Zsolt Gyalai1,3, Zsuzsanna Jeremias3, Andrea Rab1, Roxana Rudzik2, Dan Dobreanu2,3
1 Department of Cardiology, Mureş County Clinical Hospital, Târgu Mureş, Romania
2 Department of Cardiology, Emergency Institute of Cardiovascular Diseases and Transplantation, Târgu Mureş, Romania
3 University of Medicine and Pharmacy Târgu Mureş, Romania
Abstract: Cardiac resynchronization therapy (CRT) can improve left ventricular function and symptoms of heart failure by restoring synchronous left ventricular (LV) contractions. Although this improvement is achieved in the majority of patients, some 30% of those who underwent CRT remain non-responders. A number of methods have been tested to improve the rate of responders and convert non-responders into responders, among which different echocardiographic methods. The aim of this study was to establish whether echocardiographic parameters of mitral and aortic velocity time integral (VTI) can help to optimize CRT settings. 27 patients with CRT have been included in the study; demographic, clinical (blood pressure, laboratory parameters, etiology of heart failure, comorbidities, ECG), therapeutic and echocardiographic (standard measurements, LVDFT/RR, IMD, SPWMD, aortic and mitral-VTI, LVEF, dp/dt, GMI) parameters were assessed. Patients underwent echocardiography to determine dyssynchrony parameters with actual CRT, without CRT and with optimized CRT settings, which was carried out using the aortic and mitral-VTI. Our results indicate that echocardiographic optimization using VTI parameters did not improve mechanical dyssynchrony and acute left ventricular function parameters in patients with CRT
and should be considered only in selected cases of CRT non-responders, associated with other optimization methods. Keywords: CRT, echocardiograpic optimization, Mi-VTI, Ao-VTI, non-responders
INTRODUCTION
Chronic heart failure (HF) is one of the most common cardiovascular pathologies in developed countries. Besides medical treatment, interventional treatment using devices that target left ventricle dyssynchrony are gaining more and more attention. Electrical dyssynchrony (atrioventricular and interventricular conduction delays that manifest as left bundle branch block (LBBB), right bundle branch block (RBBB) or other conduction abnormalities on the ECG) and mechanical dyssynchrony (with three aspects of atrioventricular, interventricular and intraventricular dyssynchrony) leads to a spectrum of pathophysiological alterations of the LV function, causing a decrease in the efficacy of LV contractility and thus reducing stroke volume1. In HF patients who remain symptomatic despite optimal medical therapy, cardiac resynchronization the rapy is a valuable option to improve left ventricular contractility and symptoms of heart failure. Cardiac resynchronization therapy (CRT) can help improve left ventricular systolic function and symptoms of heart failure by restoring the normal AV and intraventricular conduction patterns and synchronous contractility2. According to current guidelines, CRT has class I indication in HF patients with NYHA class II-IV symptoms, who are in sinus rhythm, have a left ventricular ejection fraction (LVEF) <35%, LBBB morphology with QRS duration >120 ms and are symptomatic despite optimal medical therapy for HF3. However, about 30% of patients do not respond clinically
to CRT and 45% show no evidence of reverse remodeling or clinical improvement despite meeting the guideline criteria4,5. It is yet unclear why this issue occurs. Despite evidence that several parameters are correlated with response to CRT, none of these can accurately predict individual response to CRT6-8. The occurrence of this important category of non-responder patients, in whom CRT fails to achieve the desired response, is an intensely researched issue that remains to be solved. A number of methods to convert nonresponders into responders have been studied and
tested. CRT optimization based on ventriculo-ventricular delay (VVD) settings according to the narrowest QRS complex on ECG after implantation, and the difference between biventricular pacing and pre-implantation ECG, as tested by Vidal et al. showed a good correlation to echocardiographic parameters, but did not supply data on improvement of LVEF or reverse remodeling9. Some studies suggest that echocardiography can help optimize resynchronization settings in non-responders. Attempts have been made for echocardiographic optimization, because of its affordability and large availability. Ritter’s method, measuring largest stroke volume or residual LV dyssynchrony had only mild benefit in comparison to standard CRT settings10-12. The aim of this study was to assess whether two echocardiographic parameter (Mi and Ao-VTI) can help to optimize CRT settings and to establish which of these parameters is the most efficient. The authors have chosen to assess mitral and aortic VTI parameters because they refl ect the global function of the left ventricle and do not require special technical background or training, thus they are largely available.
