Elvis Gabriel Botu1, Horia Traila1, Catalin Usurelu1, Marin Postu2, Dirk Dreyer3
1 Department of Cardiology, Arges Emergency County Hospital, Romania
2 „Prof. Dr. C.C. Iliescu” Emergency Institute for Cardiovascular Diseases, Bucharest, Romania
3 Executive Board Straub Medical AG Switzerland
Abstract: Introduction – The abdominal aortic aneurysm (AAA) represents an irreversible dilation of the abdominal aorta. By comparison with classic surgery, abdominal aorta endovascular repair procedures (EVAR) have numerous advantages: reduced morbidity and mortality reduced procedural and hemorrhagic risk, rapid recovery. The partial or total endograft occlusion is one of the more common EVAR complications, with the occlusion being in most cases thrombotic, with the maximum incidence in the first three month after endograft insertion. Case presentation – We report the case of a72 year old male patient, with multiple cardiovascular risk factors (cigarette smoking history, hypertension, dyslipidaemia), coronary artery disease with angioplasty performed on the right coronary artery and diagnosed with partially thrombosed abdominal aortic aneurysm, extending to both common iliac arteries, for which an endovascular aneurysm repair was performed, with a bifurcated aorto-iliac supported endograft. Two month after the procedure, the patient reports to our center accusing intermittent buttock claudication on short walking distance and is diagnosed partial thrombosis of the endograft. An endovascular desobstruction approach of the endograft was decided, the repermeabilisation procedure being realized by a multidisciplinary team and consisted in rotational thrombectomy, followed by endograft remodeling with balloons and bare self-expanding and balloon expandable stents. Conclusion – the presented case highlights the importance of thrombotic load reduction prior to the conventional percutaneous angioplasty. The trombectomy with the Rotarex® device obtained the recanalization of the endograft through extraction of the thrombotic material, allowing the bare stent angioplasty with a minimum risk of acute ischemia secondary to a possible distal embolization.
Keywords: abdominal aortic aneurysm, thrombosis, rotational thrombectomy, percutaneous angioplasty, AAA, EVAR.
INTRODUCTION
The abdominal aortic aneurysm (AAA) represents an irreversible dilation of the abdominal aorta. There are a number of factors associated with the apparition and evolution of AAA – advanced age, male, smoking, hypertension and a history of atherosclerotic disease. The association between smoking and AAA deserves a special mention, as over 90% of the patients with
AAA are smokers or ex-smokers. AAA is, from an epidemiologic standpoint, only second after pulmonary cancer in terms of the correlation with smoking, having a stronger statistical association than with the cerebrovascular or coronaries disease.
By comparison with classic surgery, abdominal aorta endovascular repair procedures (EVAR) have numerous advantages: reduced morbidity and mortality reduced procedural and hemorrhagic risk, speedy recovery. EVAR have a mortality risk estimated at 1,4% compared to 4.2% for open surgery (OR 0.3; 95% CI 0.22-0.50; P < 0,0001). EVAR procedures are associated with up to a 3 fold reduction of per operatory mortality compared with patients with similar characteristics who have undergone an elective AAA surgical repair, which checks out even for younger patients with fewer comorbidities.
Still, EVAR is not a simple or risk free procedure; numerous periprocedural complications can occur – aortic rupture, endo-leaks, endo-graft migration, en-do-graft limb occlusion, high re-intervention rates. The occlusion of one of endo-grafts’ limbs or one of the extensions is one of the more frequent complications, being determined by either anatomical factors, or particularities of graft implantation (endoprosthesis size and type, implantation technique, etc.), or a combination of those. The incidence of endoprosthesis occlusion is estimated between 2 and 25%, and the nature of occlusion is usually thrombotic, with the maximum incidence for occurrence in the first two month after the implantation of the endograft.
The anatomical factors are more often implicated in graft occlusion. They are represented by: small caliber arteries (especially in women), excessive aneurysm neck angulation (over 60°), tortuous iliac arteries, iliac artery dissection, a small caliber and calcified distal aorta.
The treatment of endograft occlusion can be: endo-vascular, surgical (femoro-femoral, aorto-femoral by-pass), hybrid or conservatory. There is controversy regarding the optimal therapeutic attitude, which is why prevention of graft occlusion is of major importance – patient selection, based on a favorable anatomy, proper graft type and size selection and continuous platelet antiagregant therapy for 12 month are prophylactic recommended measures.
