospitalized Patients with Heart Failure (OPTIMIZE-HF). Journal of the American College of Cardiology 2008;52:347-56.
5 . Fonarow GC, Abraham WT, Albert NM, et al. Association between performance measures and clinical outcomes for patients hospitalized with heart failure.[see comment]. Jama 2007;297:61-70.
6 . O’Connor CM, Abraham WT, Albert NM, et al. Predictors of mortality after discharge in patients hospitalized with heart failure: an analysis from the Organized Program to Initiate Lifesaving Treatment in Hospitalized Patients with Heart Failure (OPTIMIZE-HF). American heart journal 2008;156:662-73.
7 . Lee DS, Schull MJ, Alter DA, et al. Early deaths in patients with heart failure discharged from the emergency department: a population-based analysis. Circulation Heart failure 2010;3:228-35.
8 . Schrock JW, Emerman CL. Observation unit management of acute decompensated heart failure. Heart failure clinics 2009;5:85-100, vii.
9 . Ambrosy AP, Gheorghiade M, Chioncel O, Mentz RJ, Butler J. Global perspectives in hospitalized heart failure: regional and ethnic variation in patient characteristics, management, and outcomes. Current heart failure reports 2014;11:416-27.
1 0. Trends in Hospital Readmissions for Four High-Volume Conditions, 2009-2013. HCUP, 2015. July 22, 2016, at https://www.hcup-us.ahrq.gov/reports/statbriefs/sb196-Readmissions-Trends-High-Volume-Conditions.jsp.)
1 1. Cohn JN, Johnson G, Ziesche S, et al. A comparison of enalapril with hydralazine-isosorbide dinitrate in the treatment of chronic congestive heart failure. The New England journal of medicine 1991;325:303-10.
1 2. Garg R, Yusuf S. Overview of randomized trials of angiotensin-converting enzyme inhibitors on mortality and morbidity in patients with heart failure. Collaborative Group on ACE Inhibitor Trials. Jama 1995;273:1450-6.
1 3. The Cardiac Insufficiency Bisoprolol Study II (CIBIS-II): a randomised trial. Lancet 1999;353:9-13.
1 4. Packer M, Coats AJ, Fowler MB, et al. Effect of carvedilol on survival in severe chronic heart failure. The New England journal of medicine 2001;344:1651-8.
1 5. McMurray JJ, Packer M, Desai AS, et al. Angiotensin-neprilysin inhibition versus enalapril in heart failure. The New England journal of medicine 2014;371:993-1004.
1 6. Gabet A, Juilliere Y, Lamarche-Vadel A, Vernay M, Olie V. National trends in rate of patients hospitalized for heart failure and heart failure mortality in France, 2000-2012. European journal of heart failure 2015;17:583-90.
1 7. O’Connor CM, Starling RC, Hernandez AF, et al. Effect of nesiritide in patients with acute decompensated heart failure. The New England journal of medicine 2011;365:32-43.
1 8. McMurray JJ, Teerlink JR, Cotter G, et al. Effects of tezosentan on symptoms and clinical outcomes in patients with acute heart failure: the VERITAS randomized controlled trials. Jama 2007;298:2009-19.
1 9. Massie BM, O’Connor CM, Metra M, et al. Rolofylline, an adenosine A1-receptor antagonist, in acute heart failure. The New England journal of medicine 2010;363:1419-28.
2 0. Fonarow GC, Stough WG, Abraham WT, et al. Characteristics, treatments, and outcomes of patients with preserved systolic function hospitalized for heart failure: a report from the OPTIMIZE-HF Registry. Journal of the American College of Cardiology 2007;50:768-77.
2 1. Yancy CW, Lopatin M, Stevenson LW, De Marco T, Fonarow GC. Clinical presentation, management, and in-hospital outcomes of patients admitted with acute decompensated heart failure with preserved systolic function: a report from the Acute Decompensated Heart Failure National Registry (ADHERE) Database. Journal of the American College of Cardiology 2006;47:76-84.
2 2. Peacock WFt, De Marco T, Fonarow GC, et al. Cardiac troponin and outcome in acute heart failure. The New England journal of medicine 2008;358:2117-26.
2 3. Lee DS, Stitt A, Austin PC, et al. Prediction of heart failure mortality in emergent care: a cohort study. Ann Intern Med 2012;156:767-75.
2 4. Stiell IG, Clement CM, Brison RJ, et al. A risk scoring system to identify emergency department patients with heart failure at high risk for serious adverse events. Academic emergency medicine: official journal of the Society for Academic Emergency Medicine 2013;20:17-26.
2 5. Gheorghiade M, Abraham WT, Albert NM, et al. Systolic blood pressure at admission, clinical characteristics, and outcomes in patients hospitalized with acute heart failure. Jama 2006;296:2217-26.
