Alina Bisoc1, Mariana Radoi2
1 Assistant Professor, cardiologist, Faculty of Medicine, “Transilvania” University; Emergency Clinical Hospital, Cardiology Department, Brasov, Romania
2 Professor of cardiology, Faculty of Medicine, “Transilvania” University;
Emergency Clinical Hospital, Cardiology Department, Brasov, Romania
Contact address:
Alina Bisoc. Faculty of Medicine, “Transilvania” University; Emergency Clinical Hospital, Cardiology Department, Brasov, Romania
E-mail: alina_bisoc@yahoo.com.
Abstract: Scop – Evaluarea în cardiomiopatia indusă de antracicline (CIA) a nivelelor troponinei T determinată prin tehnici de înaltă sensibilitate (hs-cTnT) corelate cu modificările ECG de efort, înainte ca scăderea FEVS să fie diagnostică pentru CIA. Metodă – 68 de pacienţi cu cancer trataţi cu antracicline au fost supravegheaţi 6 luni pentru apariţia CIA, diagnosticată prin scăderea FEVS <50% sau cu 10% unităţi. Hs-cTnT şi testul de efort s-au efectuat initial la 3 şi 6 luni. Rezultate – La 6 luni, 15 pacienţi (22,05%) au evoluat cu CIA (grupul 1) şi 53 (77,95%) fără CIA (grupul 2). După 3 luni, testul de efort a arătat la grupul 1 vs. grupul 2 scăderea capacităţii maxime de efort (p=0,0489) și creșterea incidenței subdenivelării evolutive de segment ST≥1mm (p=0,0014). Nivelul la 3 luni a hs-cTnT>0,009 ng/ml asociat cu o creştere>30% faţă de momentul iniţial este predictor pentru apariţia CIA la 6 luni (p=0,001) cu sensibilitate de 53,3%, specificitate de 100%, valoare predictiv pozitivă de 100% şi valoare predictiv negativă de 88,3%. Concluzii – La pacienţii cu cancer trataţi cu antracicline, nivelul hs-TnT la 3 luni este predictiv pentru apariţia CIA la 6 luni şi s-a asociat cu reducerea toleranţei şi apariţia subdenivelării segmentului ST la efort.
Cuvinte cheie: cardiomiopatie precoce indusă de antracicline, hs-cTnT, ECG de efort, capacitate maximă de efort, subdenivelare de segment ST la efort.
Abstract: Purpose – The evaluation of high-sensitive cardiac troponin T (hs-cTnT) levels and ECG exercise test changes in anthracyclines induced cardiomyopathy (AIC) occurring before the reduction at the diagnostic value of the left ventricle ejection fraction (LVEF). Method – 68 patients with cancer treated with anthracyclines were followed 6 months for the occurrence of AIC, diagnosed when LVEF decrease < 50% or by 10% units compared to baseline. The plasma levels of hs-cTnT and ECG exercise test were carried out at baseline, 3 and 6 months. Results – At 6 months 15 patients (22.05%) were diagnosed with AIC (group1) and 53 (77.95%) evolved without AIC (group 2). After 3 months, exercise test findings in group 1 vs. group 2 showed the reduction of maximal work capacity (p=0.0489) and significant increasing in the incidence of depression of ST segment ≥1mm (p=0.0014). At 3 months, hs-cTnT levels >0,009 ng/ml associated with percentage increased >30% is a predictor for AIC with 53.3% sensitivity, 100% specificity, 100% positive predictive value and 83.3% negative predictive value. Conclusions – In patients with cancer treated with antracyclines >hs-cTnT levels at 3 month were predictive for the occurrence of AIC at 6 months and associated with decreasing of exercise tolerance and increasing of incidence of ST segment depression.
Keywords: early anthracycline-induced cardiomyopathy, hs-cTnT, ECG exercise test, maximal work capacity, depression of ST segment at exercise ECG test.
