C. Matei1,2, I. M. Coman1,2, E. Apetrei1,2
Article received on the 5th of August 2012. Article accepted on the 17th of August 2012.
1 ”Prof. Dr. C.C. Iliescu” Emergency Institute for Cardiovascular Diseases, Bucharest
2 “Carol Davila” University of Medicine and Pharmacy, Bucharest
Dr. Costel Matei, ”Prof. Dr. C.C. Iliescu” Emergency Institute for Cardiovascular Diseases, Cardiology Clinic, IInd Unit, 258, Fundeni Road, 022328 Bucharest, Romania. E-mail: cmatei2002@yahoo.co.uk
Abstract: Heart rate variability, expression of balance between sympathetic and parasympathetic tonus, is frequently reduced in patients with heart failure due to sympathetic hyperactivity. Its use in predicting mortality risk in patients with heart failure has been studied previously, proved to be a useful non/invasive method for risk stratification. Aim – To evaluate the usefulness and prognostic value of heart rate variability (HRV) parameters in patients with dilated cardiomyopathy (DCM). Methods: Fifty-one patients (76.5% men) with dilated cardiomyopathy of various etiologies were included in the study. Time-domain heart rate variability parameters from the 24h ECG recordings were analyzed. Patients were followed clinically, ECG, echocardiography for a mean of 47.4 months (range 6-90 months); 22 deaths were observed during study period. Statistical analysis was performed with MedCalc 12.3.0.0 (Medcalc Software BVBA, Belgium). Results – 24h ECG recordings was indicated in 71% of patients as a class I indication and in 29% of patients and class IIb indication. There were no significant differences between HRV parameters between different DCM etiologies. Patients were divided in tertiles according to the HRV parameters. Death risk shows, without reaching statistical significance, a progressive decrease with rMSSD increase, for all other parameters U-shape curves were observed in the study group tertiles. Kaplan-Meier survival curves showed no survival difference between tertiles or between low or normal HRV, except rMSSD where survival was better in patients with reduced HRV. Conclusions – Our data are useful primarily to a better parameters definition of values considered to be discriminatory for patients with “low variability”. There were no statistically significant differences in HRV parameters between different causes of DCM. Statistical analysis failed to show a significant survival difference according to HRV parameters. Probably it is necessary to consider these parameters together with other factors that influence the evolution of patients with DCM and heart failure.
Keywords: heart rate variability, dilated cardiomyopathy, heart failure
Rezumat: Variabilitatea ritmului cardiac, expresie a balanţei între sistemul autonom simpatic şi parasimpatic, este frecvent redusă în cazul pacienţilor cu insuficienţă cardiacă datorită hiperactivităţii simpatice. Utilizarea acesteia în predicţia riscului de mortalitate la pacienţii cu insuficienţă cardiacă a fost studiată anterior, dovedindu-se a fi o metodă neinvazivă utilă în stratificarea riscului de moarte subită. Scopul lucrării a fost evaluarea utilităţii metodei şi a valorii prognostice a parametrilor variabilităţii ritmului cardiac rezultaţi din înregistrarea ambulatorie a electocardiogramei la pacienţii cu cardiomiopatie dilatativă. Material şi metodă – Studiul a inclus 51 de pacienţi (76,5% bărbaţi) cu cardiomiopatie dilatativă de diferite etiologii, în ritm sinusal, la care au fost analizaţi parametrii de variabilitate ritmului cardiac în domeniul timp rezultaţi din înregistrarea ECG pe 24 ore. Pacienţii au fost urmăriţi clinic, ECG, ecocardiografic pe o perioadă medie de 47,4 luni (între 6–90 luni), fiind observate 22 decese. Analiza statistică s-a făcut cu programul MedCalc 12.3.0.0 (Medcalc Software BVBA, Belgia). Rezultate – Examenul Holter ECG a fost indicat la 71% din pacienţi ca indicaţie de clasă I şi la 29% ca indicaţie de clasă IIb. Nu am înregistrat diferenţe semnificative statistic între valorile parametrilor de variabilitate între diferitele etiologii ale CMD. Pacienţii au fost împărţiţi în funcţie de valorile parametrilor de variabilitate în terţile, riscul de deces la pacienţii studiaţi arătând, fără a atinge semnificaţia statistică, o scădere progresivă a acestui risc cu creşterea rMSSD, pentru toţi ceilalţi parametri observându-se curbe de tip „U” între cele 3 terţile obţinute din lotul studiat. Curbele Kaplan-Meier nu au arătat diferenţe de supravieţuire între pacienţii din cele 3 terţile şi nici între categoriile de variabilitate redusă sau normală, cu excepţia rMSSD la care supravieţuirea a fost mai bună la pacienţii cu variabilitate redusă. Concluzii – Datele obţinute sunt utile în primul rând pentru o definire mai bună a acestor parametri şi a valorilor considerate a fi discriminatorii pentru încadrarea pacienţilor în categoria „variabilitate scăzută”. Nu am constatat diferenţe ale parametrilor HRV semnificative statistic între diferitele etiologii ale CMD. Analiza statistică nu a reuşit să evidenţieze o diferenţă semnificativă în privinţa supravieţuirii în funcţie de valoarea parametrilor de variabilitate, probabil fiind necesară considerarea acestor parametri împreună cu alţi factori care influenţează evoluţia pacienţilor cu CMD şi insuficienţă cardiacă.