MATERIALS AND METHODS
27 patients with heart failure and previously implanted CRT device were enrolled in a prospective study, aimed to establish acute hemodynamic response and optimal AV and VV delay settings for their CRT device, with the help of echocardiography. All of the patients were examined between August 2013 and December 2014, at the Department of Cardiology, Emergency Institute of Cardiovascular Diseases and Transplantation Târgu Mureş, being admitted for follow-up visits (authors own cases). Demographic (age, sex, height, weight, body mass index, smoking status), clinical (blood pressure, laboratory parameters, etiology of HF, comorbidities, ECG data including rhythm, atrioventricular and intraventricular conduction delays, characteristics of the implanted device), therapeutic and echocardiographic parameters were registered. Only patients with sinus rhythm were evaluated, VTI optimization in patients with atrial fibrillation being considered too difficult to perform. All of the patients underwent a baseline echocardiographic examination and a set of dyssynchrony parameters were recorded under four different settings: with actual CRT parameters (actual stimulation parameters after implantation), without CRT pacing (native rhythm, with device stimulation turned off) and with stimulation parameters optimized using mitral
and aortic velocity time integral (VTI). Immediately post-implant devices were programmed using electric criteria, taking in account native AV conduction (PR interval), conduction delay between RV and LV lead, intramural conduction time, width and morphology of the QRS complex (R wave in lead V1); before discharge, echocardiographic evaluation of the transmitral inflow profile also was performed. The standard schedule during this examination was the following: device interrogation with the specific programming device for each CRT (recording native rhythm, native atrio-ventricular delay, actual paced AV and VV delay), standard echocardiographic study with chamber quantifi cation and functional assessment, measurement of a first set of dyssynchrony parameters (with actual post-implantation AVD and VVD settings). The following parameters were assessed: left ventricular diastolic fi lling time/ RR ratio (LVDFT/RR), left ventricular preejection period (LVPEP), right ventricular preejection period (RVPEP), interventricular mechanical delay (IMD), septal to posterior wall motion delay (SPWMD), mitral time-velocity integral and aortic time-velocity integral, dp/dt (when mitral regurgitation was present), left ventricular ejection fraction (LVEF) using Simpson’s biplane method, isovolumetric contraction time (IVCT), isovolumetric relaxation time (IVRT), ejection time (ET) and global myocardial performance index (the sum of isovolumetric contraction time and isovolumetric relaxation time divided by
ejection time). A second set of these dyssynchrony parameters was assessed under native rhythm, after the CRT stimulation was turned off. After these recordings, an attempt for echocardiographic optimization using mitral and aortic velocity time integral (VTI) was performed: starting from a baseline AV delay of 100 msec (or below of this level, if the actual setting values were lower) and gradually increasing AV delay by 20 msec at a time (baseline AV delay, +20, +40, +60, +80 msec respectively) and measuring aortic and mitral VTI parameters for each of