Introduction
Anthracycline-induced cardiomyopathy (AIC) is a consequence of myocyte damage caused by direct cardiotoxicity and myocyte apoptosis. Studies in animal models have shown the relationship between the mycocyte injuries induced by doxorubicin and increased cardiac troponin levels (cTn) proportionally to the administered dose1, and clinical studies have shown that elevated levels of cTnT indicate cardiotoxicity2. In some studies in which dosing of troponin was done by immunoassay, the cTn level did not correlate with cardiac dysfunction assessed by decreased LVEF3,4, which was explained by the different „cut-off” values of immunoassay techniques and inaccuracy of echocardiographic estimation of the left ventricular ejection fraction (LVEF). Most clinical trials used the cTnI determination to assess patients with neoplastic disease treated with anthracyclines and reported that cTnI determined by immunoassay is a biomarker of anthracycline cytotoxicity, elevated cTnI levels were predictive for the occurrence of early AIC5,6. In recent years, clinical trials wherein cTnI was determined by ultrasensitive techniques reconfirms the increase of the cTnI level as biomarker of cardiotoxicity6,7 correlated with the degree of cardiac dysfunction6. The cTnI dynamics under therapy proved to be an indicator of cardiac dysfunction recovery8. Increase of cTnI in patients with cancer and treatment with anthracyclines has been shown to be predictive for the occurrence of early-onset AIC9, the development with clinical cardiovascular events9 and a useful indicator of initiating cardiac dysfunction therapy with converting enzyme (ACE) inhibitors and beta blockers8. Concomitant use of biomarkers and imaging techniques has increased the predictive sensitivity of such investigations in the AIC diagnosis.
Goal
Evaluation in patients with anthracycline-induced cardiomyopathy of the plasma troponin T levels determined by high sensitivity techniques (hs-TnT) correlated with changes of exercise ECG testing before LVEF decrease to be diagnostic for AIC.
Method
Prospective study which enrolled a consecutive series of patients with various malignancies and indication for treatment with doxorubicin sent from oncology and hematology centers for cardiologic evaluation before and during treatment with anthracyclines.
The patients were cardiologically monitored for the occurrence of early onset AIC. The AIC diagnostic was established by echocardiography in accordance with the recommendations of the ACC/AHA/ASE 2003 guide when LVEF decreased below the value of 50% or by 10% under the initial value10,11 in the absence of another cause of cardiac dysfunction than treatment with anthracyclines.
We studied the correlation of hs-cTnT levels with the changes in exercise ECG testing in patients diagnosed with AIC versus those that have evolved without AIC during the 6 month follow-up period.
Eligible patients were aged over 18 years and had a left ventricular ejection fraction (LVEF) above 50%.
The study protocol was approved by the local Ethics Committee and each patient signed an informed consent on enrollment.
Patients were evaluated by clinical examination, transthoracic 2D echocardiography (2D-ETT), hs-cTnT plasma levels, resting ECG and exercise ECG test at the initial moment, 3 and 6 months after starting the treatment with anthracyclines. Resting ECG was performed on a NIHON KOHDEN Cardiofax GEM cardiograph, transthoracic 2D echocardiography on an ALOKA Prosound SSD-4000SV echograph. LVEF was calculated by the modified biplane Simpson method. The hs-TnT determinations were made on the Cobas e411 device by Roche electrochemiluminescence. Exercise ECG testing was done on the treadmill, Schiller device, using the Bruce protocol and was limited by symptoms. We noted the maximal exercise capacity (METs), heart rate at maximum tolerated stress and depression of ST segment ≥1 mm in two contingent derivatives defining for the positive exercise ECG test.
Study group
There were included in the study 68 patients with cancer and anthracycline treatment indication, 27 men (39.7%) and 41 women (60.3%) with mean age 56.6 years (range 23-73 years of age). 38 patients (55.9%) had breast cancer, 21 patients (30.9%) lung cancer and 9 patients (13.2%) malignant lymphomas. The patients received a cumulative doxorubicin dose ranging between 220-280 mg/m2 up to 3 months and 420-500 mg/m2 up to 6 months. Treatment also included the therapy of cardiovascular risk factors up to „target levels” recommended for each patient after cardiovascular risk assessment using the SCORE charts for countries with high cardiovascular risk12.
The study was conducted during 2012-2014 in the Department of Cardiology of the County Emergency Hospital in Brasov.