CASE PRESENTATION
We report the case of a 72 year old male patient, with multiple cardiovascular risk factors (cigarette smoking history, hypertension, dyslipidaemia), who reports to our center accusing intermittent buttock claudication on short walking distance. The patient was known with coronary artery disease for which angioplasty with DES was performed on the right coronary artery, one year prior to the current presentation. Later, the patient was diagnosed with partially thrombosed abdominal aortic aneurysm, extending to both common iliac arteries, asymptomatic, but considered to have indication for intervention (Figure 1).
Given the heighten surgical risk, but mainly the pa-tients’ preference, a decision was taken for endovascular repair with the mounting of an aorto-biiliac stent graft, procedure performed in another clinic. Pre-procedural, the difficulty posed by the anatomic particularities of the case was acknowledged (aneurysm neck angulation, kinking and bilateral calcifications of the iliac arteries, aortic proximal angle of 120°), with high risk de periprocedural complications and possibly graft limb occlusion. An E-Tegra® system (Jotec Gbmh) stent graft was implanted, with bilateral iliac extensions, of 19 mm proximal and 15 mm distal diameters. The main body prosthesis was deployed through right femoral aboard. The control injection after stent graft implantation showed a type I endoleak proximally, solved through balloon post dilatation.
Two month after the EVAR procedure, the patient becomes symptomatic with intermittent high claudication (pain in the right buttock) with low threshold/ on short distance (under 50 m) and lack of the pulse at the right femoral artery. With the history of recent aortic stent grafting (2 month prior the current presentation), the suspicion of EVAR complication was razed, with the location of the pain suggesting a distal critical lesion or occlusion of the vascular endoprosthesis.
An angio CT of the abdominal aorta and iliac ar-teries showed a thrombosed aneurismal sac, with no endoleak, the thrombosis of the prosthesis’ right limb and of the extension attached to it (Figure 2, 3 and 4). At the level of the right limb prosthesis, the axial image shows what appears to be the intraluminal fol-ding of the metal struts of the stent grafts’ limb inside the right common iliac artery (Figure 5). It also shows important under expanding and significant stenosis at the level of the left stent grafts’ limb extension. (Figure 3).
The diagnostic was completed through angiographic exploration of the abdominal aorta and iliac arteries, which confirmed the angio CT diagnostic, showing the occlusion of the right limb of the stent graft and the extension attached to it (Figure 6). Refill through collaterals from the left internal iliac artery, with slow flow and delayed filling of the arteries of the inferior right limb (Figure 6). The angiographic aspect is suggestive for intraprosthetic thrombosis, at the same time showing a tight stenosis of the left limb extension of the stent graft, secondary to a significant under-expanding of the endoprosthesis at this level, in a kink bend (Figure 6).
We considered as possible causes for endopros-thesis occlusion the presence of sever iliac arteries kinking, associated with a possible oversizing of the stent graft during the procedure and inadequate apposition of the prosthesis to the vessel walls, causing secondary intraluminal perimeter folding of the metal, which produced intraprosthetic obstacle and significant hemodynamic slowdown of the blood flow and rise the risk of thrombosis.
Given the highly symptomatic character of the lesions, we took into consideration several therapeutic options: surgical revascularization with creation of an extra-anatomic bypass, tempting an endovascular prosthesis recanalisation, or even the option of hybrid revascularization, with endovascular correction of the left limb extension stenosis and femoro-femoural extra-anatomic bypass. The presence of high intra-protetic thrombotic load was carrying a high risk of distal embolization in case of percutaneous revascularization.
With those considerations in mind, we decided to still approach the case percutaneously, but securing the procedure by the use of a device realizing mechanical thrombectomy with simultaneously thrombaspiration, followed by bare stents angioplasty of the remaining subjacent lesions. Taking into account the time elapsed from the first intervention till occurrence of present symptoms, we considered the thrombus being sufficiently organized so it wouldn’t be suitable for thrombaspiration with a Penumbra® (Penumbra Inc, USA) type device. On the other hand, a hybrid thrombectomy with Fogarty balloon would have carried a high risk of stentgraft dislodgement. All those considered, we opted for a strategy of initially using a rotational thrombectomy device from Rotarex® (Straubmedical Inc./CHE), considered the elective device for older thrombotic occlusions, completed with conventional angioplasty.