these AV delay, concomitantly with different VV delay settings, ranging from -20 msec (“RV fi rst”), through 0, +20, +40, +60 and fi nally +80 msec or “LV only” in devices that allowed this setting. After establishing the best mitral and aortic VTI parameters, a third set of dyssynchrony parameters were assessed with matching stimulation parameters (AV delay and VV delay optimized for “Best Mi” VTI) and a fourth set of dyssynchrony parameters were determined based on “Best Ao” VTI optimized AV and VV delay. Echocardiographic evaluation and measurements
were performed with a General Electric Vivid S5TM device (System SW v3.0.16, using SW v10.3.0 build 144 application), by the same examiner in each of the cases to reduce inter-examiner variations, with patients in supine position with the reading/programmer head placed above the central unit of the CRT device. Three programmer devices were used, namely BiotronikTM
(PSW 1402.A/1 software), MedtronicTM (2090/2.9 software) and St. Jude MedicalTM (3330 software v21.1.2). In the case of aortic and mitral VTI measurements, a pause of 3 minutes was interposed after each adjustment of AV and VV delay to allow proper hemodynamic adaptation, after which VTI parameters were assessed in inspiratory apnea. Echocardiographic parameters were measured according to previously validated methods, as follows: – LVDFT/RR: left ventricular diastolic fi lling time is measured based on the transmitral pulsed wave velocity curve, from apical four chamber view, from the start of the E wave until the end of the A wave, RR interval is measured between the peaks of two successive QRS complexes. – IMD (interventricular mechanical delay): is quantifiable as the time difference between pre-ejection periods for left and right ventricles, (i.e. the interval from the beginning of the QRS complex to the beginning of ejection through the aortic/ pulmonary valves) measured by PW-Doppler method, IMD = left ventricular pre-ejection period (LVPEP) – right ventricular pre-ejection period (RVPEP)
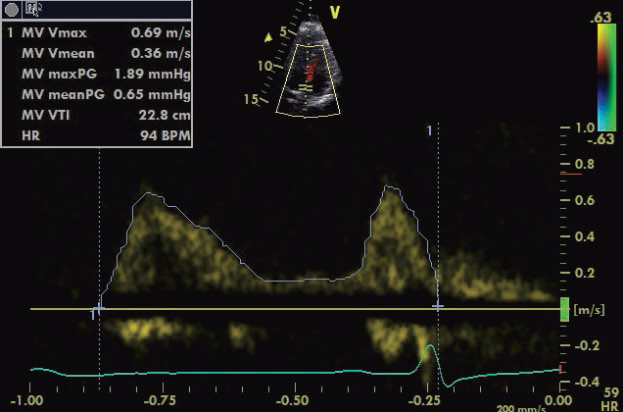
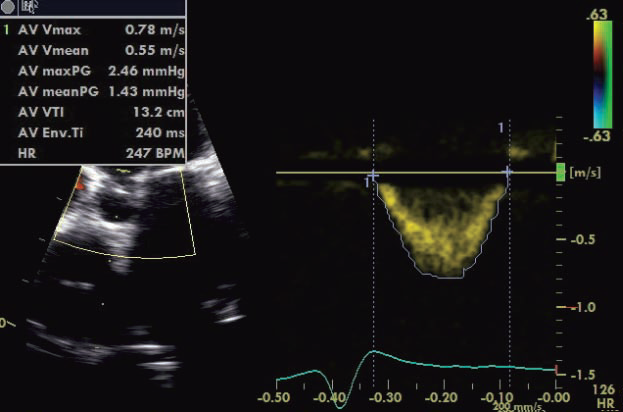
SPWMD (septal-to-posterior wall motion delay) is obtained in parasternal short axis sections at the level of papillary muscles in 2D guided – M mode – Mi-VTI (mitral velocity-time integral): determined by pulsed wave transmitral velocity curves, from apical four chamber view, as shown in Figure 1. – Ao-VTI (aortic velocity-time integral): determined by pulsed wave Doppler method at the left ventricular ejection tract, below the aortic valves, from apical 5 chamber view, as shown in Figure 2 – dp/dt: measured based on the CW Doppler curve, where mitral regurgitation was present – LVEF was determined using Simpson’s biplane method from apical four and two chamber views – GMI (global myocardial index): calculated as the sum of IVCT and IVRT, divided by ET, according to the formula: GMI=(IVCT+IVRT)/ET, measured by pulsed wave Doppler, from apical 5 chamber view. The study was approved by the hospital’s ethics committee, written informed consent was obtained from each of the enrolled patients.
Figure 1. Echocardiographic measurement method of Mi-VTI.
Figure 2. Echocardiographic measurement method of Ao-VTI.
Data management and statistical analysis: data was recorded in Microsoft Offi ce Excel 2003. Data are represented as mean ± SD. Calculations and sta tis tical analysis were performed using Microsoft Office Excel 2003 and GraphPad InStat 3.06, applying Kolmogorov-Smirnov’s goodness-of-fi t test and using Student’s paired t-test for comparing the native and basal-paced data group, respectively repeated measure ANOVA with Bonferroni post-test or Friedman test with Dunn post-test for comparing the basal-paced and the two optimized (“best with Mi-VTI” and “best
with Ao-VTI”) data group.