Statistical analysis
The database was prepared using the Microsoft Excel program. Statistical analysis was performed using the GraphPad InStat 3 and SPSS 20.0 programs. Data were summarized using the median value and percentiles 25 and 75 due to non-Gaussian distribution. Nominal variables were expressed as a percentage (%). In order to compare nominal variables we used Fisher’s exact test or X2. The nonparametric Mann-Whitney test was applied for quantitative variables. Dynamic assessment of changes derived from exercise testing parameters and hs-cTnT plasma levels at baseline, 3 and 6 months in patients with AIC was performed by using the nonparametric Friedman test. Spearman correlation test was used for quantitative variables and Chi-squared test for nominal variables. p<0.05 value was considered statistically significant.
Results
After 6 months of treatment with anthracyclines, 15 patients of the 68 patients included in the study were diagnosed with asymptomatic AIC (22.05%) (group 1), 53 patients (77.95%) evolving without decrease of LVEF diagnostic for AIC (group 2). There were no significant differences in the cumulative dose of anthracyclines between the two groups. Incidence of cardiovascular risk factors (hypertension, diabetes, smoking, dyslipidemia) and cardiac medication (inhibitors of angiotensin converting enzyme/ARBs, beta-blockers, statins, aspirin) showed no statistically significant differences between the two groups (Table 1).
Table 1. Demographic data, cardiovascular risk factors and cardiac treatment
| Parameter | Group 1 (n=15) | Group 2 (n=53) | p |
| Number of patients | 15 (22.05%) | 53 (77.95%) | |
| Men | 5 (33.3%) | 22 (42%) | 0.7762 |
| Women | 10 (66.7%) | 31 (58%) | |
| Age (years) (mean ± DS) | 62.5±7.2 | 54.9±10.4 | 0.0090 |
| Smokers | 5 (33.3%) | 21 (39.6%) | 0.7686 |
| Arterial hypertension under treatment | 12 (80%) | 30 (56.6%) | 0.1362 |
| Diabetes | 6 (40%) | 12 (22.6%) | 0.1983 |
| Atherogenic dyslipidemia | 9 (60%) | 33 (62.3%) | 1.0000 |
| Obesity | 6 (40%) | 14 (26.4%) | 0.2388 |
| Cardiac treatment | |||
| ACE inhibitors/Satrani | 12 (80%) | 34 (64.2%) | 0.3528 |
| Beta-blockers | 9 (60%) | 21 (39.6%) | 0.5544 |
| Statins | 9 (60%) | 33 (62.3%) | 1.0000 |
| Aspirin | 12 (80%) | 39 (73.6%) | 0.7754 |
| ACE inhibitors = angiotensin converting enzyme inhibitors | |||
Patients in group 1 were older than those in group 2.
There were no significant changes in the ST segment on the resting electrocardiogram at baseline and under evolution, arrhythmias or impaired driving.
At the moment of enrollment in the trial, plasma hs-cTnT levels were not significantly different between the two groups (Table 2).
Table 2. Parameters obtained from exercise testing and the hs-cTnT value at the time of enrollment
| Parameter | Group 1 (n=15) | Group 2 (n=53) | p |
| Heart rate at rest (beats/min) | 74 (70;75) | 77 (65;92) | 0.2027 |
| Heart rate at maximum stress tolerated (beats/min) | 135 (125;143) | 150 (123,5;158) | 0.1016 |
| Maximal exercise capacity (METs) | 7.8 (7;9.2) | 9.6 (6.9;12.9) | 0.2421 |
| Positive exercise ECG test (no. of patients) | 6 (40%) | 6 (11%) | 0.0186 |
| Hs-cTnT (ng/ml) | 0.00713 (0.00430;0.00787) | 0.00435 (0.00381;0.00748) | 0.0848 |
| Data are expressed as median and percentiles 25 and 75 | |||
Assessment on enrollment by exercise ECG test showed a significantly higher incidence of positive exercise ECG tests in patients in group 1 versus those in group 2 (p=0.0186), with no differences in the maximal exercise capacity and heart rate obtained at maximum stress tolerated (Table 2). Patients with positive exercise ECG test on enrollment were considered with exercise-induced myocardial ischemia.
