Mihaela Grecu1, Mariana Floria2,3, Grigore Tinica1,3
1 Cardiovascular Disease Institute, Iasi, Romania
2 Emergency Clinical Hospital Iasi, Romania
3 ”Grigore T. Popa” University of Medicine and Pharmacy, Iasi, Romania
Abstract: Autonomic nerve activity through major epicardial ganglionated plexi (GP) plays an important role in the initiation and maintenance of atrial fi brillation (AF). GP modulating may contribute to paroxysmal AF control. Objective – We aimed to assess the impact of vagal response of GP stimulation on success rate in patients with endocardial catheter pulmonary vein isolation. Methods – Mapping of GP and immediate effect of GP ablation were tested using endocardial high frequency stimulation. A positive vagal response of GP was defi ned as pauses and/or more than 50% R-R intervals increasing during 5 seconds. Results – We included prospectively 63 patients (57 ± 8 years, 66.7% men) with symptomatic refractory paroxysmal AF. Success rate at 6 months after one single ablation procedure was 84%; recurrences were in 10 (15.9%) patients. High frequency stimulation of GP induced a positive vagal response to high frequency stimulation of at least one GP before pulmonary vein isolation in 40/63 patients (64%). All patients witch have had a positive response of at least one GP during procedure were in sinus rhythm at 6 months. Conclusions – Ganglionated plexi may be elicited by endocardial approach. Major ganglionated plexi ablation/modulation could have better outcomes in paroxysmal AF ablation.
Keywords: ganglionated plexi, ablation, atrial fibrillation.
INTRODUCTION
More than 30% of patients with arrhythmias at hospital admission have atrial fibrillation (AF)1. Major strides have been made in the treatment of AF in last ten years offering the potential to cure it. Intrinsic cardiac autonomic nervous system may serve as triggers or perpetuators for AF2,3. Both components of autonomic nervous system are present in ganglionated plexi (GP) embedded in the fat pads on the epicardial surface4,5. GP function is an integration centre that modulates the autonomic innervation between extrinsic and intrinsic cardiac autonomic nervous system6. The parasympathetic activation induces widening of the window of atrial vulnerability while the sympathetic stimulation enhances automatic firing within the pulmonary vein and/or promote atrial ectopic beats at the veno-atrial junction6. The response of GP stimulation is primarily vagal because the parasympathetic fibres represent the majority7. GP are located in epicardial fat pads at the border of the pulmonary vein antrum, and can be localized at the time of ablation using endocardial or epicardial high frequency stimulation at specific sites in left atrium to elicit slowing of the ventricular rate during AF8. Prior studies showed that four major epicardial GP (anterior right – ARGP, inferior right – IRGP, superior left – SLGP and inferior left – ILGP), as part of intrinsic cardiac autonomic nervous system, were identified on the left atrial posterior wall9-13. Still controversies exist regarding the benefit of atrial denervation on AF. The relationship of major GP response during endocardial approach of paroxysmal AF ablation and success rate is unknown.
MATERIAL AND METHODS
This single center prospective clinical study was aimed to evaluate the relationship between of major GP response during pulmonary vein isolation and success rate in symptomatic refractory paroxysmal AF during endocardial catheter ablation procedure.
Patient population
In this single center prospective study patients with paroxysmal AF were referred to undergo pulmonary vein isolation using fi rst generation cryo-balloon or by means of radiofrequency catheter ablation. Patients selection and inclusion in the study was made according with the current guidelines1,14, between January 2013 and December 2016. We enrolled prospectively and consecutively patients with symptomatic refractory paroxysmal AF referred for endocardial catheter ablation. Paroxysmal AF was defi ned as AF that terminates spontaneously or with intervention within 7 days of onset14. Persistent AF was defi ned as recurrent AF that is sustained for seven days14. In addition, patients with continuous AF who undergo cardioversion within seven days were classified as having paroxysmal AF if the cardioversion was performed within 48 hours of AF onset, and persistent AF if the cardioversion was performed more than 48 hours after AF onset14. Inclusion criteria were: symptomatic patients age ³ 18 years and documented refractory paroxysmal AF referred for endocardial catheter ablation. Patients were fully informed about the nature of the study and provided written informed consent which was approved by the local Ethics Committee. Exclusion criteria were: patients with valvular AF, patients with advanced left ventricular systolic function impairment (ejection fraction <35%), patients with significant chronic obstructive pulmonary disease (peak expiratory fl ow <40%), co-morbidity with reduced life expectancy, anticipated protocol non-compliance that would limit follow-up, presence of left atrial or left atrial appendage thrombus (detected by bi-dimensional transesophageal echocardiography), pregnant women or planned to become pregnant during the study, and patients who refused to sign informed consent.
