Nelis Asan1, E. Apetrei1,2, D. Deleanu1, V. A. Iliescu1,2, I. M. Coman1,2
Article received on the 6th of February 2013. Article accepted on the 24th of February 2013.
1 “Prof. Dr. CC. Iliescu” Institute of Emergency for Cardiovascular Diseases, Bucharest
2 “Carol Davila” University of Medicine and Pharmacy, Bucharest
Prof. Dr. Eduard Apetrei, “Prof. Dr. CC. Iliescu” Institute of Emergency for Cardiovascular Diseases, Bucharest, 258 Fundeni Street, District number 2. E-mail: eapetrei@gmail.com
Abstract: Hypertrophic obstructive cardiomyopathy is a fascinating disease entity and a source of controversy since the first modern descriptions by Brock in 19572. Recognition and understanding the significance of left ventricle outflow obstruction has indeed been a “winding road”, but one that has eventually provided clinically relevant answers directly related to patient management. We report a case of hypertrophic obstructive cardiomyopathy (resting left ventricular outflow tract gradient of 136 mmHg) and mitral valve abnormalities in a 63 year-old woman.
Case study
A 63 year-old woman presented with fatigue and progressive shortness of breath on exertion (class III NYHA). The patient reported remained asymptomatic until age 55, when she was diagnosed with severe subaortic stenosis. Transthoracic echocardiography, at that time, showed left ventricle (LV) hypertrophy – intraventricular septum (IVS) 13 mm and LV posterior wall (PP) 13 mm, LV outflow tract gradient of 107/43 mmHg, mitral regurgitation grade I-II and 50% ejection fraction (EF). There is no any data available related with the morphology of aortic or mitral valve. The patient had several cardiovascular risk factors, such as 6- year history of moderate hypertension, dyslipidemia, 6-year history of diabetes mellitus type II treated with oral antidiabetic agents and family history of sudden cardiac arrest (father died of sudden cardiac arrest when he was 65 years). She followed the treatment as prescribed in 2005 with betablockers and non-dihydropyridine calcium channel blocker and had a favorable clinical evolution. Over a period of 3 months, the patient readmitted to the hospital because of dyspnea on minimal-moderate exertion. Clinical examination revealed stable patient with a blood pressure of 120/70 mmHg, pulse rate of 80 bpm, the first sound is soft at the apex, the second sound is normal, mydsistolic ejection murmur heard best at third left parasternal space and at apex, increased in orthostatism; without systemic or pulmonary congestion signs. The electrocardiogram (Figure 1) indicated sinus rhythm 70bpm, QRS axis +20 grade, left ventricle hypertrophy, ST segment depression 3.5 mm with negative T wave in V4-V6, DI, DII and aVL. Chest X-ray showed slight enlargement of the heart silhouette with cardiothoracic ratio of 55% and mild accentuation of pulmonary arterial vessels.
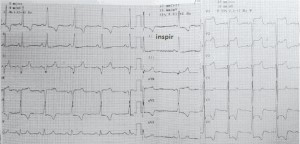
Figure 1. Electrocardiogram at admission (see text).
Transthoracic echocardiography (TTE) showed that the interventricular septum and LV posterior wall are thickened (Figure 3), left ventricular outflow tract peak gradiente (Figures 4 and 7), intrinsic abnormalities of the mitral valve – direct papillary insertion into the anterior mitral leaflet and a particular structure of interventricular septum (Figure 5).
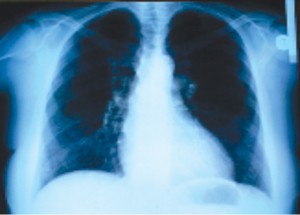
Figure 2. The posterior – anterior view of chest X ray.
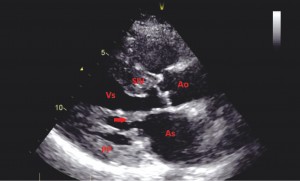
Figure 3. Transthoracic echocardiography parasternal long-axis showing insertion of papillary muscle into the anterior mitral leaflet (see arrow). LA – left atrium. LV- left ventricle. PP – posterior wall. IVS – interventricular septum. Ao – aorta.
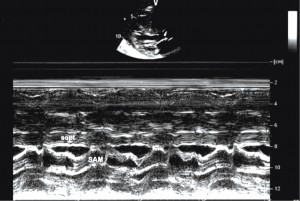
Figure 4. Two dimensional transthoracic echocardiography long axis (up) and M-mode (down). Left ventricular outflow tract (subaortic stenosis) caused by anterior systolic displacement of anterior mitral leaflet and subvalvular mitral apparatus.
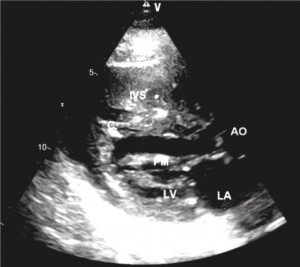
Figure 5. Two dimensional transthoracic echocardiography long axis in diastole showed trabeculae 4mm and 5mm thick (C) insert into IVS and free wall of LV. Left atrium dilated. LA left – atrium, LV – left ventricle, AML – anterior mitral leaflet, PML – posterior mitral leaflet, IVS – interventricular septum, Ao – aorta, PM – papillary muscle.