After 3 months of treatment with anthracyclines, nonspecific ST-T changes occurred in the resting electrocardiogram in all patients. In the exercise ECG testing, the patients in group 1 versus group 2 had a significantly higher incidence of depression of ST segment ≥1mm [11 pts (73.3%) vs. 7 pts (13.2%), p=0.0001], associating a decreased maximal exercise capacity [7(6.4;8) METs vs. 9.2(6.7;12.4) METs, p=0.0489] and heart rate at maximum stress tolerated [120(110;131) beats/minute vs. 145(124;150.5) beats/minute (p=0.0051)] (Table 3). Compared to the initial assessment, at the exercise test after 3 months they showed ECG criteria for positive test in another 6 patients, 5 (33.3%) of group 1 and 1 patient (1.9%) of group 2, with significant difference between the two groups (p=0.0014). Patients with depression of ST segment during exercise occurred after 3 months of treatment with anthracyclines were considered with cytotoxic myocardial injury caused by the treatment with anthracyclines.
After 3 months of treatment, in patients in group 1 versus group 2 there was a significant increase of the median value of hs-cTnT (0.00998 ng/ml vs. 0.00498 ng/ml, p=0.0003). The percentage of increase compared to the initial value of hs-cTnT was 38.5% in group 1 versus 0.6% in group 2 (p=0.0001) (Table 3).
Table 3. Parameters obtained from exercise testing and hs-cTnT value at 3 months
| Parameter | Group 1 (n=15) | Group 2 (n=53) | p |
| Heart rate at rest (beats/min) | 73 (68;78) | 77 (68;80.5) | 0.4130 |
| Heart rate at maximum stress tolerated (beats/min) | 120 (110;131) | 145 (124;150.5) | 0.0051 |
| Maximal exercise capacity (METs) | 7 (6.4;8) | 9.2 (6.7;12.4) | 0.0489 |
| Positive exercise ECG test (no. of patients) | 11 (73.3%) | 7 (13.2%) | ?0.0001 |
| Exercise ECG test turned to positive at 3 months compared with the initial assessment (no. of patients) | 5 (33.3%) | 1 (1.9%) | 0.0014 |
| Hs-cTnT (ng/ml) | 0.00998 (0.00737;0.01354) | 0.00498 (0.00387;0.00830) | 0.0003 |
| Plasma levels of hs-cTnT?0.009 ng/ml (no. of patients) | 10 (66.7%) | 9 (17%) | 0.0005 |
| Percentage of increase of hs-cTnT value at 3 months compared to the value at the time of enrollment | 38.5% (27.9%;80.8%) | 0.6% (-8.1%;14.5%) | ?0.0001 |
| Percentage increase ?30% of hs-cTnT at 3 months compared to the initial value (no. of patients) | 11 (73.3%) | 5 (9.4%) | ?0.0001 |
| Data are expressed as median and percentiles 25 and 75 | |||
After 6 months of initiation of treatment with anthracyclines, the resting electrocardiogram showed no notable changes in the ST segment and T wave compared with the changes at 3 months. In the exercise test, depression of ST segment ≥1mm was significantly more frequent in patients in group 1 versus those in group 2 [12 pts (80%) vs. 7 pts (13.2%), p=0.0001)] and associated with an important and statistically significant decrease in maximum exercise capacity [6(4.6;6.8) METs vs. 8.8(6.2;12.1) METs, (p=0.0009)] and the heart rate at maximum stress tolerated [110(90;123) beats/minute vs. 130(126;148.5) beats/minute, p=0.0001]. Compared with the assessment at 3 months, the exercise test at 6 months had ECG criteria for positive test in 1 more patient (8.5%) in group 1 and no patient in group 2.
There was a significant decrease of the median values of hs-cTnT found in the patients of group 1 versus group 2 (0.01006 ng/ml vs. 0.00643 ng/ml, p=0.0001) (Table 4).