We performed a right brachial aboard with a 5F sheath, and bilateral femoral aboard: 6F sheath on the left and 8F on the right side, later changed to an 8F left, respectively 10F right, the later necessary for the introduction of the 10F Rotarex® thrombectomy catheter. The arterial punction of the right femoral artery was performed under anatomic and fluoroscopic guidance. We succeeded the crossing of the right stenosis in retrograde approach, with a hydrophilic stiff guide wire, using a 5F vertebral catheter for support. Later we exchanged the guide wire with the dedicated 0.025’’ guide wire of the Rotarex® thrombectomy system, over which we started to cross the intraprosthetic thrombus in the right extension with the 10F thrombectomy catheter. Upon penetration into the iliac segment/ right limb of the endoprosthesis the progression became strenuous, caused by the severe kinking and probably by the obstacle created by the perimeter folding at this level. Forcing the progression we were faced with the fracture of the 0.025’’ guide wire (Figure 7). Using a snare type recovery device introduced through the left femoral sheath we successfully extracted the broken guide wire from the descending aorta. We decided to continue the thrombectomy procedure using a new guide wire placed in the abdominal aorta, after a predilation with a Chronus Advanced 4/80 mm Rontis® (Rontis Medical) balloon in the area of maximum stenosis where the guide wire fracture was recorded, in order to reduce the risk of repeating the incident and to allow an easier access for the thrombectomy catheter through that area (Figure 8). Subsequently we succeeded the passage with the thrombectomy catheter and we obtained recanalization of the vascular axis from the level of the right iliac artery up to the abdominal aorta, through the endo-graft, extracting a lot of thrombus (Figure 9). We continued intervention with 8/60 mm Chronus Advanced Rontis® balloon dilatation, along the right limb and extension of the endoprosthesis and we implanted proximally inside the prosthesis right limb, a self-expandable stent Zeus® (Rontis Medical) 11/60 mm (Figure 10). Further on, we used the kissing stents technique with two balloon expandable Dynamic® (Biotronik) stents of 10/56 mm in the right limb and 10/25 mm in the left extension (Figure 11). We finally implanted another self-expandable 10/60 mm stent Protege EV3® (Medtronic) at the level of the distal right external iliac artery, forced by a flow limiting dissection area, secondary the insertion of the 10F sheath in the extremely tortuous right iliac artery (Figure 12). The final angiographic result, was considered excellent at the end of the procedure, without any flow limiting areas of stenosis and with excellent distal blood flow (Figure 13).
At the end of the procedure, the left femoral punction sites was closed with an Angioseal® (Terumo Eu-rope NV) 8F arterial closing device and through surgical suture on the right side.
The patient’s ulterior evolution was very good, with mobilization from the next day following the procedure. The pulse was present on palpation both at right common femoral artery and right popliteal artery levels. On day 3 after the procedure, the patient was evaluated with Doppler ultrasound with the presence of triphasic flow of normal amplitude at femuro-po-pliteal, peroneal and posterior tibial levels. Patient was discharged with triple therapy: with oral anticoagulant (the patient being in permanent atrial fibrillation) and double antiplatelet therapy, which we decided to maintain for three month, and considering the long term continuation of the association of oral anticoagulant and one antiplatelet drug (favoring the use of clopidogrel).
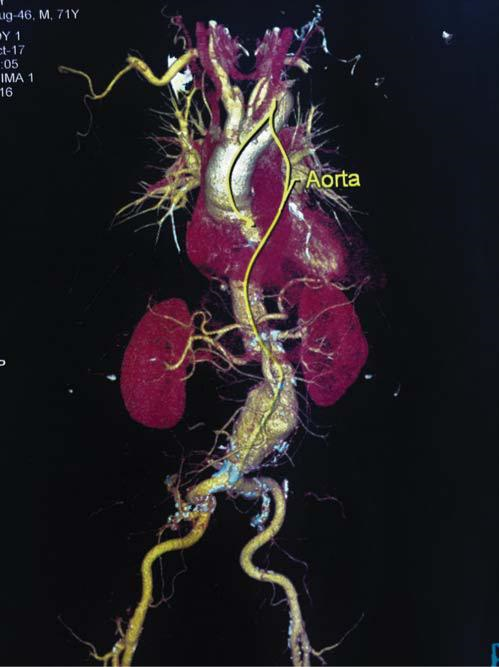
Figure 1. Abdominal aortic aneurysm extending to both common iliac arteries.