Endocardial catheter ablation and ganglionated plexi stimulation The ablation procedure consisted of endocardial antral isolation of each pulmonary vein antrum by radiofrequency energy using a Lasso™catheter (BiosenseWebster, CA, USA) and an irrigated tip Celsius™ Thermo Cool (Biosense Webster, CA, USA) or cryoablation (ICE® Cryoablation System, Medtronic, USA), as currently recommended15. After pulmonary vein isolation at each site of positive vagal response to high frequency stimulation (20 Hz, 2 ms, 20 mA) by pacing with a quadripolar electrophysiological catheter type (2-2-2 mm, St. Jude Medical) additional endocardial applications energy was applied. High frequency stimulation was repeated and additional energy applications were applied until the vagal response to high frequency stimulation was eliminated. In patients with cryotherapy we did not use another position for cryoballoon than conventional. High frequency stimulation was applied after cryoballoon was retired; the same transseptal puncture and quadripolar electrophysiological catheter were used for high frequency stimulation in patients with cryotherapy. A positive vagal response of major GP clustered on the left atrial posterior wall to high frequency stimulation was defi ned as R-R intervals increasing with ≥50% during 5 seconds and/or as pauses. Antral pulmonary vein isolation was obtained at 35W (by radiofrequency energy) and minus 40° (by cryotherapy). In patients with paroxysmal AF longer than 24 hours we used exclusively radiofrequency energy and ablation procedure was completed by complex fractionated atrial electrogram and cavo-tricuspid istmus ablation (if common atrial fl utter was confi rmed before or during procedure). Both procedures were done using local anesthesia by femoral access and deep sedation with morphine fractionated intravenous during energy application.
Pre and Postablation Care and Follow-up
Before the procedure a 12 leads ECG recording, polysomnography, transthoracic and transesophageal echocardiography and computer-tomography of the pulmonary veins were performed in all patients. Antiarrhythmic medications except amiodarone was stopped more than fi ve half-lives prior to ablation. All patients were taking acenocumarol during a minimum 4-8 weeks before intervention. Documented INR (International Normalized Ratio) between 2 and 3 was mandatory for at least 3 times prior to the procedure. Acenocumarol was continued during the procedure for an INR value about 2. Transesophageal echocardiography was performed 24 hours before intervention and during the procedure. An intracardiac thrombus was ruled out in all patients. After the procedure the patients were clinically monitored during in-hospital stay for 2 or 3 days. The follow-up planned at 1, 3 and 6 months consisted in: clinical examination, 12 leads ECG, transthoracic echocardiography and 24 hours Holter monitoring. Adverse events were evaluated during hospitalization, at every follow-up and anytime during the study by the cardiologist. Antiarrhythmic and anticoagulation drugs were continued in all patients until the fi rst followup. A success rate at 6 months was defi ned as stable sinus rhythm confi rmed by 24 hours ECG Holter monitoring. A redo procedure was decided at 6 months depending on AF burden revealed by 24 hours ECG Holter monitoring. This study is part of a larger one analyzing the efficacy of catheter ablation in refractory symptomatic AF who has been approved by the local Hospital Ethics Committee.
Statistical analysis
Data are presented as frequency distributions and simple percentages. Continuous variables are expressed as mean ± standard deviation. Statistical analysis was performed using SPSS 11.5 for Windows (SPSS Inc., Chicago, IL, USA). A p value ≤0.05 was considered significant.