Table 4. Parameters obtained from exercise testing and the hs-cTnT value at 6 months
| Parameter | Group 1 (n=15) | Group 2 (n=53) | p |
| Heart rate at rest (beats/min) | 82 (74;84) | 71 (69;86) | 0.0670 |
| Heart rate at maximum stress tolerated (beats/min) | 110 (90;123) | 130 (126;148.5) | 0.0001 |
| Maximal exercise capacity (METs) | 6 (4.6;6.8) | 8.8 (6.2;12.1) | 0.0009 |
| Positive exercise ECG test (no. of patients) | 12 (80%) | 7 (13.2%) | 0.0001 |
| Hs-cTnT (ng/ml) | 0.01006 (0.01006;0.01620) | 0.00643 (0.00442;0.00920) | 0.0001 |
| Data are expressed as median and percentiles 25 and 75 | |||
In patients diagnosed with AIC at 6 months, the dynamic assessment of changes in exercise testing and plasma hs-cTnT levels during the three evaluations is shown in Table 5. The data revealed that patients who developed AIC at 6 months showed a significant and progressive decrease in maximal exercise capacity from the initial moment to 3 months (p=0.0001) and from 3 to 6 months (p=0.0001) associated with progressive decrease in heart rate at maximum stress tolerated from the initial moment to 3 months (p=0.0001) and from 3 to 6 months (p=0.0001). In these patients the incidence of depression of ST segment ≥ 1mm on exercise ECG increased progressively during the follow-up period from 40% at the initial moment to 73.3% at 3 months (p=0.1394) and to 80% at 6 months with statistical significance between the initial moment and assessment at 6 months (p=0.0495). The plasma hs-cTnT levels increased progressively under treatment with anthracyclines, significantly from the initial moment at 3 months (p=0.0001) and from 3 to 6 months (p=0.0041).
Table 5. Evolution of parameters obtained from exercise testing and Hs-cTnT value during the 6 follow-up months in patient who evolved with AIC
| Parameter | Initial assessment (n=15) | Assessment at 3 months (n=15) | p | Sperman (rho) | Assessment at 6 months (n=15) | p | Sperman (rho) |
| HR* rest | 74 (70;75) | 73 (68;78) | 0.0001 | 0.8521 | 82 (74;84) | 0.0001 | 0.7964 |
| HR effort | 135 (125;143) | 120 (110;131) | 0.0001 | 0.7887 | 110 (90;123) | 0.0001 | 0.7776 |
| METs | 7.8 (7;9.2) | 7 (6.4;8) | 0.0001 | 0.8518 | 6 (4.6;6.8) | 0.0001 | 0.7402 |
| Positive exercise ECG test | 6 (40%) | 11 (73.3%) | 0.1394 | 12 (80%) | 1.000 | 0.049 | |
| Hs-cTnT (ng/ml) | 0.00713 (0.00430;0.00787) | 0.00998 (0.00737;0.01354) | 0.0001 | 0.7093 | 0.01006 (0.01006;0.01620) | 0.0041 | 0.5088 |
| Data are expressed as median and percentiles 25 and 75; HR* = heart rate | |||||||
Analysis of ROC curves in monitored patients of hs-cTnT values at baseline and at 3 months, and the percentage increase of hs-cTnT in the first 3 months shows that the hs-cTnT level at 3 months (AUC=0.806, 95% CI 0.665-0.946, p=0.0001) and the percentage increase of hs-cTnT in the first 3 months (AUC=0.849, 95% CI 0.714-0.984, p=0.0001) are predictors for the development of AIC at 6 months (Figure 1, Table 6).
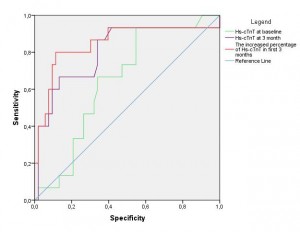
Figure 1. ROC curves: the hs-cTnT value on enrollment, at 3 months, and the percentage increase of hs-cTnT in the first 3 months to predict the evolution of AIC.
Table 6. Area under the curve
| Variables tested | Area | Standard error | Significance level (p) | 95% Confidence Interval | |
| Minimum limit | Maximum limit | ||||
| Hs-cTnT on enrollment | 0.647 | 0.072 | 0.084 | 0.506 | 0.788 |
| Hs-cTnT at 3 months | 0.806 | 0.072 | <0.0001 | 0.665 | 0.946 |
| Percentage increase of hs-cTnT in the first 3 months | 0.849 | 0.069 | <0.0001 | 0.714 | 0.975 |
For a cut-off value of hs-cTnT at 3 months above 0.009 ng/ml associated with a percentage increase of over 30% in the first 3 months, we obtained a sensitivity of 53.3% and a specificity of 100% for the evolution to AIC at 6 months, with a positive predictive value of 100% and a negative predictive value of 88.3%.