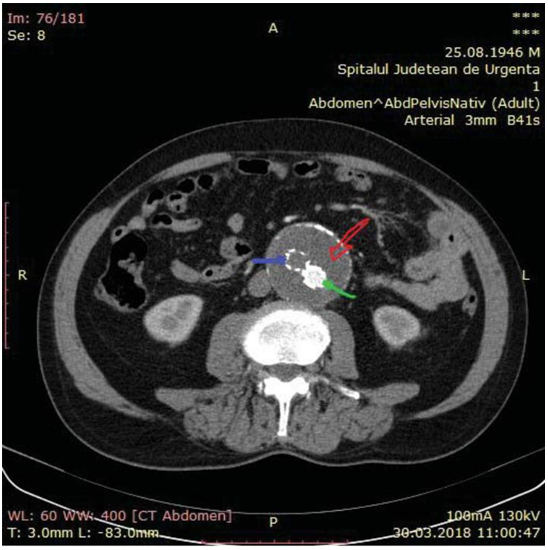
Figure 2. Thrombosis in the right limb of the endoprosthesis (purple ar-row). The left limb loads the contrast substance (green arrow).
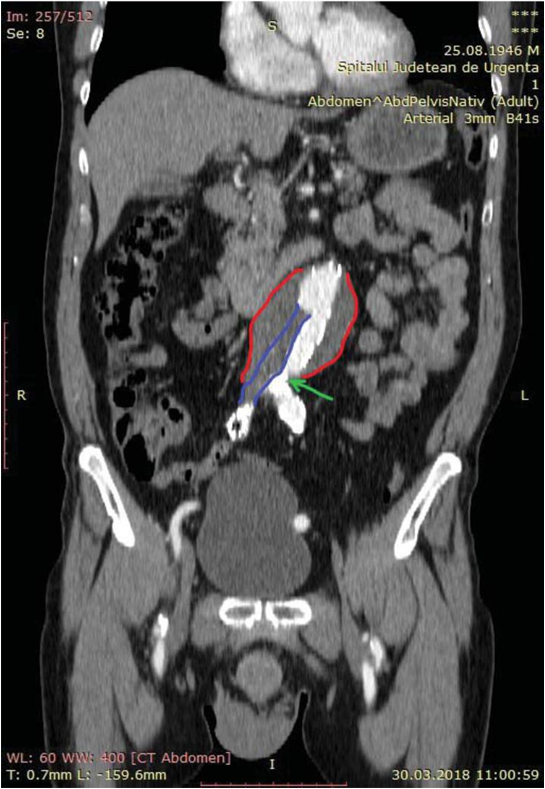
Figure 3. Thrombosis in the right limb and right extension of the endo-prosthesis (purple) and narrow left extension stenosis (green arrow).
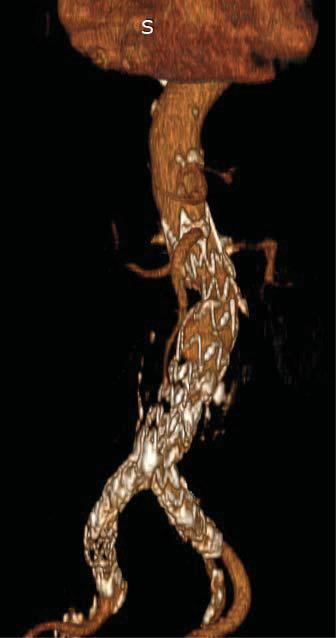
Figure 4. CT reconstruction – lack of loading with contrast substance in the right limb and right extension of the prosthesis.