RESULTS
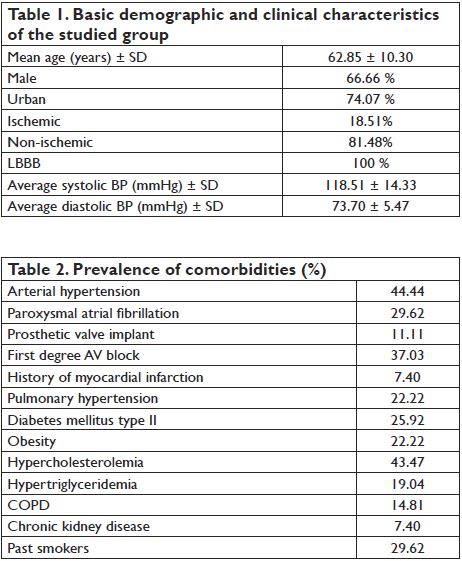
Demographic and clinical characteristics of the patients are summarized in Table 1. Relevant comorbidities of the patients are included in Table 2. The most frequent comorbidities were arterial hypertension and hypercholesterolemia. Besides CRT, patients received medical therapy for heart failure according to current ESC guideline recommendations, as shown in Table 3. All patients received beta-blocker therapy with carvedilol, an average daily dose of 22.1 mg. 88.8% of patients received RAAS antagonists, mostly ramipril (70.37%, average dose of 4.16 mg), 2 patients were treated with perindopril and 3 patients with candesartan. All patients were treated with spironolactone (average daily dose 43.75 mg) and 81.48 % received furosemide (average daily dose 44
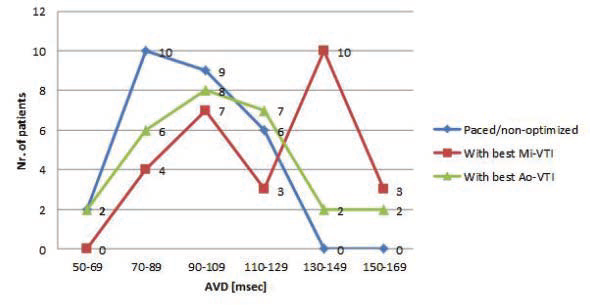
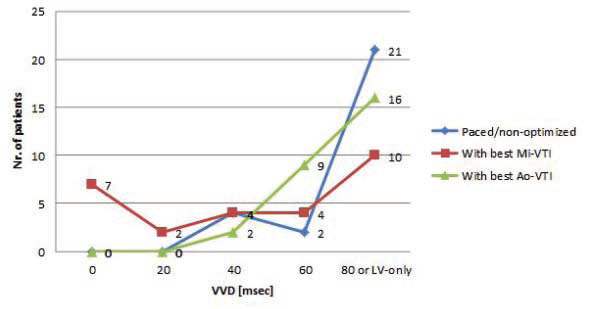
mg). Basic echocardiographic parameters are shown in Table 4. Mean LV ejection fraction was 32.25% (SD: 6.79 %, limits 20-43 %). The average time passed from the implantation, at the moment of examination, was 1.3 years (between 3 months and 4 years). Figure 3 and Figure 4 show the distribution of AVD and VVD in our group, under three different settings: basal-paced and the two best settings determined by VTI optimization (”best with Mi-VTI” and “best with Ao-VTI” respectively). The mean AVD under the three different setting was (msec. ± SD): 92.92 ± 20.50 for
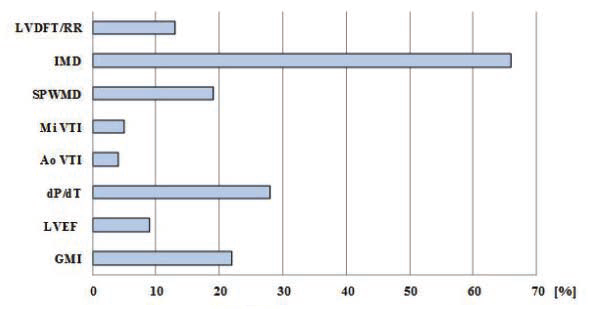
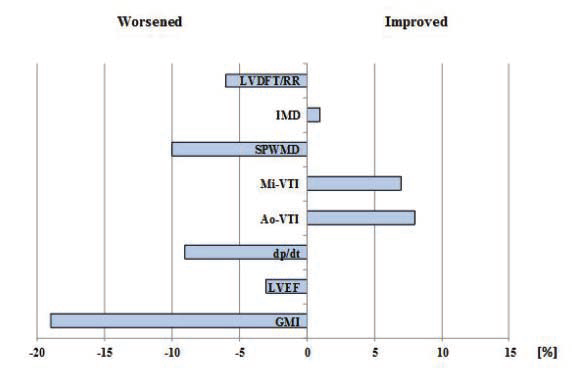
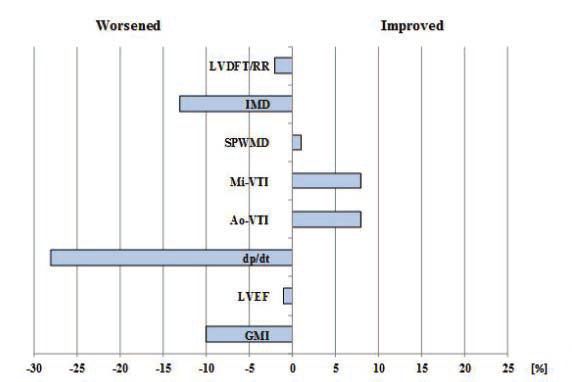
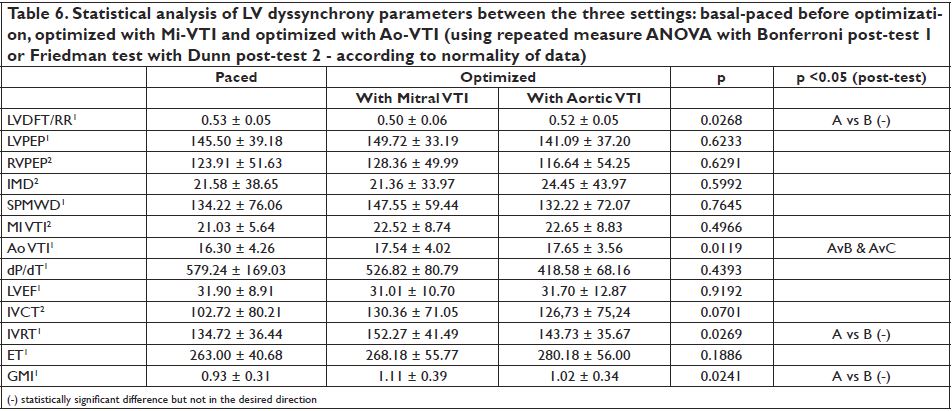
basal-paced setting, 115.91 ± 26.53 for Mi-VTI optimization and 110.45 ± 26.88 for Ao-VTI optimization. The most frequent VVD found was +80 msec or “LV only” under each of the settings (with 77.77%, 37.03% and 59.25 % respectively). Figure 5 shows the percentual improvement of dyssyncrony parameters with basal-paced CRT rhythm (before optimisation) compared to native rhythm (with the CRT device turned off). Table 5 shows the statistical (p) values for these dyssynchrony parameters. Figure 6 and Figure 7 shows the improvement or worsening of LV dyssynchrony parameters after optimization of AVD and VVD with Mi-VTI and Ao-VTI respectively, expressed in percents, compared to basal- paced setting before optimization. Table 6 summarizes
the statistical (p) values for these dyssynchrony parameters.
Figure 3. Distribution of the atrio-ventricular delay under the three settings.
Figure 4. Distribution of the ventriculo-ventricular delay under the three
settings.
DISCUSSIONS
As shown in Figure 5 and Table 5, cardiac resynchronization therapy proved its benefi ts on each and every studied parameter, though the difference was statistically signifi cant only in the case of LVDFT/RR, IMD and GMI. As in many other studies, CRT proved to have also in this case series a positive acute effect on mechanical dyssynchrony and LV function parameters. Regarding optimization, the results were far below expectations. As Figure 6 and Figure 7 shows, practically the only positive effect was demonstrated on the two VTI parameters, quite explicable due to the fact that these were the parameters based on, and for which the CRT settings were optimized. In case of all the other parameters, except small benefits on IMD (with Mi-VTI) and SPWMD (with Ao-VTI) respectively, the results were negative both with Mi-VTI and Ao-VTI optimization, furthermore in some cases the worsening was even statistically significant (LVDFT/RR, IVRT