Cuvinte-cheie: variabilitatea ritmului cardiac, cardiomiopatie dilatativă, insuficienţă cardiacă
Introduction
Dilated cardiomyopathy (DCM), a myocardial tissue disease clinically manifested by signs and symptoms of heart failure (HF), is burdened by an increased risk of morbidity and mortality, especially due to ventricular arrhythmias, a third to half of patients presenting sudden death during disease progression or resuscitation after cardiac arrest or sustained ventricular tachycardia (VT)1,2. In the era of the implantable cardiac defibrillator, selection of patients with maximum of benefit from this type of treatment is a challenge in terms of cost-effectiveness, related to the still increased costs of cardiac devices.
Heart rate variability (HRV), an expression of balance between sympathetic and parasympathetic autonomic system, is frequently affected in patients with heart failure due to sympathetic hyperactivity often found in this category of patients3. Using heart rate variability in prediction of mortality risk in patients with heart failure has been studied previously, proved to be a useful noninvasive method for sudden death risk stratification2,4. Compared with electrophysiological studies (EPS), an invasive method with high cost as the main disadvantage, which proved useful for risk stratification in patients with ischemic cardiomyopathy, but not in those with idiopathic DCM (less than 5% of patients with inducible VT during EPS1), HRV has the advantage of being relatively easy to measure through a long-term ECG recording and its analysis by a dedicated software. Methods, recording techniques and analysis, data interpretation, as well as their interpretation have been extensively described in the European Society of Cardiology guidelines3.
Arrived into common use in recent years, implantable devices used in heart failure patients treatment (implantable cardiac defibrillator or cardiac resynchronization devices) incorporated technology for acquisition, analysis and transmission (via telemetry or query in the specialized departments) of data related to heart rate variability, data which can be used in context to early detect the need for hospital care5.
Actual data needs to be studied, especially in connection with HRV analysis in a particular subset of patients with heart failure, those with dilated cardiomyopathy.
Objectives
The study objective was to evaluate the usefulness of the method and the prognostic value of parameters derived from heart rate variability analysis from 24 hours ambulatory ECG recordings (Holter ECG) in patients with dilated cardiomyopathy of various etiologies.
Material and methods
Patients in our study were selected from the database of patients with dilated cardiomyopathy which were hospitalized in the Emergency Institute for Cardiovascular Disease “Prof. Dr. C.C. Iliescu” in Bucharest between Jan 2003 and Dec 2007. Of the 562 patients in the database, 118 patients (21% of total) were referred for Holter ECG examination at the attending physician indication. We select only those in sinus rhythm (73 patients) and HRV was interpreted only in 51 patients. Patients with poor technical quality recordings and those where the presence of a large number of supraventricular or ventricular arrhythmias did not permit HRV analysis were excluded from the analysis. The study group consisted of 51 patients, 76.5% men, with mean age of 55.4 ± 14.1 years. Considering the etiology of DCM, patients in the study group were diagnosed as having idiopathic DCM (49.0%), ischemic DCM (33.3%), alcoholic DCM (9.8%), and other etiologies (7.9%).
Clinical parameters (NYHA class, 6 minutes walk test), traditional cardiovascular risk factors (hypertension, diabetes mellitus, smoking, and dyslipidemia), electrocardiographic parameters (QRS duration, presence of conduction disturbances), echocardiographic parameters (LV ejection fraction, LV and LA dimensions, presence and degree of diastolic dysfunction) and treatment (drug classes) were noted at baseline.