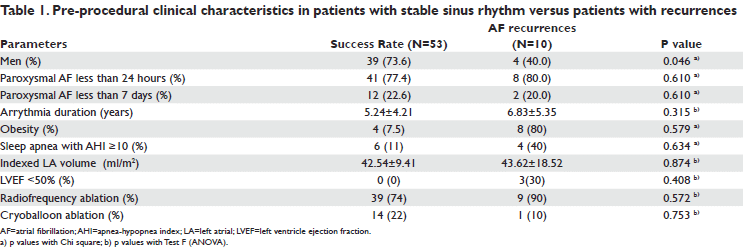
RESULTS
A totally of 63 patients were included; all patients were with paroxysmal AF; mean age of 57±8 years; 66.7% men; mean BMI 28.7±4 kg/m2; 61% have had arterial hypertension, 34% coronary heart disease and 19% diabetes mellitus. Mean paroxysmal AF duration was 4.7±1.7 months. Circumferential or segmental pulmonary vein isolation was performed by radiofrequency energy in 48 (76%) patients and cryotherapy in 15 (24%) patients. At 6 months 53 (84%) patients were in stable sinus rhythm, 18 (34%) out of those patients being on antiarrhythmic drugs. At 6 months AF recurrences was unregistered in 10 (15.9%) patients. A redo (second) procedure was therefore decided in these patients. Clinical characteristics in patients with stable sinus rhythm versus patients with recurrences are presented in Table 1. AF recurrences were more frequently in obese patients, with sleep apnea and low left ventricle ejection fraction. Cryoballoon ablation seems to be more effi cacy than radiofrequency. Recurrences were more frequently in radiofrequency group (23% versus 7%; p=0.037), without any significant complication in both groups. High frequency stimulation of GP induced a positive vagal response to high frequency stimulation of at least one GP before pulmonary vein isolation in 40/63 patients (64%). SLGP was most frequently implicated in a positive response to high frequency stimulation (n=34). RIGP, ARGP (Figure 1) and ILGP responded in 19, 16 and 9 patients, respectively. Eighteen out of 78 positive vagal responses were elicited in patients with cryotherapy. There were no differences in responses number in patients with radiofrequency ablation (60 responses in 48 patients) versus cryoablation (18 responses in 15 patients). After procedure, during high frequency stimulation only non-sustained AF or no responses were observed after pulmonary vein isolation. All patients witch have
had a positive response of at least one GP during procedure were in sinus rhythm at 6 months.
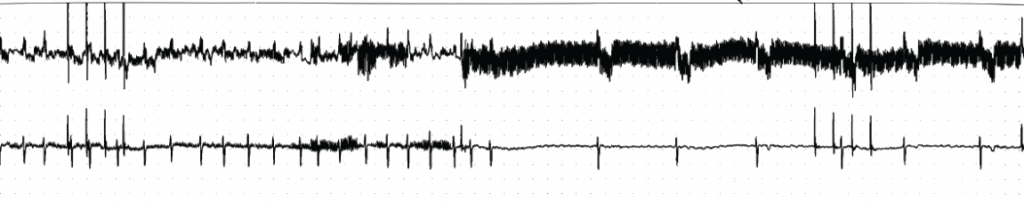
Figure 1. Significant positive vagal response to high frequency stimulation (larger segment of ECG in black) near to left superior pulmonary vein (the anatomic site of SLGP) in a male patient of 50 years old.
DISCUSSION
Treatment of AF rests a corner stone for every cardiologist. The persistent activity of some active epicardial non isolated GP could explain the recurrence rate of AF after surgical pulmonary veins isolation alone 16. It seems that electrical exclusion of the left atrial posterior wall that include the pulmonary veins (as trigger and substrate of AF) and hers adjacent major GP (as
a possible persistence factor in AF) with the complex interconnection neural network could leads at elimination of persistent AF17-20. This study aimed to assess the response of GP stimulation through an endocardial approach during catheter ablation in patients with refractory paroxysmal AF. Adding GP to other ablation targets may improve ablation success 21. This approach could allow for the elimination of AF in 2 ways: by modulation/ablation of GP (as component of intrinsic autonomic nervous system) and pulmonary veins for the all known cardiac autonomic nervous system infl uences by creation of a contiguous encircling lesion around the pulmonary veins and, by modifying in the same time the substrate, eliminating a potential perpetuation of AF through an electrical and structural reverse remodeling19,20. This procedure allowed a minimal amount of atrium myocardium destroyed. In addition, if “the octopus hypothesis”22 is true, energy applied on to the fat pads could directly destroys nerve cells rather than only axons. If the GP are not completely destroyed, partial injury of GP or interruption of the axons extending from the GP do probably occur. The communications between GP, as a potential trigger factor for AF, could be affected, which may alter autonomic input to the sinus node. However it has been shown that changes in sinus node activity are similar in patients with only circumferential ablation (without GP ablation) to patients following GP ablation (without circumferential pulmonary veins ablation)21. Probably the active GP could be an important maintaining factor in AF persistence. Therefore interaction between the major GP could be more intricate and implicate in persistence of AF than already shown. In this study all patients with positive GP response were in sinus rhythm at 6 months. The absence of any response of GP before ablation in some patients could be due to diffi culty in reach GP in endocardial approach. The negative response after catheter ablation is probably related to extensive endocardial radiofrequency applications on the pulmonary veins-atrium junction followed by GP areas destruction. LSGP was more frequently implicated in positive responses to high frequency stimulation because is more accessible during endocardial approach. However using an endocardial approach is more diffi cult to identify the major GP than the epicardial one because direct visualization is combined with high frequency stimulation. AF recurrences were more frequently in patients with risk factors for AF. It is well know that aggressive risk factor management improved