and GMI after optimization with Mi-VTI).
Based on this case series, echocardiographic optimization of cardiac resynchronization therapy using Mi and Ao-VTI parameters showed no benefi t and should be considered only in individual cases, especially in non-responders to CRT with standard pacing settings, perhaps correlated with other optimization methods, such as TDI Doppler imaging techniques using myocardial strain and strain rate 13 and cardiac MRI, which can help better identify patterns of dyssynchrony 14, although these methods have limited applicability due to their lack of availability and high costs.
In addition, there is a concern regarding reproducibility and averaging of mitral and aortic VTI measurements for multiple beats15. Furthermore, the timeconsuming nature of echocardiographic optimization and relatively high variability of parameters, partially dependent also on the degree of experience of the examiner, is considered one of the major disadvantages of the procedure, thus limiting the use of these parameters in routine clinical practice. This is one of the reasons why many authors consider that it should be used only in selected cases, where standard settings of CRT fail to achieve the expected response3. When echocardiographically assessing CRT patients, especially non-responders, demonstrating mechanical dyssynchrony is the main target, but other aspects as clinical response, dynamic dyssynchrony, contractile reserve and the presence of myocardial scar tissue should not be disregarded. In addition, lead placement and its optimization is also an important issue and should always be considered when assessing non-responders, especially in patients with ischemic cardiomyopathy16. Optimization based on avoidance of apical lead
placement and targeting latest activated area showed likely benefi t by reducing hospitalization for HF and improving the rate of responders17-20. Our results correspond partially with findings from the literature, which in itself consists of controversioptimizatial conclusions in this fi eld. Small randomized clinical trials and experimental studies found improvement in the left ventricular systolic function, heart failure symptoms, reducing hospitalizations and a positive change in the quality of life after optimization with AV and VV delay. Sawhney et al. have identified that AV delay optimization using the aortic-VTI improves the three-month clinical outcome more than a 120 ms programmed empiric AV delay10. Similar results were described by Kerlan et al., who compared echocardiographic AV delay optimization guided by the aortic- VTI versus the mitral inflow method and concluded that AV delay optimization with aortic-VTI for patients with severe heart failure, provides considerably more improvement21. Other studies emphasize the importance of mitral-VTI. Jansen et al. and Thomas et al. had similar findings when comparing the use of mitral-VTI to aortic-VTI guided optimization, claiming that the mitral- VTI method is reliable and affordable in everyday clinical practice and adequate in improving response to CRT15,22.