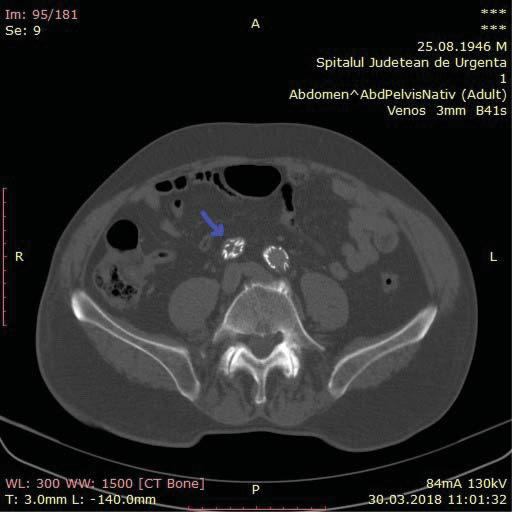
Figure 5. At the level of the right limb prosthesis, the axial image shows what appears to be the intraluminal folding of the metal struts of the stent grafts’ limb inside the right common iliac artery (purple arrow).
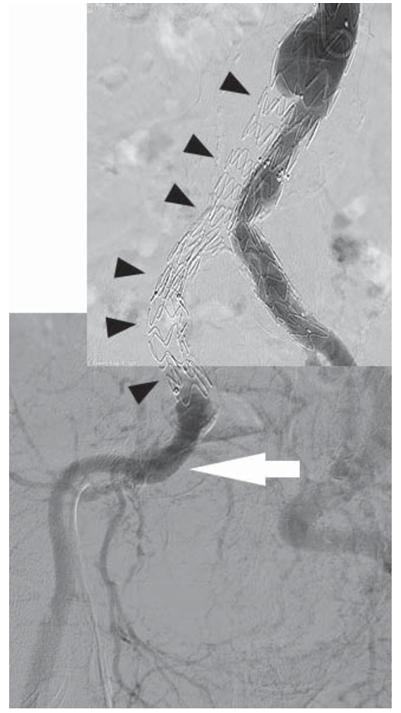
Figure 6. The occlusion of the right limb of the stent graft and the extension attached to it (black arrows). Refill through collaterals from the left internal iliac artery, with slow flow and delayed filling of the arteries of the inferior right limb (white arrow).
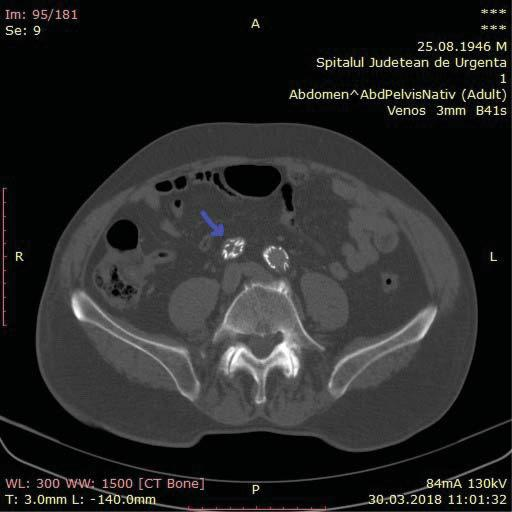
Figure 7. In the attempt to perform the first passage of thrombectomy through the prosthesis, the fracture of the Rotarex device guide is observed.
Figure 8. Low diameter balloon preload.
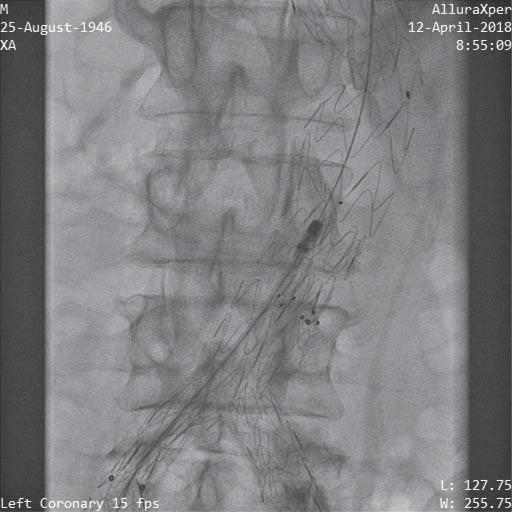
Figure 9. Thrombectomy passage through the entire occlusion area of the prosthesis.