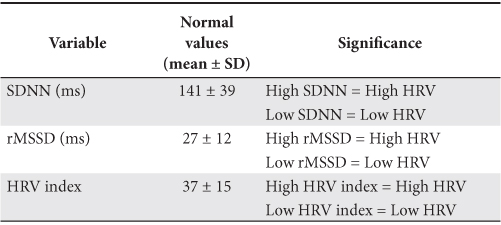
Twenty-four hours Holter ECG was recorded with a digital portable MT-100 device and recorded ECG signal analysis was performed with MT-200 software (Schiller AG, Switzerland) with a sampling rate of 128 ms (RR intervals were measured with incremental range of 8 ms). Heart rate variability was assessed only in time domain with the dedicated module of the above mentioned software, using parameters determined by statistical and geometric methods as were recommended by the European Society of Cardiology Guidelines3. Normal values for some of these parameters are presented in Table 1. Statistical parameters were calculated for the of day-time and night-time period as well as for whole duration of the recording.
Table 1. Normal values of HRV parameters3
Patients were followed for an average of 47.4 ± 20.7 months (range 6-90 months), their final status (death/survival) and follow-up duration being determined by the DCM patients follow-up algorithm described in detail previously6. When available, the same data recorded at baseline were tracked at the end of follow-up.
The database was statistically analyzed using MedCalc 12.3.0.0 (Medcalc Software BVBA, Belgium). Continuous variables were expressed as mean ± standard deviation. Comparison between groups was performed with Mann-Whitney test or Chi-square test. Univariate regression analysis was performed by Cox proportional-hazards regression. Cut-off values for HRV parameters were determined using Receiver Operator Characteristics (ROC) curves and sensitivity (St), specificity (Sp), positive predictive value (PPV) and negative predictive value (NPV) were calculated. Survival differences between selected categories were presented by Kaplan-Meier survival curves. A p value <0.05 was considered statistically significant.
Results
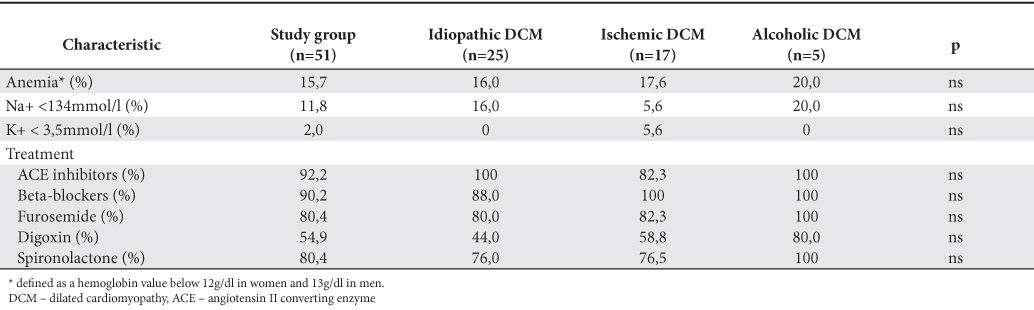
The main characteristics of the study group, global and by dominant etiologies above mentioned are shown in Table 2 (clinical data and echocardiography parameters) and Table 3 (blood samples and treatment). There were no significant differences of prevalence of coronary heart disease risk factors between different etiologies of DCM, even on therapeutic classes used in the treatment or changes of the factors associated with unfavorable outcome (anemia, chronic kidney disease, hyponatremia).