Figure 5. Percentual improvement of dyssynchrony parameters with basal- paced CRT rhythm compared to native rhythm (with the CRT device turned off).
Figure 6. Percentual improvement or worsening of LV dyssynchrony parameters, after optimization with Mi-VTI compared to basal-paced setting before optimization.
Figure 7. Percentual improvement or worsening of LV dyssynchrony parameters after optimization with Ao-VTI compared to basal-paced setting before optimization.
Contrarily, results from large multicentre trials, that assessed the utility of aortic and mitral VTI for optimization of CRT, showed no signifi cant benefi t compared to other optimization methods. According to the FREEDOM23,24, CLEAR25, SMART-AV12, Adaptive CRT26,27, DECREASE-HF 1 and Responde CRT28 trials, the difference of benefi ts between automatic electrical, devicebased algorithms (SmartDelay and QuickOpt for AV delay and Expert-Ease, Quick-Opt, Peak endocardial acceleration for VV delay-based optimization) and echocardiographic CRT optimization remains uncertain. Correspondingly, our results suggest that AV and VV delay optimization do not provide improvement in echocardiographic parameters of patients with CRT.
Therefore, as stated in ESC guidelines, current evidence does not support AV and VV optimization routinely in all patients receiving CRT. However, in nonresponders, evaluation of AV and VV delay may be recommended, in order to correct suboptimal device settings3. The same conclusions were published in a recent, leading review conducted by Daubert, so that the systematic routine optimization of the AV and VV delays in all CRT system recipients is not warranted29. In actual practice, ESC guidelines recommend to first programme a fi xed 100–120 ms AV delay, without
VV interval. However, in subgroups of patients, especially in the presence of a long interatrial delay, the intervals should be optimized after the implantation. Further echocardiographic evaluations and optimizations are advised in case of non-responders to CRT3,29. Most studies agree that one parameter cannot be singled out as a predictor of favorable outcome or successful optimization, instead a series of parameters should be used when echocardiographically evaluating CRT patients3. In conclusion, echocardiographic optimization using VTI parameters did not improve mechanical dyssynchrony and acute LV function parameters in patients with CRT, thus should be considered only as a complementary tool in selected cases of CRT non-responders,
associated with other optimization methods.
Conflict of interest: none declared.
References
1. Rao RK, Kumar UN, Schafer J, Viloria E, De Lurgio D, Foster E. Reduced ventricular volumes and improved systolic function with cardiac resynchronisation therapy: a randomized trial comparing simultaneous biventricular pacing, sequential biventricular pacing, and left ventricular pacing. Circulation 2007 Apr 24;115(16):2136-44
2. Pouleur AC, Knappe D, Shah AM, Uno H, Bourgoun M, Foster E, Mc- Nitt S, Hall WJ, Zareba W, Goldenberg I, Moss AJ, Pfeffer MA, Solomon SD. Relationship between improvement in left ventricular dyssynchrony and contractile function and clinical outcome with cardiac resynchronization therapy: the MADIT-CRT trial. Eur Heart J 2011 Jul;32(14):1720-1729
3. Brignole M, Auricchio A, Baron-Esquivias G, Bordachar P, Boriani G, Breithardt OA, Cleland J, Deharo JC, Delgado V, Elliott PM, Gorenek B, Israel CW, Leclercq C, Linde C, Mont L, Padeletti L, Sutton R, Vardas PE. 2013 ESC Guidelines on cardiac pacing and cardiac resynchronization therapy: the Task Force on cardiac pacing and resynchronization therapy of the European Society of Cardiology (ESC). Developed in collaboration with the European Heart Rhythm Association (EHRA). Eur Heart J 2013 Aug;34(29):2281-329