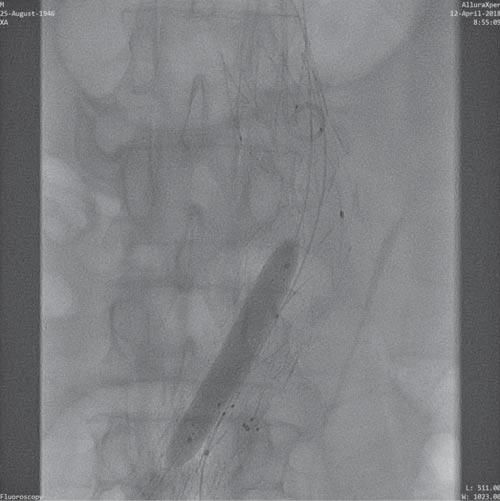
Figure 10. Post-deployment self-expanding stent right limb of the prosthesis.
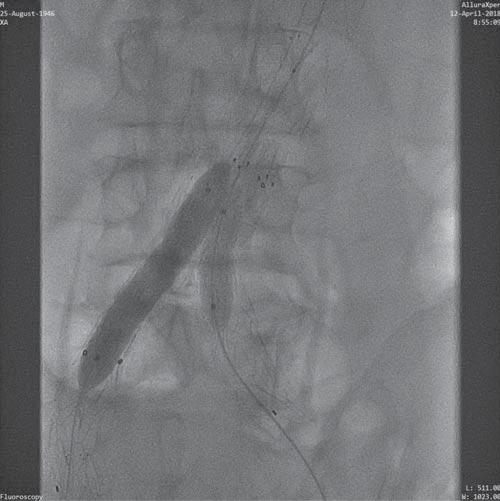
Figure 11. Stent implantation on bilaterally intraprotetic balloon by kissing stents technique.
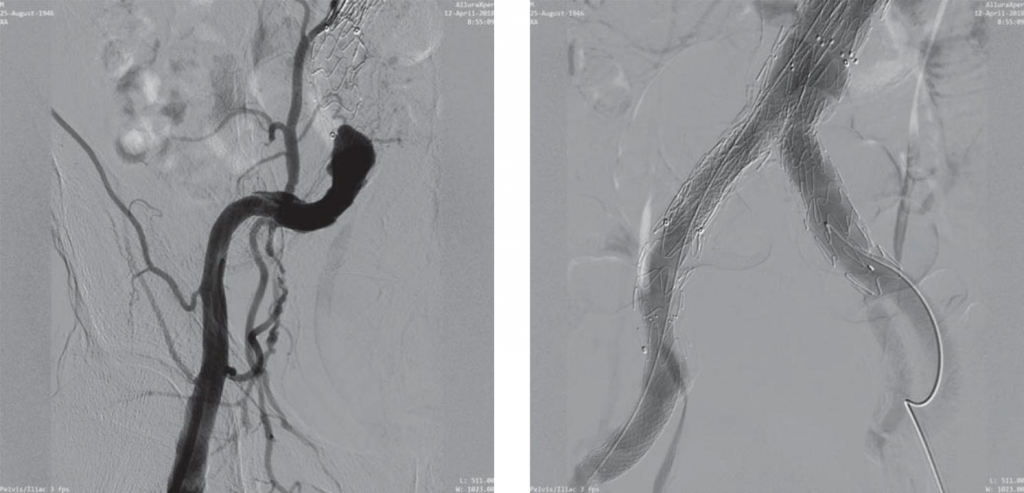
Figure 12. Iatrogen dissection of external iliac artery secondary to implantation of the 10F sheath.
Figure 13. The final angiographic result was considered excellent without any flow limiting areas of stenosis and with excellent distal blood flow.
DISCUSSIONS
Literature data suggests that up to 20% of the patients who had undergone an EVAR procedure will require reinterventions, the majority being represented still by endovascular procedures. The thrombosis of one of the endoprosthesis limbs is a complication with a reported incidence between 2 and 25%. Predictors for this complication are generally linked with the implantation of a long endoprosthesis into a relatively short vessel, the presence of important kinking and tortuosity and severe vascular calcifications. Also, the occlusion can be produced in case of a reduced distal run-off or an unnoticed vessel wall dissection produced during endograft deployment.