Table 2. The main clinical and echo characteristics of study group (at baseline)
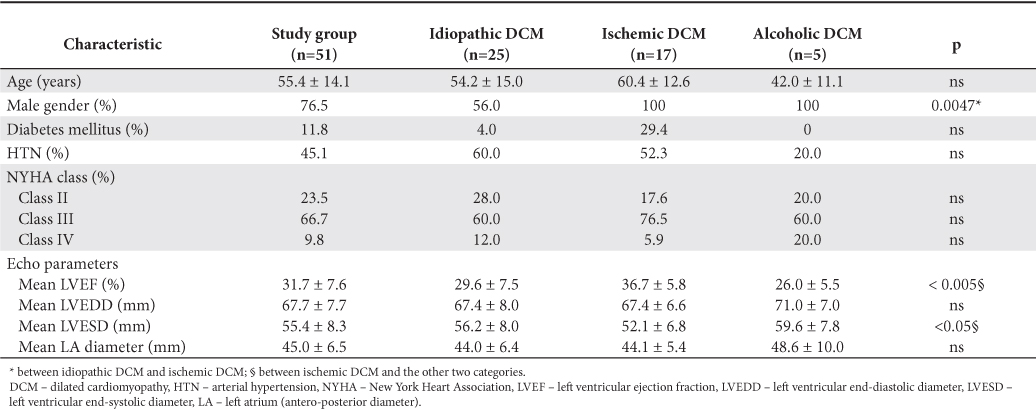
Table 3. Blood samples results and therapeutic classes used
Twenty-four ambulatory ECG recording was indicated in 71% of patients as a class I indication according to ACC/AHA guidelines7 (79% in idiopathic DCM, 72% in the ischemic DCM, 40% in the alcoholic DCM). The remaining recordings were a class IIb indication, falling within the category of arrhythmic risk evaluation in patients with heart failure without symptoms attributable to arrhythmias.
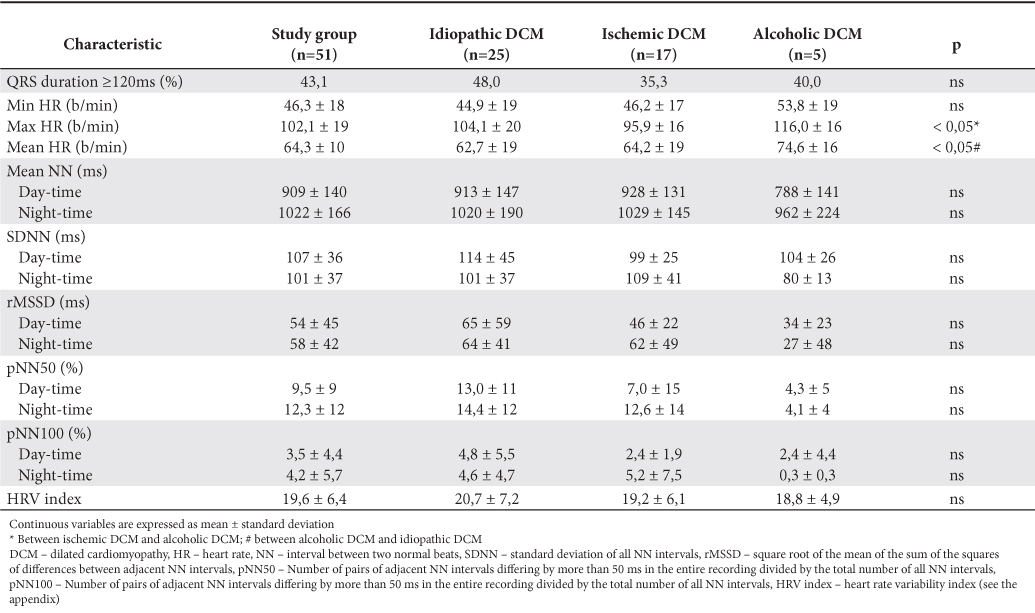
Data from Holter ECG recordings are summarized in Table 4. We observed an average of maximum heart rates during Holter recording higher in patients with alcoholic DCM (116.0±16 b/min) than in ischemic DCM (95.9±16 b/min) or idiopathic DCM (104.1±20 b/min) with statistically significant difference between the first two categories (p <0.05). There were no statistically significant differences in the percentage of patients with wide QRS complex (>120 ms) in each subgroup and between HRV parameter values in each subgroup. The values considered “normal” for HRV parameters established by ESC Guidelines3 are presented in Table 1.