4. Abraham WT, Fisher WG, Smith AL, Delurgio DB, Leon AR, Loh E, Kocovic DZ, Packer M, Clavell AL, Hayes DL, Ellestad M, Trupp RJ, Underwood J, Pickering F, Truex C, McAtee P, Messenger J; MIRACLE Study Group. Multicenter InSync Randomized Clinical Evaluation Cardiac resynchronization in chronic heart failure. N Engl J Med 2002 Jun 13;346(24):1845-53
5. Chung ES, Leon AR, Tavazzi L, Sun JP, Nihoyannopoulos P, Merlino J, Abraham WT, Ghio S, Leclercq C, Bax JJ, Yu CM, Gorcsan J 3rd, St John Sutton M, De Sutter J, Murillo J. Results of the Predictors of Response to CRT (PROSPECT) trial. Circulation 2008 May 20;117(20):2608- 16
6. Goldenberg I, Moss AJ, Hall WJ, Foster E, Goldberger JJ, Santucci P, Shinn T, Solomon S, Steinberg JS, Wilber D, Barsheshet A, McNitt S, Zareba W, Klein H; MADIT-CRT Executive Committee. Predictors of response to cardiac resynchronization therapy in the Multicenter Automatic Defibrillator Implantation Trial with Cardiac Resynchronization Therapy (MADIT-CRT). Circulation 2011 Oct 4;124(14):1527-36
7. Verhaert D, Grimm RA, Puntawangkoon C, Wolski K, De S, Wilkoff BL, Starling RC, Tang WH, Thomas JD, Popović ZB. Long-term reverse remodeling with cardiac resynchronization therapy: results of extended echocardiographic follow-up. J Am Coll Cardiol 2010 Apr 27;55(17):1788-95
8. Köbe J, Dechering DG, Rath B, Reinke F, Mönnig G, Wasmer K, Eckardt L. Prospective evaluation of electrocardiographic parameters in cardiac resynchronization therapy: Detecting nonresponders by left ventricular pacing. Heart Rhythm April 2012;9(4):499–504
9. Vidal B, Tamborero D, Mont L, Sitges M, Delgado V, Berruezo A, Diaz- Infante E, Tolosana JM, Pare C, Brugada J. Electrocardiographic optimization of interventricular delay in cardiac resynchronization therapy: a simple method to optimize the device. J Cardiovasc Electrophysiol 2007;18:1252–1257
10. Sawhney NS, Waggoner AD, Garhwal S, Chawla MK, Osborn J, Faddis MN. Randomized prospective trial of atrioventricular delay programming for cardiac resynchronization therapy. Heart Rhythm 2004;1:562–567
11. Leon AR, Abraham WT, Brozena S, Daubert JP, Fisher WG, Gurley JC, Liang CS, Wong G. Cardiac resynchronization with sequential biventricular pacing for the treatment of moderate-to-severe heart failure. J Am Coll Cardiol 2005;46:2298–2304
12. Ellenbogen KA, Gold MR, Meyer TE, Fernndez Lozano I, Mittal S, Waggoner AD, Lemke B, Singh JP, Spinale FG, Van Eyk JE, Whitehill J, Weiner S, Bedi M, Rapkin J, Stein KM. Primary results from the Smart- Delay determined AV optimization: a comparison with other AV delay methods used in cardiac resynchronization therapy (SMART-AV) trial: a randomized trial comparing empirical, echocardiography-guided, and algorithmic atrioventricular delay programming in cardiac resynchronization therapy. Circulation 2010;122:2660–2668
13. Sogaard P, Egeblad H, Pedersen AK, Kim WY, Kristensen BO, Hansen PS, Mortensen PT. Sequential versus simultaneous biventricular resynchronization for severe heart failure: evaluation by tissue Doppler imaging. Circulation 2002 Oct 15;106(16):2078-84
14. Heydari B, Jerosch-Herold M, Kwong RY. Imaging for Planning of Cardiac Resynchronization Therapy. J Am Coll Cardiol Img 2012;5(1):93- 110
15. Jansen AH, Bracke FA, van Dantzig JM, Meijer A, van der Voort PH, Aarnoudse W, van Gelder BM, Peels KH. Correlation of echo- Doppler optimization of atrioventricular delay in cardiac resynchronization therapy with invasive hemodynamics in patients with heart failure secondary to ischemic or idiopathic dilated cardiomyopathy. Am J Cardiol 2006 Feb 15;97(4):552-7
16. Ypenburg C, van Bommel RJ, Delgado V, Mollema SA, Bleeker GB, Boersma E, Schalij MJ, Bax JJ. Optimal left ventricular lead position predicts reverse remodeling and survival after cardiac resynchronization therapy. J Am Coll Cardiol 2008 Oct 21;52(17):1402-9