the long-term success of AF ablation23. Despite of non-conversion to sinus rhythm during the procedure, the absence of extrinsic and intrinsic ANS infl uences could results in a reversal structural and electrical remodelling, with spontaneously conversion to sinus rhythm or paroxysmal AF within the 3 or even 6 months post-procedure. We observed a statistically non-signifi cant reduction of LA volume in patients who converted and remained in sinus rhythm (form 42.54±9.41 ml/m2 versus 38±10 mL, p=0.62). However we had fewer patients to be a statistically signifi cant LA volume reduction. Despite of this it means a profound modifi cation of the substrate by the GP modulation/ablation procedure that could permit conversion in sinus rhythm. This study suggests that modulation/ablation of GP by endocardial catheter approach could be an effective and benefi cial therapeutic intervention for patients with refractory symptomatic AF, and might have better long-term outcomes. The clinical evidence to date seems to support the use of GP ablation as an adjunctive procedure in AF ablation. It is very diffi cult to identify in this small clinical study the relationship between GP responses, AF duration and sinus rhythm conversion. The results of other study are concordantly with similar studies published in the literature. For example, a study with similar number of patients and GP modulation technique shown that addition of anatomic GP modifi cation to pulmonary vein isolation confers signifi cantly better outcomes than pulmonary vein isolation alone during a 12 months or even 2-year followup period24,25. On the contrary, other authors shown that the presence of GP responses after extensive pulmonary vein isolation was signifi cantly associated with increased AF recurrence after ablation in patients with paroxysmal AF26. In addition, GP ablation during other more invasive techniques like thoracoscopic surgery for advanced AF has no detectable effect on AF recurrence but causes more major adverse events, major bleeding, sinus node dysfunction, and pacemaker implantation27. In the literature there are some important gender differences in AF ablation. For example, female sex was found to be a predictor of recurrence of AF28. In addition, it seems that females have more bleeding complications than males29. In this small study we did not analyze the predictors of recurrences depending on gender, due to the small number of patients. However, we could not confirm more complications in females. According with literature data, fi rst-line ablation for paroxysmal AF is safe and effective with either radiofrequency or cryoballoon ablation, with is a trend for more recurrences and complications after radiofrequency ablation than cryotherapy30. In our study due to the small number of patients with cryotherapy (n=15) it is difficult to assess more comparative data between the two groups. However, cryotherapy seems to have higher efficacy without more severe complications. However, the efficacy of this approach on the longterm in curing refractory persistent AF remains to be demonstrated.
Limitations of the study
This is a small prospective non-randomized study, without a control group. All patients included in the study were with paroxysmal AF; 49 patients (78%) have had paroxysmal AF less than 24 hours and 14 patients (22%) have had paroxysmal AF less than 7 days. Therefore, it might be discussed about a kind of inhomogeneous study group even all patients according with current definition were with paroxysmal AF. The small number of patients included in the study made more difficult interpretation of the results in the subgroups study. For example, it cannot be excluded the contribution of obesity or left ventricular dysfunction or AF duration when analyzing comparative data in patients with recurrences versus success rate, even the results are non-signifi cant statistically. This technique has been used in vagally mediated AF but also investigated in paroxysmal and non-paroxysmal AF. Clinical studies demonstrate significant discrepancy related with detection of vagal ganglionated plexi sites or ablation targets and definition of procedural end-points. The relationship between AF duration, sinus rhythm conversion and GP responses remains unclear. Obviously learning curve had an important role for the first experience with this technique. However, when considering ablation of GP, it is important to recognize that it is currently not possible to selectively ablate GPs without ablating atrial myocardium (as substrate of AF) and therefore without modulating the substrate.
CONCLUSION
Successful identification of autonomic ganglia through an endocardial approach can be reliably achieved. The absence of any response of ganglionated plexi to high frequency stimulation, immediately after major ganglionated plexi ablation, is probably due to the complete destruction of ganglionated plexi. However this seems to have better outcomes in patients with paroxysmal
AF.
Conflict of interest: none declared
Funding: This work was supported by the Project entitled: Expanding and upgrading an Atrial Fibrillation Treatment Research Center as a method of preventing heart failure by developing the research and development infrastructure.