From a clinical standpoint, the acute occlusion of one of the endoprosthesis limbs manifests itself rather through buttock claudication than critical ischemia, with the condition that the prosthesis limb should be placed proximal to the origin of the internal iliac artery, in order to facilitate the flow from collaterals and into external iliac artery.
The treatment for this type of complication has been amply described in the literature and includes a various array of procedures: surgical thrombectoy with Fogarty balloon, hybrid procedures (Fogarty thrombectomy followed by angioplasty), trombolysis followed or not by stenting, percutaneous thrombectomy associated or not with thrombosis and followed by stenting, primary angioplasty with stent or open surgical revascularization with anatomic by-pass (aorto-femoral) or extra-anatomic (femoro-femoral or axilo-femoral). For the time being there are no randomized studies evaluating the efficiency of angioplasty in the treatment of endograft occlusion.
Each one of the above techniques are caring procedural risks. Surgical revascularization techniques through by-pass are usually associated by high mortality. In the case of surgical thrombectomy, the difficulties are tied to the need of surgical access and the risk of modification of the prosthesis architecture, dislodgement or endoleaks following the passage of the Fo-garty catheter. On the other hand the thrombectomy alone isn’t enough as it doesn’t correct the cause that lead to thrombosis. Thrombolysis – a similar approach as repermeabilisation through surgical thrombectomy – is encumbered by the hemorhagic risk, while also leaving uncorrected an underlying mechanical cause. Even though through conventional percutaneous angioplasy a vessel repermeabilization is obtained while also correcting the local cause for thrombosys (underexpanded stent/disection/ residual stenosis), the risk of distal embolization of the thrombotic material in case of heavy thrombotic load is verry high. Thus, an approach which combines a „debulking” technique reducing the thrombotic load, with one which corrects the endoprosthesys defect through endovascular remodeling seems to be the optimal approach. Literature data are reporting as successful, even though quite rare, the of the Rotarex® device in the treatment of sub-acute or older thrombosis in native vessels or intraprosthetic. The device is composed of a rotational trombectomy system functioning at rotational speeds of 40.000-60.000 rpm coupled electromagnetically to the catheter and associated with a continuous aspiration system, which is triturating and extracting the thrombotic material, without risk of distal embolization. The contraindications of using such system, are severe calcifications, small caliber vessels, subintimal passage of guide wire, the impossibility of crossing the occlusion or severe vascular spasm.
CONCLUSIONS
We report the case of a aorto-biiliac endoprosthesis desobstruction, by using a strict endovascular approach, which combined the rotational thrombectomy and conventional angioplasty, with excellent immediate result. We can thus conclude that the interventional treatment with rotational thrombectomy and continuous aspiration followed by stent angioplasty represents a viable solution in the case aorto-iliac endograft occlusions, with good initial results, reduced procedural risks and high comfort for the patient. More studies, on a bigger number of patients with long-term follow-up are necessary in order to adequately evaluate the procedural efficiency, the frequency and the type of possible complications.
Disclosures: Dirk Dreyer is Director Global Sales & Marketing, and Member of the Executive Board Straub Medical AG Switzerland
References
1. Chaikof EL, Dalman RL, Eskandari MK, Jackson BM, Lee A, et al. (2018) The Society for Vascular Surgery practice guidelines on the care of patients with an abdominal aortic aneurysm. J Vasc Sur 67: 2-77.
2. Guoquan Wang, Shuiting Zhai, Tianxiao Li, Xuan Li,Danghui Lu, Bo Wang, Dongbin Zhang, Shuaitao Shi,Zhidong Zhang, Kai Liang, Kewei Zhang, Xiaoyang Fu, Kun Li, and Weixiao Li, Limb graft occlusion following endovascular aortic repair: Incidence, causes, treatment and prevention in a study cohort, Exp Ther Med. 2017 Aug; 14(2): 1763-1768.
3. Tatli E, Tokatli A, Vatan MB, Aksoy M, Can Y, et al. (2016) Percutaneous approach to the treatment of a totally occluded abdominal aortic stent graft. Perfusion 31: 521-524.
