Andreea Cuculici1, Adrian Mereuta1, Carmen Ginghina1
1 Emergency Institute for Cadiovascular Diseases “Prof. Dr. C. C. Iliescu”, Bucharest, Romania
Abstract:
Patients presenting with non ST-segment elevation myocardial infarction (NSTEMI) represent a wide spectrum of coronary artery disease severity and, therefore, have major differences in the outcome. Rapid risk stratification in patients with NSTEMI is crucial for appropriate management, especially for very high risk patients, who benefi t from an early invasive approach. The ECG remains the most accessible used diagnostic tool for guiding emergent treatment strategies. The ECG recorded during acute myocardial ischemia has diagnostic, therapeutic and prognostic significance. When confronted with ST – T abnormalities, the main challenge is to differentiate between a primary and secondary ST depression due to other pathologies. If there is a clinical high index of myocardial infarction and the ECG is not diagnostic, serial ECGs at 5-10 min intervals are needed.
Keywords: non ST-segment elevation myocardial infarction, ECG patterns, risk stratification
The Third Universal Definition of myocardial infarction (MI) consensus document defines MI by the evidence of myocardial necrosis in a clinical setting suggestive for acute myocardial ischemia, proved by a rise and/or fall of cTn, with at least one value above the 99th percentile of a normal reference population in the presence of at least one of the following: a) symptoms
consistent with myocardial ischemia; b) new or presumed new signifi cant ST-segment or T-wave changes or new left bundle-branch block; c) development of pathological Q waves on the electrocardiogram; d) imaging evidence of new loss of viable myocardium such as new regional wall motion abnormalities; or e) identification of an intracoronary thrombus either by angiography or autopsy1. Myocardial ischemia may occur during two pathophysiologic processes: decreased blood supply, in which a coronary artery has been acutely occluded by a thrombus or vasospasm, or a mismatch between increased demands due to excess cardiac work (caused by exercise or other stress) and inadequate blood supply in the presence of nonoclusive coronary artery disease (CAD).
On the basis of the above criteria, MI is diagnosed in instances in which a supply/demand imbalance leads to myocardial injury with necrosis that is not caused by acute coronay syndrome (ACS), including arrhythmias, aortic dissection, severe aortic valve disease, hypertrophic cardiomyopathy, shock, respiratory failure, severe anemia, hypertension with or without left ventricular
hypertrophy, coronary spasm, coronary embolism or vasculitis, and coronary endothelial dysfunction without significant CAD2,4. Patients with myocardial ischemia secondary to a decreased supply typically present with two types of electrocardiogram (ECG) patterns: a) predominant ST segment elevation acute coronary syndrome, and are classified as having either “aborted myocardial infarction (MI)” or ST-elevation MI (STEMI) based on the presence or absence of biomarkers of myocardial necrosis; and b) patients without predominant ST segment elevation on the 12-lead ECG – non-ST elevation ACS5. Patients presenting with NSTEMI represent a wide spectrum of CAD severity and, therefore, have major differences in the outcome. Urgent reperfusion with thrombolytic therapy has been proven to be beneficial only in patients presenting with ST-segment elevation, whereas in the general group without ST-segment elevation, including those with ST-segment depression (Figure 1,2), fl at or negative T wave and even normal or unchanged ECG, thrombolytic therapy may even be harmful, while an early invasive strategy (percutaneous coronary intervention/urgent CABG) is superior to a conservative approach in high-risk patients with NSTEMI. Rapid risk stratifi cation in patients with NSTEMI is crucial for appropriate management, especially for higher-risk patients, who benefit from an early invasive approach. Therefore, the timing of angiography and revascularization should be based on patient risk profile3.