Table 4. Parameters from Holter ECG recordings analysis
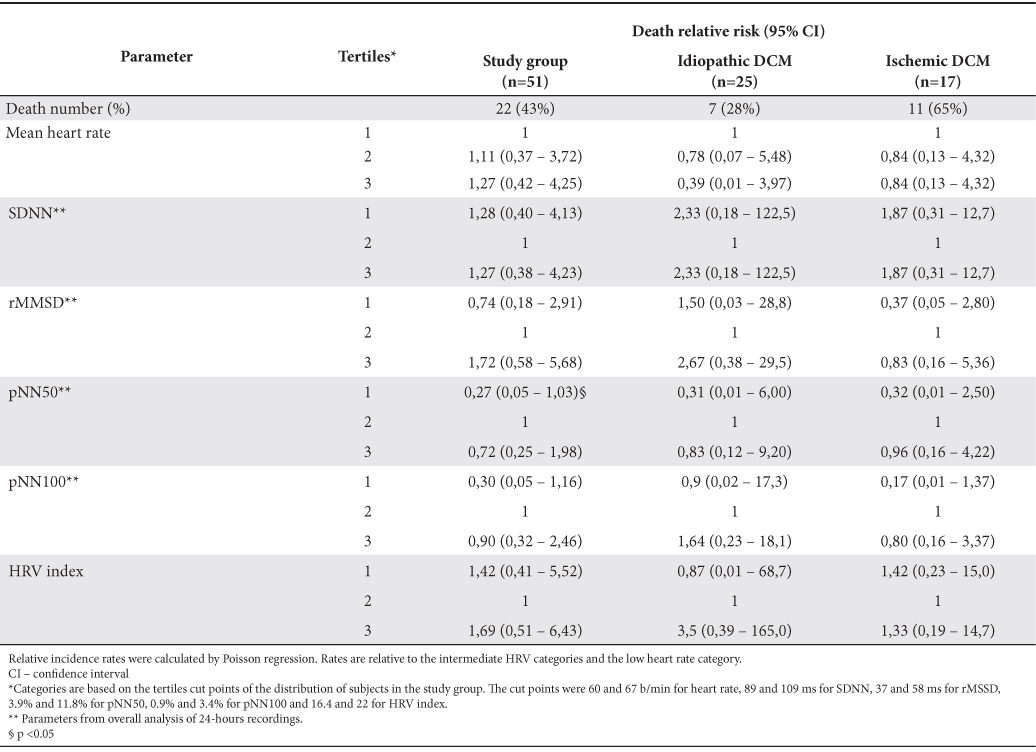
For each of the HRV parameters studied the patients in the study group were divided into tertiles and the prognostic value of these parameters for death risk was followed in every subgroup (Table 5).
Table 5. The relative risk for death associated with HRV parameters
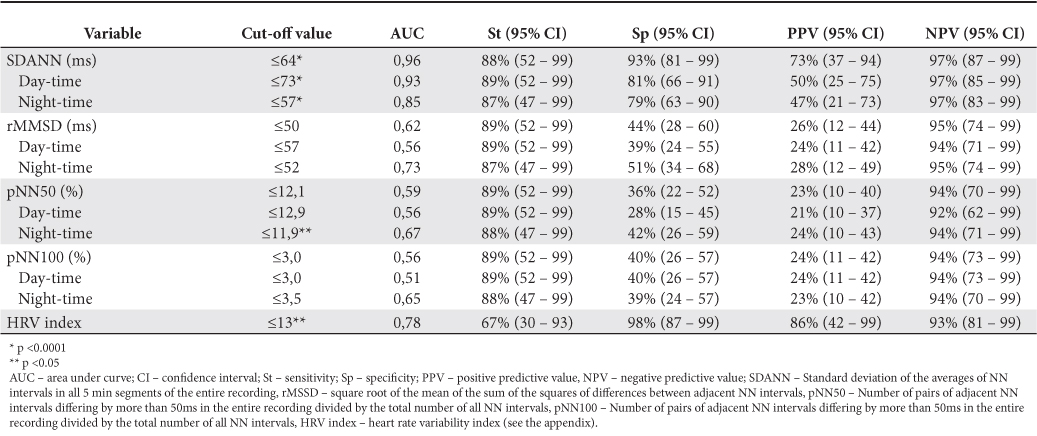
There are few data in literature about the values considered “normal” for heart rate variability parameters, and even fewer data about “cut-off” values of the various parameters that quantify variability to define “low HRV patients”. Therefore, based on consideration of a value of SDNN <80 ms as a unfavorable prognostic indicator in patients with DCM, as Karcz et al. shown in their article8, we defined patients with low HRV as those patients who meet this criterion. Based on this classification of patients we studied other parameters of variability in terms of “cut-off” values used to assert low HRV values. Sensitivity, specificity and predictive values for each of these “cut-off” values are shown in Table 6.