4. Fabio Augusto Cypreste Oliveira1, Fabio Lemos Campedelli1, Car-los Eduardo de Sousa Amorelli1, José Eduardo da Costa Filho2, Dan-iel Resende Gibbon2, Juliana Caetano Barreto3, Philippe Moreira da Silva, Endovascular treatment of iliac limb occlusion of a bifurcated abdominal aortic stent graft – rotational and aspiration thrombec-tomy followed by primary angioplasty and stenting, J Vasc Bras 2012, Vol. 11, Nº 3
5. Paulo Eduardo, Ocke Reis Marcello Rotolo, Alessandra Viz Veiga, Jean Moura Netto, Vitor Nascimento Maia, Lys Nunes dos Santos, Irlandia Figueira Ocke Reis, Fernando De Azambuja Fontes, Thadeu Mozella, Gustavo Solano, Daniel Paixao, Luciana Cristina Ximenes, Amanda Barata Reis and Pietro de Almeida Sandri, Total Abdominal Aortic Stent Graft Occlusion, Journal of Vascular and Endovascular Surgery ISSN 2573-4482, Vol.2 No.S1:37
6. Liaw JV, Clark M, Gibbs R, Jenkins M, Cheshire N, Hamady M. Up-date: Complications and management of infrarenal EVAR. Eur J Ra-diol. 2009;71:541-551.
7. Sivamurthy N, Schneider DB, Reilly LM, Rapp JH, Skovobogatyy H, Chuter TA. Adjunctive primary stenting of Zenith endograft limbs during endovascular abdominal aortic aneurysm repair: Implications for limb patency. J Vasc Surg. 2006;43:662-670. doi:
8. Greenhalgh RM, Brown LC, Kwong GP, Powell JT, Thompson SG (2004) Comparison of endovascular aneurysm repair with open re-pair in patients with abdominal aortic aneurysm (Evar trial 1), 30-day operative mortality results: randomized controlled trial. Lancet
364: 843-848.
9. Prinssen M, Verhoeven EL, Buth J, Cuypers PW, van Sambeek MR, et al. (2004) A randomized trial comparing conventional and endo-vascular repair of abdominal aortic aneurysms. N Engl J Med 351: 1607-1618.
10. Sampram ES, Karafa MT, Mascha EJ, Clair DG, Greenberg RK, et al. (2003) Nature, frequency, and predictors of secondary procedures after endovascular repair of abdominal aortic aneurysm. J Vasc Surg
37: 930-937.
11. MantasGK, Antonopoulos CN, Sfyroeras GS, Moulakakis KG, Kaki-sis JD, et al. Factors Predisposing to Endograft Limb Occlusion after Endovascular Aortic Repair. Eur J Vasc Endovasc Surg 49: 39-44.
12. Mehta M, Sternbach Y, Taggert JB, et al. Longterm outcomes of sec-ondary procedures after endovascular aneurysm repair. J Vasc Surg 2010; 52:1442-1449
13. Liaw JV, Clark M, Gibbs R, Jenkins M, Cheshire N, Hamady M. Up-date: complications and management of infrarenal EVAR. Eur J Ra-diol 2009; 71:541-551
14. Giorgos S. Sfyroeras, Dimitris Maras, Vassileios Andrikopoulos – „Abdominal Endograft Collapse with Acute Bilateral Lower Limb Ischemia”- DOI: 10.1016/j.jvir.2010.12.026
15. Pasqualino Sirignano – “Preoperative Intrasac Thrombus Load Pre-dicts Worse Outcome after Elective Endovascular Repair of Ab-dominal Aortic Aneurysms” DOI: 10.1016/j.jvir.2015.07.005
16. Andrew C. Picel, Nikhil Kansal – „Essentials of Endovascular Ab-dominal Aortic Aneurysm Repair Imaging: Preprocedural Assess-ment” DOI:10.2214/AJR.13.11735, AJR:203, October 2014
17. Yolanda Bryce, Philip Rogoff, Donald Romanelli, Ralph Reichle – „En-dovascular Repair of Abdominal Aortic Aneurysms: Vascular Anato-my, Device Selection, Procedure, and Procedure-specific Complica-tions”, RadioGraphics • Volume 35 Number 2, March-April 2015
 This work is licensed under a
This work is licensed under a