In Figure 3, two dimensional TTE long axis plane identified left ventricle (LV) with end-diastolic diameter 37 mm, end-systolic diameter 20mm, left atrium diameter 43mm, left ventricular hypertrophy – IVS 21mm at base and 17mm at middle, PP 13mm. The thickness of the anterior wall of the right ventricle is 7 mm.
Left ventricular outflow tract obstruction is showed in Figure 4 and Figure 7. Continuous-wave (CW) Doppler in apical 5 chambers recorded a left ventricular outflow tract peak gradient of 138 mmHg (Figure 7).
The anomalies of mitral subvalvular apparatus include (Figure 3 and Figure 5) nodular calcifications on anterior mitral valve chordae, thickening chordae, anomalous papillary muscle with direct insertion into the anterior mitral leaflet. Aortic valve are thickening and stiffness (calcification), but with normal leaflet mobility. Tricuspid and pulmonary valves are normal. Color Doppler shows high velocities across the left ventricular outflow tract in mosaic color and mitral regurgitation jet of III-IV grade (Figure 6).
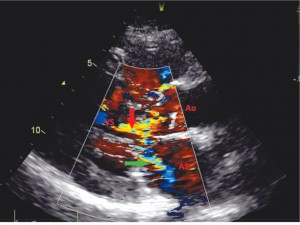
Figure 6. Transthoracic echocardiography long axis Color Doppler – systolic turbulent flow in LV outflow tract (red arrow) and posteriorly directed jet of mitral regurgitation within the left atrium (green arrow).
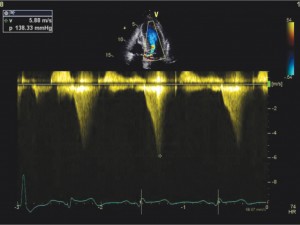
Figure 7. Transthoracic echocardiography apical 5 chambers (up) and continuous-wave Doppler (CW) (down) – the concave-to-the – left contour of the Doppler CW jet. Peak gradient in LVOT is 138 mmHg.
Assessment of LV diastolic function – mitral inflow shows a restrictive inflow pattern. LV systolic function is normal with a LV ejection fraction of 65%.
Particularly is the structure of interventricular septum (Figure 5). In long axis plane, we observed 2 thickening trabeculae 4mm-5mm inserting into LV free wall at apex and into the interventricular septum. The trabeculaes are parallel with interventricular septum and can be “part” of interventricular septum. IVS measured 17mm without trabeculae.
Following the clinical profile, electrocardiography and transthoracic echocardiography data and cardiovascular risk factors, coronary angiography was performed. Epicardial coronary arteries were normal. Left ventricular catheterization and angiography revealed VS/Ao pressure gradient of 96 mmHg, mitral regurgitation grade III, left ventricle ejection fraction of 70% and telediastolic LV pressure of 30 mmHg.
DISCUSSIONS
Structural intrinsic and functional mitral valve or subvalvular abnormalities have been reported in left ventricular outflow tract obstruction in hypertrophic cardiomyopathy. The most frequent is an anomalous papillary muscle insertion directly into the anterior mitral leaflet, an entity that has been well documented by Klues et al. in 1991. Of 78 mitral valve specimens from patients with hypertrophic obstructive cardiomyopathy, 10 were identified as having direct insertion of one or both left ventricular papillary muscles into the anterior mitral leaflet8. Is a congenital malformation, consequence of selective failure of chordal development at about 12 weeks gestation. Normally, at this stage, the papillary muscles are contiguous with the mitral leaflets. The cephalad portions of the papillary muscles are first transformed into a thick muscular chordae, which finally become fibrous chordae positioned between the mitral leaflets and the papillary muscles. The arrest in normal embryological development is not pathognomonic of obstructive hypertrophic cardiomyopathy8.
Other anomalies of mitral subvalvular apparatus include abnormal chordae tendineae that attach to the ventricular septum or left ventricular free wall and accessory papillary musclese, all which may tether the mitral leaflets toward the septum and produce LVOT obstruction9.
The choice of optime procedure must be individualizad based on age, comorbidities, suitability of coronary anatomy, intrinsec mitral valve disease, patient preferente and operator experience4.
The conventional septal myectomy (Marrow procedure) is not the optimal procedure.
After the classic septal myectomy, the patients with HOCM and anomalies of the mitral subvalvular apparatus can lead to intraoperative death, incomplete or only temporary relief of LVOT obstruction, persistent of SAM and mitral regurgitation3,8.
Additional procedures are represented by: anterior mitral leaflet excision or plicating, “false chordae” excision and papillary muscle realignment2,6,10.
Rankin J at al. described o new surgical concept: septal myectomy, excision of papillary muscle incriminated in LVOT obstruction, chordae excision and mitral ring anuloplasty; the mitral valve was repaired by connecting the left aspect of the leaflets to the posterior papillary muscle, using Gore-Tex artificial chords11.