Table 6. Cut-off values for HRV parameters in DCM patients
Death prediction by HRV parameters was studied by Cox proportional-hazard regression. Only rMSSD (daytime rMSSD: HR = 0.37, 95% CI = 0.16 to 0.86, p <0.05; night-time rMSSD: HR = 0.35, 95% CI = 0.14 to 0.87, p <0.05; overall rMSSD: 0.31, 95% CI = 0.13 to 0.71, p <0.01) proved to be statistically significant. Kaplan-Meier survival curves are showed in Figure 1 (normal vs. low variability) and Figure 2 (1st tertile –low variability– vs. 2nd tertile –intermediate variability– vs. 3rd tertile – increased variability–).
From the data obtained we see that, except rMSSD, the studied parameters of HRV showed no significant value to differentiate DCM patients with increased mortality risk. rMSSD, the only parameter that reached statistical significance, showed a better outcome in patients with lower variability than those with normal or increased variability, data which is somehow inconsistent with data from existing literature2,9. We have to note that the relatively small number of patients did not allow statistical analysis within different etiological subgroups (idiopathic DCM vs. ischemic DCM vs. alcoholic DCM).
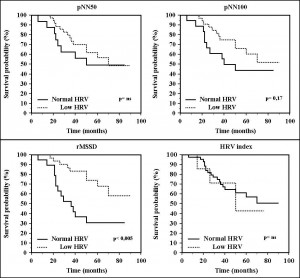
Figure 1. Kaplan Meier survival curves in patients with low compared to normal HRV (different parameters of HRV)
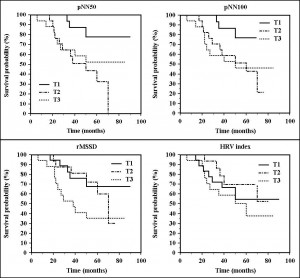
Figure 2. Kaplan Meier survival curves for the different HRV parameters (T1 – 1st tertile – low variability, T2 – 2nd tertile – intermediate variability, T3 – 3rd tertile – increased variability)
Assessment of risk of death in patients studied showed, but without reaching statistical significance, a progressive increase in risk with average heart rate during 24-hours Holter ECG recording and a progressive decrease in risk with increasing rMSSD. For all other parameters “U” shaped are observed between the three tertiles of the study group (Figure 3). Possible explanations of these results will be commented in detail below.
During follow-up, 5 patients (10% of total) received cardiac pacemaker as cardiac resynchronization therapy indication and permanent atrial fibrillation was found in 4 patients (9% of patients), all fits in 2nd and 3rd tertiles of HRV parameters).
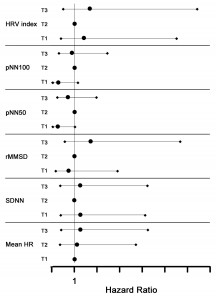
Figure 3. Relative risk for death according to HRV parameters
Discussion
Mortality in dilated cardiomyopathy, as well as in heart failure, remains high despite advances in pharmacological therapy. Device therapy in heart failure (cardiac resynchronization therapy and implantable cardiac defibrillator) brings a new hope for this category of patients. For DCM patients risk stratification, more objective methods, invasive or noninvasive, for prognostic determination of individual mortality risk is one of the major concerns in this area. Despite previous attempts, prediction of the risk of overall mortality and sudden death remains a challenge, especially due to complex interactions between different factors.
The clinical importance of HRV, expression of sympathetic and parasympathetic balance, was studied since the late 1980s, when it was demonstrated that HRV is a strong and independent predictor of mortality after acute myocardial infarction10,11. Later studies have shown potential usefulness of HRV for risk stratification in various other physiological or pathological conditions, heart failure being one of these entities.
Should also be noted that, although the existing data in literature shows that low HRV represents a high risk of mortality associated to increased sympathetic tone, there is no data to support that high HRV, given by a parasympathetic dominance, is a good prognostic marker. For example, Bettoni and Zimmermann12 showed significant changes in HRV on Holter ECG recordings before the onset of an episode of paroxistic atrial fibrillation (PAF) in terms of a primary increase adrenergic tone 20 minutes before PAF, followed by a rapid switch to vagal dominance immediately before the onset of PAF. It was also demonstrated that vagal stimulation shortens the atrial refractory period and facilitates atrial reentry, this effect being used to induce or maintain AF in experimental models.