Using the binomial logistic regression model we analyzed the positivation of exercise test and hs-cTnT level >0.009 ng/ml associated with a percentage increase >30% at 3 months compared to baseline. The final regression model showed that the positivation of exercise stress test at 3 months did not correlate with the occurrence of AIC at 6 months (p=0.086), whereas the hs-cTnT value >0.009 ng/ml at 3 months associated with an increase of >30% from baseline predicts the development of AIC at 6 months (p=0.001).
The binomial logistic regression analysis of the occurrence of ST segment depression ST ≥1mm on exercise testing at 3 months compared to baseline and exercise test at 6 months compared to baseline showed that ST segment changes during exercise correlate with the diagnosis of AIC (p=0.05).
Discussions
Studies conducted in animal models of disease showed that in anthracycline-induced cardiotoxicity the histopathological changes were correlated with plasma levels of cTnT13. Clinical studies that determined cTnT in assessing anthracycline cardiotoxicity revealed that elevated plasma cTnT levels were predictive of left ventricular dilatation detected by echocardiography14 and decrease of LVEF15, which made the determination of cTnT to be used for assessing the effectiveness of cardioprotection with dexrazoxane in patients at high risk for the occurrence of AIC16.
Using new ultrasensitive techniques for measuring extremely small quantities of cTnI (cTnI-u) brought substantial information on the biomarker value of cTnI-u in assessing anthracycline cardiotoxicity6. The negative predictive value of cTnI low levels makes cTnI-u levels important in identifying patients with low risk of AIC occurrence9. Assessment of cTnI levels by highly sensitive techniques (hs-TnI), simultaneously with the imaging study of cardiac dysfunction by modern echocardiography techniques such as „spackle tracking” and tissue Doppler showed that the increase of the hs-cTnI level and decrease of longitudinal „foreign” ≤19% at 3 months are predictors of LVEF reduction at 3 months17.
In our study, dynamic assessment of plasma hs-cTnT levels, tolerance and ST segment depression ≥1mm during exercise combines two accessible and cost-effective methods for the diagnosis of cardiotoxicity. The reduction at 3 months of the exercise tolerance and heart rate at maximum stress tolerated appear to be predictive for the decrease of LVEF diagnosed for AIC at 6 months. Increase of hs-cTnT levels at 3 months was predictive for AIC at 6 months, the cut-off value above 0.009 ng/ml associated with a percentage increase of over 30% in the first 3 months had a sensitivity of 53.3%, a specificity of 100%, a positive predictive value of 100% and a negative predictive value of 88.3% for the evolution of AIC at 6 months. In this context the occurrence at 3 months of the ST segment depression ≥1mm at maximum stress tolerated associate with a significant and progressive increase at 3 months of the hs-cTnT level was interpreted as secondary for the myocyte cytotoxic lesions induced by treatment with anthracyclines.
The importance of measuring biomarkers in the diagnosis of cardiomyopathy caused by chemotherapy is emphasized in „The position statement from the Heart Failure Association of ESC” published in 2011, where the use of biomarkers is strongly recommended by highlighting the fact that biomarkers cannot substitute the information provided by echocardiography or other imaging techniques in the diagnosis of cardiomyopathy18. The importance of determining cTn in assessing cardiotoxicity is revealed by the follow-up algorithm of patients receiving anthracyclines, proposed by Giulia Bacchiani and Daniela Cardinale in 2012 by recommending concomitant assessment of TnI levels and 2D-ETT changes19. Recommendation for using 2D-ETT in clinical practice is due to the fact that echocardiographic techniques such as „spackle tracking” and tissue Doppler are still not readily available, which proved to be more refined and sensitive in assessment of geometry and abnormalities of contraction/relaxation of the LF (left ventricle), including diagnosis of AIC.
Conclusions
In cancer patients treated with doxorubicin, hs-TnT levels at 3 months and the percentage increase in the first 3 months are predictive for the occurrence at 6 months of anthracycline-induced asymptomatic cardiomyopathy.
In patients diagnosed at 6 months with anthracycline-induced asymptomatic cardiomyopathy, the increase at 3 months of the hs-TnT levels was associate with reduction of tolerance and occurrence of ST segment depression during exercise, as a possible expression of doxorubicin cardiotoxicity.
Conflict of interest: none declared.
References
1. Herman EH, Zhang J, Lipshultz SE, et al. Correlation between serum levels of cardiac troponin-T and the severity of the chronic cardiomyopathy induced by doxorubicin. J Clin Oncol 1999;17:2237.