2 6. Auble TE, Hsieh M, Gardner W, et al. A prediction rule to identify low-risk patients with heart failure. Academic emergency medicine: official journal of the Society for Academic Emergency Medicine 2005;12:514-21.
2 7. Hsieh M, Auble TE, Yealy DM. Validation of the Acute Heart Failure Index. Annals of emergency medicine 2008;51:37-44.
2 8. Collins SP, Jenkins CA, Harrell FE, Jr., et al. Identifi cation of Emergency Department Patients With Acute Heart Failure at Low Risk for 30-Day Adverse Events: The STRATIFY Decision Tool. JACC Heart failure 2015;3:737-47.
2 9. Greig D, Austin PC, Zhou L, et al. Is chemic electrocardiographic abnormalities and prognosis in decompensated heart failure. Circulation Heart failure 2014;7:986-93.
3 0. Diercks DB, Peacock WF, Kirk JD, Weber JE. ED patients with heart failure: identification of an observational unit-appropriate cohort. Am J Emerg Med 2006;24:319-24.
3 1. Januzzi JL, Jr., Filippatos G, Nieminen M, Gheorghiade M. Troponin elevation in patients with heart failure: on behalf of the third Universal Definition of Myocardial Infarction Global Task Force: Heart Failure Section. European heart journal 2012;33:2265-71.
3 2. Pang PS, Teerlink JR, Voors AA, et al. Use of High-Sensitivity Troponin T to Identify Patients With Acute Heart Failure at Lower Risk for Adverse Outcomes: An Exploratory Analysis From the RELAX-AHF Trial. JACC Heart failure 2016;4:591-9.
3 3. High Sensitivity cTnT Rules Out Cardiac Insufficiency Trial (TACIT). clinicaltrials.gov, 2016. (Accessed July 19, 2016, at https://clinicaltrials.gov/ct2/show/NCT02592135?term=Pang+AND+heart+failure&rank=1.)
3 4. Maisel AS, Krishnaswamy P, Nowak RM, et al. Rapid measurement of B-type natriuretic peptide in the emergency diagnosis of heart failure. The New England journal of medicine 2002;347:161-7.
3 5. Januzzi JL, Jr., Camargo CA, Anwaruddin S, et al. The N-terminal Pro-BNP investigation of dyspnea in the emergency department (PRIDE) study. The American journal of cardiology 2005;95:948-54.
3 6. Maisel A, Mueller C, Adams K, Jr., et al. State of the art: using natriuretic peptide levels in clinical practice. European journal of heart failure 2008;10:824-39.
3 7. Januzzi JL, van Kimmenade R, Lainchbury J, et al. NT-proBNP testing for diagnosis and short-term prognosis in acute destabilized heart failure: an international pooled analysis of 1256 patients. European heart journal 2006;27:330.
3 8. Fonarow GC, Peacock WF, Horwich TB, et al. Usefulness of B-type natriuretic peptide and cardiac troponin levels to predict in-hospital mortality from ADHERE. The American journal of cardiology 2008;101:231-7.
3 9. Schneider HG, Lam L, Lokuge A, et al. B-type natriuretic peptide testing, clinical outcomes, and health services use in emergency department patients with dyspnea: a randomized trial. Ann Intern Med 2009;150:365-71.
4 0. Pfi sterer M, Buser P, Rickli H, et al. BNP-guided vs symptom-guided heart failure therapy: the Trial of Intensifi ed vs Standard Medical Therapy in Elderly Patients With Congestive Heart Failure (TIME-CHF) randomized trial. Jama 2009;301:383-92.
4 1. Singer AJ, Birkhahn RH, Guss D, et al. Rapid Emergency Department Heart Failure Outpatients Trial (REDHOT II): a randomized controlled trial of the effect of serial B-type natriuretic peptide testing on patient management. Circulation Heart failure 2009;2:287-93.
4 2. Lokuge A, Lam L, Cameron P, et al. B-type natriuretic peptide testing and the accuracy of heart failure diagnosis in the emergency department. Circulation Heart failure 2010;3:104-10.
4 3. Miro O, Levy PD, Mockel M, et al. Disposition of emergency department patients diagnosed with acute heart failure: an international emergency medicine perspective. European journal of emergency medicine : official journal of the European Society for Emergency Medicine 2016.
4 4. Collins SP, Storrow AB. Moving Toward Comprehensive Acute Heart Failure Risk Assessment in the Emergency Department: The Importance of Self-Care and Shared Decision Making. JACC Heart failure 2013;1:273-80.
4 5. Collins S, Storrow A. Moving Towards Comprehensive Acute Heart Failure Risk Assessment in the Emergency Department. J Am Coll Cardiol HF 2013;1:273-80.
 This work is licensed under a
This work is licensed under a