17. Saxon LA, Olshansky B, Volosin K, Steinberg JS, Lee BK, Tomassoni G, Guarnieri T, Rao A, Yong P, Galle E, Leigh J, Ecklund F, Bristow MR. Infl uence of left ventricular lead location on outcomes in the COMPANION study. J Cardiovasc Electrophysiol 2009;20:764–768
18. Thebault C, Donal E, Meunier C, Gervais R, Gerritse B, Gold MR, Abraham WT, Linde C, Daubert JC. Sites of left and right ventricular lead implantation and response to cardiac resynchronization therapy observations from the REVERSE trial. Eur Heart J 2012;33:2662–2671
19. Singh JP, Klein HU, Huang DT, Reek S, Kuniss M, Quesada A, Barsheshet A, Cannom D, Goldenberg I, McNitt S, Daubert JP, Zareba W, Moss AJ. Left ventricular lead position and clinical outcome in the multicenter automatic defibrillator implantation trial-cardiac resynchronization therapy (MADIT-CRT) trial. Circulation 2011;123:1159–1166
20. Khan FZ, Virdee MS, Palmer CR, Pugh PJ, O’Halloran D, Elsik M, Read PA, Begley D, Fynn SP, Dutka DP. Targeted left ventricular lead placement to guide cardiac resynchronization therapy; the TARGET study: a randomized, controlled trial. J Am Coll Cardiol 2012; 59:1509–1518
21. Kerlan JE, Sawhney NS, Waggoner AD, Chawla MK, Garhwal S, Osborn JL, et al. Prospective comparison of echocardiographic atrioventricular delay optimization methods for cardiac resynchronization therapy. Heart rhythm : the offi cial journal of the Heart Rhythm Society. 2006 Feb;3(2):148-54
22. Thomas DE, Yousef ZR, Fraser AG. A critical comparison of echocardiographic measurements used for optimizing cardiac resynchronization therapy: stroke distance is best. European journal of heart failure. 2009 Aug;11(8):779-88
23. Abraham W T, Gras D, Yu CM, Guzzo L, Gupta MS; FREEDOM Steering Committee. Rationale and design of a randomized clinical trial to assess the safety and effi cacy of frequent optimization of cardiac resynchronization therapy: the Frequent Optimization Study Using the QuickOpt Method (FREEDOM) trial. Am Heart J. 2010;159:944–948
24. Abraham WT, Calo` L, Islam N, Klein N, Alawwa A, Exner D, Goodman J, Messano L, Clyne C, Pelargonio G, Hasan A, Seidl K, Sheppard R, Yu CM, Herre J, Lee LY, Boulogne E, Petrutiu S, Birgersdotter-Green U, Gras D. Randomized controlled trial of frequent optimization of cardiac resynchronization therapy: results of the Frequent Optimization Study Using the QuickOptTM Method (FREEDOM) Trial. Heart Rhythm 2010;7:2–3
25. Ritter P, Delnoy PP, Padeletti L, Lunati M, Naegele H, Borri-Brunetto A, Silvestre J. A randomized pilot study of optimization of cardiac resynchronization therapy in sinus rhythm patients using a peak endocardial acceleration sensor vs. standard methods. Europace 2012; 14:1324–1333
26. Martin DO, Lemke B, Birnie D, Krum H, Lee KL, Aonuma K, et al. Investigation of a novel algorithm for synchronized left-ventricular pacing and ambulatory optimization of cardiac resynchronization therapy: results of the adaptive CRT trial. Heart rhythm : the offi cial journal of the Heart Rhythm Society. 2012 Nov;9(11):1807-14
27. Birnie D, Lemke B, Aonuma K, Krum H, Lee KL, Gasparini M, et al. Clinical outcomes with synchronized left ventricular pacing: analysis of the adaptive CRT trial. Heart rhythm : the offi cial journal of the Heart Rhythm Society. 2013 Sep;10(9):1368-74
28. Brugada J, Brachmann J, Delnoy PP, Padeletti L, Reynolds D, Ritter P, et al. Automatic optimization of cardiac resynchronization therapy using SonR-rationale and design of the clinical trial of the SonRtip lead and automatic AV-VV optimization algorithm in the paradym RF SonR CRT-D (RESPOND CRT) trial. American heart journal. 2014 Apr;167(4):429-36
29. Daube rt C, Behar N, Martins RP, Mabo P, Leclercq C. Avoiding nonresponders to cardiac resynchronization therapy: a practical guide. European heart journal. 2016 Jul 1.
 This work is licensed under a
This work is licensed under a