2. Kilickap S, Barista I, Akgul E, et al. cTnT can be a useful marker for early detection of anthracycline cardiotoxicity. Ann Oncol. 2005; 16:798-804.
3. Auner HW, Tinchon C, Linkesch W, et al. Prolonged follow-up of troponin T for the detection of anthracycline cardiotoxicity in adults with hematological malignancies. Ann Hematol 2003; 82:218.
4. Germanakis I, Anagnostatou N, Kalmanti M. Troponins and natriuretic peptides in the follow-up of anthracycline cardiotoxicity. Pediatr Blood Cancer 2008; 51:327
5. Morris PG, Chen C, Steingart R, et al. Troponin I and C-reactive protein are commonly detected in patients with breast cancer treated with dose-dense chemotherapy incorporating trastuzumab and lapatinib. Clin Cancer Res. 2011;17:3490–3499.
6. Cardinale D, Sandri MT, Martinoni A, et al. Left ventricular dysfunction predicted by early troponin I release after high-dose chemotherapy. J Am Coll Cardiol 2000;36:517.
7. Ky B, Putt M, Sawaya H, et al. Early increases in multiple biomarkers predict subsequent cardiotoxicity in patients with breast cancer treated with doxorubicin, taxanes, and trastuzumab. J Am Coll Cardiol 2014;63:809.
8. Cardinale D, Sandri MT, Colombo A, et al. Prognostic value of troponin I in cardiac risk stratification of cancer patients undergoing high-dose chemotherapy. Circulation 2004;109:2749.
9. Cardinale D, Colombo A, Torrisi R, et al. Trastuzumab-induced cardiotoxicity: clinical and prognostic implications of troponin I evaluation. J Clin Oncol 2010;28:3910-6.
10. Cheitlin MD, Armstrong WF, Aurigemma GP, et al. ACC/AHA/ASE 2003 guideline update for the clinical application of echocardiography: summary article: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (ACC/AHA/ASE Committee to Update the 1997 Guidelines for the Clinical Application of Echocardiography). Circulation. 2003;108(9):1146-1162.
11. Pai VB, Nahata MC. Cardiotoxicity of chemotherapeutic agents: incidence, treatment and prevention. Drug Saf. 2000;22(4):263-302.
12. Conroy R, Pyorala K, Fitzgerald AP et al. Estimation of ten-year risk of fatal cardiovascular disease in Europe: the SCORE project. Eur Heart J 2003;24:987-1003.
13. Herman E, Lipshultz S, Rifai N, et al. Use of cardiac troponin T levels as an indicator of doxorubicin-induced cardiotoxicity. Cancer Res. 1998;58:195-197.
14. Lipshultz SE, Lipsitz SR, Sallan SE, et al. Chronic progressive cardiac dysfunction years after doxorubicin therapy for childhood acute lymphoblastic leukemia. J Clin Oncol 2005;23:2629.
15. Auner HW, Tinchon C, Linkesch W, et al. Prolonged follow-up of troponin T for the detection of anthracycline cardiotoxicity in adults with hematological malignancies. Ann Hematol 2003; 82:218.
16. Lipshultz SE, Scully RE, Lipsitz SR, et al. Assessment of dexrazoxane as a cardioprotectant in doxorubicin-treated children with high-risk acute lymphoblastic leukaemia: long-term follow-up of a prospective, randomised, multicentre trial. Lancet Oncol 2010;11:950-61.
17. Sawaya H, Sebag IA, Plana JC, et al., Early detection and prediction of cardiotoxicity in chemotherapy-treated patients. Am J Cardiol, 2011; 107(9):1375–80.
18. Eschenhagen T, Force T, Ewer MS, et al. Cardiovascular side effects of cancer therapies: a position statement from the Heart Failure Association of the European Society of Cardiology. Eur J Heart Fail. 2011; 13(1):1-10.
19. Giulia Bacchiani, Daniela Cardinale. Using biomarkers and early prophylactic treatment to prevent cardiotoxicity in cancer patients on chemotherapy. Biomarkers:detection and treatment of cardiotoxicity. Spring 2012;112(4):250-260.
 This work is licensed under a
This work is licensed under a