On the other hand, in multivariate analysis, HRV did not prove to be a significant predictor of arrhythmic events as shown Iacoviello et al. in a study of idiopathic DCM patients13. In their study, QRS duration, QTc interval duration and heart rate variability were not predictive of arrhythmic events. Therefore, a risk stratification algorithm based on a combination of three parameters – ejection fraction, presence of TV unsubstantiated slope QT / RR – each reflecting different mechanisms that could lead to arrhythmic events it is proposed14.
Our data, although apparently seem to be in contradiction with the existing literature about prognostic potential of HRV parameters, deserves some comments.
First, given the context of multifactorial determinants of mortality risk in DCM, it is difficult to believe that a single parameter that characterizes the “heart rate variability” can provide a clear dichotomy between the categories of high and low risk patient. The difficulty is even greater since, even for values defining “normal”, there is no generally accepted consensus. Moreover, the definition of reference values in certain situations (i.e. heart failure) can mean different values than those accepted in normal subjects. That is why our attempt to determine the cut-off values of the other HRV parameters starting from a previously determined value of SDNN may be questionable in terms of initially selected reference marker.
The second reason that could explain the data obtained in the present study is that study group was with patients with DCM only, with different degrees of heart failure. The presence of a comparison group with subjects without heart failure might have been useful in determining more accurate cut-off values for HRV parameters, something which we will follow in a future study.
The structure of study group could explain also the data obtained. The presence of high percentage of patients with beta-blocker medication (90%), class of drugs known to modulate sympathetic tone usually increased in patients with heart failure, and ACE inhibitors (92.2%), also demonstrated to increases heart rate variability15,16, can explain the results not similar with literature data (La Rovere et al. study had only 6% of patients in the determination group and 31% of patients in the validation group with beta-blockers therapy2). High number of deaths observed during follow-up (43% in the whole group, 28% in the patients with idiopathic DCM, 65% in patients with ischemic DCM) may have prevented accurate determination of “cut-off” value of the HRV parameters (in the aforementioned study, after three years of follow-up, overall mortality was 37% in the determination group and 22% in the validation group, of which sudden deaths were 9.4% and 8%, respectively2).
Finally, methodology of heart rate variability study (time domain analysis with parameters determined by statistical or geometrical methods vs. frequency domain analysis with parameters measured by parametric or non-parametric methods3) can explain the different results obtained in different studies. Most studies in the literature used frequency domain analysis parameters to classify HRV in the groups studied (low variability vs. normal). Therefore, comparing the data obtained by different methods can lead to such conflicting data.
Study limitations were related to number of patients in each etiology of DCM that did not allow statistical analysis between different categories of patients with DCM. Dominance of class I indication (symptomatic arrhythmia detection – 71%) over class IIb indication (arrhythmic risk assessment in patients with heart failure without signs and symptoms of arrhythmia – 29%) for Holter ECG monitoring in patients studied may contribute to conflicting results with data existing in literature. Also, because no information about patient’s death mechanism (arrhythmic vs. non-arrhythmic), most of the deaths occurring at home, we were unable to correlate HRV parameters with the arrhythmic death risk.
Conclusions
Data from the analysis of various parameters of heart rate variability are useful primarily for a better definition of these parameters and for establishing the value considered to be discriminatory for patient classification in the “low variability” category which it is considered, based on existing data literature, to have a worse prognosis. In the present research we did not find statistically significant differences in HRV parameters between different DCM etiologies. Statistical analysis failed to show a significant survival difference depending on heart rate variability parameters. Probably it is necessary to consider these parameters together with other factors that influence the clinical evolution course of patients with DCM and heart failure.
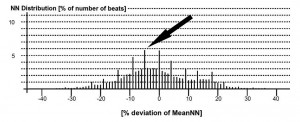
Figure 4. Gaussian distribution around the average of NN interval value on Holter ECG recording. The highest frequency observed (black arrow) is 5.8%.
Appendix
Example of HRV index calculation: the value is obtained by dividing the total number of recorded NN intervals to the number of the most frequently observed NN interval during recording3. The most frequent number of NN interval it is measured as it is showed in Figure 4, i.e. 5.8% of total NN intervals.