References
1. ACC/AHA/ESC 2006 Guidelines for the Management of Patients with Atrial Fibrillation. A Report of the American College of Cardiology/ American Heart Association Task Force on Practice Guidelines and the European Society of Cardiology Committee for Practice Guidelines (Writing Committee to Revise the 2001 Guidelines for the Management of Patients With Atrial Fibrillation). Circulation 2006;114 :257-354.
2. Ardell JL: Structure and function of mammalian intrinsic cardiac neurons. In: Armour JA, Ardell JL, eds: Neurocardiology. Oxford Press: New York, 1994, pp. 95-114.
3. Horikawa-Tanami T, Hirao K, Furukawa T, Isobe M. Mechanism of the Conversion of a Pulmonary Vein Tachycardia to Atrial Fibrillation in Normal Canine Hearts: Role of Autonomic Nerve Stimulation. J Cardiovasc Electrophysiol 2007(18):534-541
4. Patterson E, Po SS, Scherlag BJ, Lazzara R. Triggered firing in pulmonary veins initiated by in vitro autonomic nerve stimulation. Heart Rhythm 2005;2:624-631.
5. Verrier RL, Antzelevitch C. Autonomic aspects of arrhythmogenesis: The enduring and the new. Curr Opin Cardiol 2004;19:2-11.
6. Hou Y, Scherlag BJ, Lin J, Zhou J, Song J, Zhang Y, Patterson E, Lazzara R, Jackman W M, Po SS. Interactive atrial neural network: Determining the connections between ganglionated plexi. Heart Rhythm 2007;4:56-63.
7. Hou Y, Scherlag BJ, Lin J, Zhang Y, Lu Z, Truong K, Patterson E, Lazzara R, Jackman WM and Po SS. Ganglionated Plexi Modulate Extrinsic Cardiac Autonomic Nerve Input Effects on Sinus Rate, Atrioventricular Conduction, Refractoriness, and Inducibility of Atrial Fibrillation J Am Coll Cardiol, 2007;50(1):61–68.
8. Nakagawa H, Scherlag BJ, Lockwood D, Wolf RK, Peyton M, Wu R, Yokoyama K, Po SS, Herring L, Lazzara R, Jackman WM, and Armour JA: Localisation of Left Atrial Autonomic Ganglionated Plexuses using Endocardial and Epicardial High Frequency Stimulation in Patients with Atrial Fibrillation. Heart Rhythm 2005;2(5):S10-S11.
9. Arora RC, Waldmann M, Hopkins DA, Armour JA.. Porcine intrinsic cardiac ganglia. Anat Rec 2003;271A:249–58.
10. Quan KJ, Lee JH, Geha AH, Biblo LA, Van Hare GF, Mackall JA, Carlson MD. Characterization of sinoatrial parasympathetic innervation in humans. J Cardiovasc Electrophysiol 1999;10:1060 –1065.
11. Quan KJ, Lee JH, Van Hare GF, Biblo L, Carlson M. Identifi cation and characterization of atrioventricular parasympathetic innervation in humans. J Cardiovasc Electrophysiol 2002;13:735-739.
12. Yuan BX, Ardell JL, Hopkins DA, Losier AM, Armour JA. Gross and microscopic anatomy of the canine intrinsic cardiac nervous system. Anat Rec 1994;239:75–87.
13. Po SS, Scherlag BJ, Yamanashi WS, Edwards J, Zhou J, Wu R, Geng N, Lazzara R, Jackman WR. Experimental model for paroxysmal atrial fi – brillation arising at the pulmonary vein-atrial junction. Heart Rhythm 2006;3:201-208.
14. American College of Cardiology/American Heart Association Task Force on Practice Guidelines. 2014 AHA/ACC/HRS guideline for the management of patients with atrial fi brillation: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines and the Heart Rhythm Society. J Am Coll Cardiol 2014;64(21):e1-76.
15. 2012 HRS/EHRA/ECAS Expert Consensus Statement on Catheter and Surgical Ablation of Atrial Fibrillation: recommendations for patient selection, procedural techniques, patient management and
follow-up, defi nitions, endpoints, and research trial design. Europace 2012;14(4):528-606.
16. Bagge L, Blomstrom P, Nilsson L, Einarsson GM, Jideus L, Blomstrom- Lundqvist C. Epicardial off-pump pulmonary vein isolation and vagal denervation improve long-term outcome and quality of life in patients with atrial fi brillation. J Thorac Cardiovasc Surg 2009;137:1265-71.