Mitral valve replacement is possible in mitral regurgitation associated with chordae rupture, mitral valve prolapse, mitral ring dilatation or in valvular morphological disease like restrictioned, thickened, calcifications or myxomatous valve9,12.
In conclusion, we reported a patient with obstructive hypertrophic cardiomyopathy (LVOT peak gradient of 138 mmHg on transthoracic echocardiography and 96 mmHg on left ventricular catheterization) and severe mitral regurgitation of III-IV grade. A spectrum of mitral leaflet abnormalities and systolic displacement of mitral anterior leaflet and subvalvular apparatus have been related to the mechanism of mitral regurgitation.
The patient is at increased risk for sudden death (SCD). We have identified 2 risk factors for SCD: LV septal hypertrophy and family history of sudden death. Three members of family (the daughter and 2 nephew) were evaluated or screened with ecg and ecocardiography, which were normal. In our case report, percutaneous alcohol septal ablation, method we perform currently in our institute, is constrained by mitral valve abnormalities1. The myectomy has the advantage of direct visualization of complex LV outflow tract anatomy and recognition of all structural abnormalities that contribuite to mechanical subaortic obstruction.
European Society of Cardiology and American College of Cardiology12 recommend surgical septal myectomy as the gold standard treatment for most drug-refractory and severely symptomatic HOCM patients. However, in Europe after the introduction of alcohol septal ablation 15-20 years ago, septal myectomy has essentially disappeared, alcohol septal ablation being promoted as a superior and easier treatment strategy for decrease or abolition of the LVOT obstruction1,2,4.
Surgical myectomy is performed in only 5 European centres (3 in Italy)6.
Recent the Italian – Bergamo Center published a report about 124 patients with obstructive HCM and heart failure symptoms who underwent myectomy. The LV outflow gradient decreased from 95 ± 36 mmHg before surgery to 12 ± 6 mmHg post surgery. Periprocedural mortality was 0.8% (1 patient) and 0.8%, 3.3% at 1 and 5 years respectively5. The study of Iacovoni et al. has important implications for the management of obstructive HCM in Europe and should be a stimulus for other institutions elsewhere in Europe to initiate HCM programmes13.
Conflict of interests: The authors declare that no conflict of interest exists.
References
1. Apetrei E, Seggewiss H, Deleanu D şi col. Ablaţia miocardică septală percutană în cardiomiopatia hipertrofică obstructivă. Revista Română de Cardiologie, Vol IX; nr 3-4;1999.
2. Maron BJ, Maron MS, Douglas Wigle E, Braunwald E. The 50-year history, controversy and clinical implications of left ventricular outflow tract obstruction in hypertrophic cardiomyopathy: from idiopathic hypertrophic subaortic stenosis to hypertrophic cardimyopathy, J Am Coll Cardiology 2009;54:191-200.
3. Minakata K, Dearani JA, Nishimura RA et al. Extended septal myectomy for hypertrophic obstructive cardiomyopathy with anomalous mitral papillary muscles or chordae J Thorac Cardiovasc Surg 2004; 127:481-489.
4. Carolyn Ho Y. Hypertrophic Cardiomyopathy in 2012 Circulation 2012;125:1432-1438.
5. Iacovoni A, Spirito P, Simon C, Ferrazzi PA et al. A contemporary European experience with surgical septal myectomy in hypertrophic cardiomyopathy Eur Heart J 2012;33:2080-2087.
6. Maron BJ, Yacoub M, Dearani JA. Benefits of surgery in obstructive hypertrophic cardimyopathy: bring septal myectomy back for European patients Eur Heart J 2011 32: 1055-1058.
7. Nagueh SF, Bierig SM, Budoff MJ et al. American Society of Echocardiography clinical recommendations for multimodality cardiovascular imaging of patients with hypertrophic cardiomyopathy J Am Soc Ecocardiogr 2011:24:473-98.
8. Klues GH, Robert WC, Maron BJ. Anomalous insertion of papillary muscle directly into anterior mitral leaflet in hypertrophic cardiomyopathy significance in producing left ventricular outflow obstruction. Circulation 1991;84:1188-1197.
9. Imoto Y, Kado H, Yasuda H, et al. Subaortic stenosis caused by anomalous papillary muscle of the mitral valve Ann Thorac Surg 1996; 62:
1858-1860
10. Bryant R, Smedira NG Papillary muscle realignment for symptomatic left ventricular outflow tract obstruction J Thorac Cardiovasc Surg 2008;135:223-224.
11. Rankin JS, Binford RS, Johnston TS, et al. A new mitral valve repair strategy for hypertrophic obstructive cardiomyopathy. The Journal of heart valve disease 2008;17:642-647.
12. Maron BJ, McKeena WJ, Danielson GK et al. American College of Cardiology/European Society of Cardiology Clinical Expert Consensus Document on Hypertrophic Cardiomyopathy. A report of the American College of cardiology Foundation task Force on Clinical Expert Consensus Documents and the European Society of Cardiology Committee for Practice Guidelines. J Am Coll Cardiol 2003; 42:1687-1713.
13. Dearany J.A. Septal myectomy remains the gold standard. Eur Heart J 2012;33:1999-2000.
 This work is licensed under a
This work is licensed under a