HRV index= 100%: 5.8%= 17.24
Abbreviations
ACEI angiotensin II converting enzyme inhibitors
AF atrial fibrillation
b/min beats per minute
CI confidence interval
DCM dilated cardiomyopathy
EPS electrophysiologic study
HF heart failure
HR heart rate
HRV heart rate variability
HTN arterial hypertension
LA left atrium
LV left ventricle
NN normal-to-normal beats interval
PAF paroxistic atrial fibrillation
pNN100 number of pairs of adjacent NN intervals
differing by more than 50ms in the entire
recording divided by the total number of all
NN intervals
pNN50 number of pairs of adjacent NN intervals
differing by more than 50ms in the entire
recording divided by the total number of all
NN intervals
rMSSD square root of the mean of the sum of the
squares of differences between adjacent NN
intervals
SDANN standard deviation of the averages of NN
intervals in all 5 min segments of the entire
recording
SDNN standard deviation of all NN intervals
VT ventricular tachycardia
Conflicts of interests: none.
Reference List
1. Stevenson WG, Epstein LM. Predicting Sudden Death Risk for Heart Failure Patients in the Implantable Cardioverter-Defibrillator Age. Circulation 2003;107:514-6.
2. La Rovere MT, Pinna GD, Maestri R et al. Short-Term Heart Rate Variability Strongly Predicts Sudden Cardiac Death in Chronic Heart Failure Patients. Circulation 2003;107:565-70.
3. Malik M, Bigger JT, Camm AJ et al. Heart rate variability. Standards of measurement, physiological interpretation, and clinical use. Eur Heart J 1996;17:354-81.
4. Adamson PB, Smith AL, Abraham WT et al. Continuous Autonomic Assessment in Patients with Symptomatic Heart Failure. Circulation 2004;110:2389-94.
5. Hasan A, Paul V. Telemonitoring in chronic heart failure. Eur Heart J 2011;32:1457-64.
6. Matei C, Pop I, Badea M, Saraolu A, Coman IM, Apetrei E. Predictive factors for atrial fibrillation appearance in dilated cardiomyopathy. Rev Rom Cardiol 2012;22:97-106.
7. Crawford MH, Steven JB, Deedwania PC et al. ACC/AHA Guidelines for Ambulatory Electrocardiography: Executive Summary and Recommendations: A Report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Committee to Revise the Guidelines for Ambulatory Electrocardiography) Developed in Collaboration With the North American Society for Pacing and Electrophysiology. Circulation 1999 August 24;100:886-93.
8. Karcz M, Chojnowska L, Zareba W, Ruzillo W. Prognostic significance of heart rate variability in dilated cardiomyopathy. Int J Cardiol 2003;
87:75-81.
9. Dekker JM, Crow RS, Folsom AR et al. Low Heart Rate Variability in a 2-Minute Rhythm Strip Predicts Risk of Coronary Heart Disease and Mortality From Several Causes. Circulation 2000;102:1239-44.
10. Kleiger RE, Miller JP, Bigger JT, Moss AJ, and the MulticenterPost-Infarction Research Group. Decreased heart rate variability and its association with increased mortality after acute myocardial infarction. Am J Cardiol 1987;59:256-62.
11. Malik M, Farrell T, Cripps T, Camm AJ. Heart rate variability in relation to prognosis after myocardial infarction: selection of optimal processing techniques. Eur Heart J 2012;10:1060-74.
12. Bettoni M, Zimmermann M. Autonomic Tone Variations Before the Onset of Paroxysmal Atrial Fibrillation. Circulation 2002;105:2753-9.
13. Iacoviello M, Forleo C, Guida P et al. Ventricular Repolarization Dynamicity Provides Independent Prognostic Information Toward Major Arrhythmic Events in Patients With Idiopathic Dilated Cardiomyopathy. J Am Coll Cardiol 2007;50:225-31.
14. Zareba W. Holter electrocardiogram parameters in risk stratification of arrhythmic events in idiopathic dilated cardiomyopathy. More studies are needed. J Am Coll Cardiol 2007;50:232-3.
15. Binkley PF, Haas GJ, Starling RC et al. Sustained augmentation of parasympathetic tone with angiotensin converting enzyme inhibitor in patients with congestive heart failure. J Am Coll Cardiol 1993;21:655-61.
16. Townend JN, West JN, Davies MK, Littles WA. Effect of quinapril on blood pressure and heart rate in congestive heart failure. Am J Cardiol 1992;69:1587-90.
 This work is licensed under a
This work is licensed under a