17. Misaki T, Fukahara K, Recent topics on the surgical treatment for atrial fibrillation. Ann Thorac Cardiovasc 2004;10:277-80.
18. Zhou J, Scherlag B, Edwards J, WM Jackman, Lazzara R, Po SS. Gradients of atrial refractoriness and inducibility of atrial fi brillation due to stimulation of ganglionated plexi. J Cardiovasc Electrophysiol 2007;18:83-90.
19. Scanavacca M, Pisani CF, Hachul D, Lara S, Hardy C, Darrieux F, Trombetta I, Negrão CE, Sosa E. Selective atrial vagal denervation guided by evoked vagal refl ex to treat patients with paroxysmal atrial fi brillation. Circulation 2006;114:876-885.
20. Nadeau R, Cardinal R, Armour JA, Kus T, Richer L-P, Vermeulen M, Yin Y, Pagé P. Cervical vagosympathetic and mediastinal nerves activation effects on atrial arrhythmias formation. Anadolu Kardiyol Derg 2007:7Suppl1:34-6.
21. Zhang Y, Wang Z, Zhang Y, Wang W, Wang J, Gao M, Hou Y. Effi cacy of cardiac autonomic denervation for atrial fi brillation: a meta-analysis. J Cardiovasc Electrophysiol 2012;23:592–600.
22. Hwang M, Lee HS, Pak HN, Shim EB. Inducibility of human atrial fibrillation in an in silico model refl ecting local acetylcholine distribution and concentration. Korean J Physiol Pharmacol 2016;20(1):111-7.
23. Pathak RK, Middeldorp ME, Lau DH, Mehta AB, Mahajan R, Twomey D, Alasady M, Hanley L, Antic NA, McEvoy RD, Kalman JM, Abhayaratna WP, Sanders P. Aggressive risk factor reduction study for atrial fi brillation and implications for the outcome of ablation: the ARRESTAF cohort study. J Am Coll Cardiol 2014;64(21):2222-31.
24. Katritsis DG, Giazitzoglou E, Zografos T, Pokushalov E, Po SS, Camm AJ. Rapid pulmonary vein isolation combined with autonomic ganglia modifi cation: a randomized study. Heart Rhythm 2011;8(5):672-8.
25. Katritsis DG, Pokushalov E, Romanov A, Giazitzoglou E, Siontis GC, Po SS, Camm AJ, Ioannidis JP. Autonomic denervation added to pulmonary vein isolation for paroxysmal atrial fi brillation: a randomized clinical trial. J Am Coll Cardiol 2013;62(24):2318-25.
26. Kurotobi T, Shimada Y, Kino N, Ito K, Tonomura D, Yano K, Tanaka C, Yoshida M, Tsuchida T, Fukumoto H. Features of intrinsic ganglionated plexi in both atria after extensive pulmonary isolation and their clinical signifi cance after catheter ablation in patients with atrial fi brillation. Heart Rhythm 2015;12(3):470-6.
27. Driessen AHG, Berger WR, Krul SPJ, van den Berg NWE, Neefs J, Piersma FR, Chan Pin Yin DRPP, de Jong JSSG, van Boven WP, de Groot JR. Ganglion Plexus Ablation in Advanced Atrial Fibrillation: The AFACT Study. J Am Coll Cardiol 2016;68(11):1155-1165.
28. Curtis AB, Narasimha D. Arrhythmias in women. Clin Cardiol 2012;35:166-171.
29. Patel D, Mohanty P, Di Biase L, Sanchez JE, Shaheen MH, Burkhardt D, Bassouni M, Cummings J, Want Y, Lewis WR, Diaz A, Horton RP, Beheiry S,Hongo R, Gallinghouse GJ, Zagrodzky JD, Bailey SM, Al-Ahmad A, Wang P, Schweikert RA, Natale A. Outcome and complications of catheter ablation for atrial fi brillation in females. Heart Rhythm 2010;7:167-172.
30. Straube F, Dorwarth U, Ammar-Busch S, Peter T, Noelker G, Massa T, Kuniss M, Ewertsen NC, Chun KR, Tebbenjohanns J, Tilz R, Kuck KH, Ouarrak T, Senges J, Hoffmann E; FREEZE Cohort Investigators. Firstline catheter ablation of paroxysmal atrial fi brillation: outcome of radiofrequency vs. cryoballoon pulmonary vein isolation. Europace 2016;18(3):368-75.
 This work is licensed under a
This work is licensed under a