Patients at very high risk (haemodynamic instability or cardiogenic shock, recurrent or ongoing chest pain refractory to medical treatment, life-threatening arrhythmias including conduction disturbances or cardiac arrest, mechanical complications of MI, acute heart failure, recurrent dynamic ST-T wave changes (Figure 3,4), particularly with intermittent ST-elevation) should
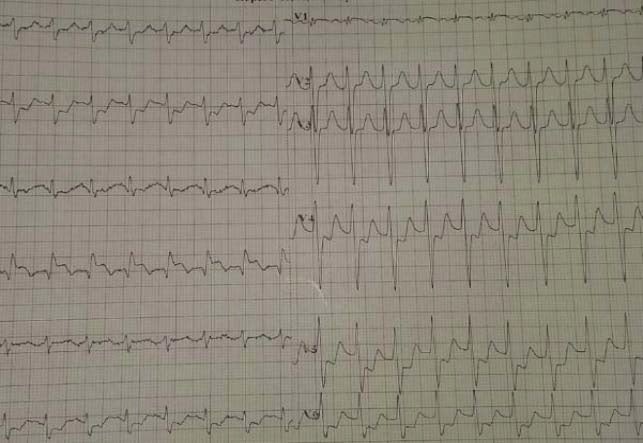
be considered for urgent coronary angiography (in less than 2 hours). In patients at high risk (at least one of the following criteria: rise or fall in cardiac troponin compatible with MI, dynamic ST- or T-wave changes (symptomatic or silent – Figure 5), GRACE score >140) an early invasive strategy within 24 hours appears to be the reasonable strategy. In intermediate-risk cases
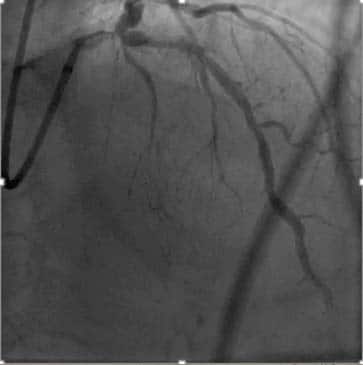
(with a GRACE risk score under 140, diabetes mellitus, LVEF<40% or congestive heart failure, the invasive evaluation can be delayed without increased risk but should be performed preferably within 72 hours of admission (Figure 6,7). In low-risk patients without recurrent symptoms (none of the above criteria met), a noninvasive assessment of inducible ischaemia should
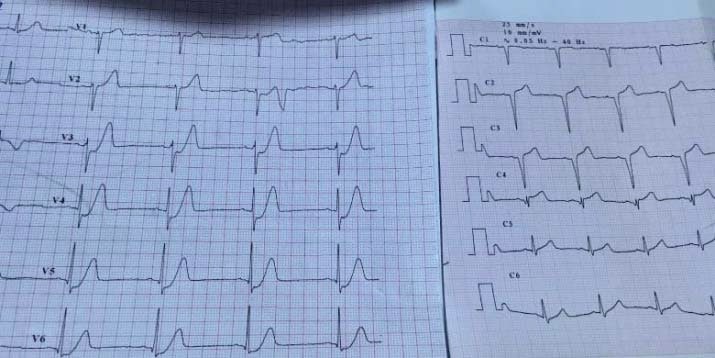
be performed before hospital discharge3. The ECG remains the most accessible used diagnostic tool for guiding emergent treatment strategies. The ECG recorded during acute myocardial ischemia has diagnostic, therapeutic and prognostic significance. ECG changes may include transient ST-segment elevation, persistent or transient ST-segment depression, T-wave inversion, flat T waves or pseudo-normalization of T waves or the ECG may be normal. Therefore, it is paramount to indentify the patients having anatomically or functionally severe coronary obstruction based on standard 12- lead ECG interpretation. When ischemia is confined primarily to the subendocardium, the overall ST vector typically faces the inner ventricular layer and the ventricular cavity such that the surface ECG leads show ST-segment depression. This subendocardial ischemic pattern is a frequent fi nding during spontaneous episodes of rest angina. In cases of severe extensive subendocardial ischemia, as in acute subtotal occlusion of the left main coronary artery (LM), the injury vector may be seen as ST-segment depression in the majority of the ECG leads but as ST-segment elevation in lead aVR6. Localization of subendocardial ischemia from the ECG changes is not as straight-forward as in the case of regional transmural ischemia due to total vessel occlusion, but ST-segment depression and lead aVR ST-segment elevation have been established as ECG markers of poor outcome in NSTEMI7. The number of leads showing ST depression and the magnitude of ST depression are indicative of the extent of ischaemia and correlate with worse prognosis, while benefi tting from an early invasive treatment strategy. When confronted with ST – T abnormalities, the main challenge is to differentiate between a primary and secondary ST depression due to other pathologies. With primary ST depression, the T wave abnormalities are accompanied by a normal QRS, and likely reflect myocardial ischemia. In secondary ST depression, the T wave abnormalities are in response to QRS complex abnormalities or ventricular hypertrophy and are not reflecting ischemia (LBBB, RBBB). An interesting variant of hyperacute T-waves was first described in 2009 by Verouden and colleagues (Figure 8) and was found to represent complete LAD occlusion and therefore a STEMI-equivalent) (Figure 9). This pattern of up sloping ST-segment depression paired with a tall, prominent T-wave (de Winter T-waves) is present in about 2% of patients with LAD occlusion and these patients require immediate reperfusion8. Another pattern suggestive for subepicardial ischemia is the T-wave pseudonormalization (equivalent of hyperacute T waves). This phenomenon may occur when a patient has a re-occlusion of a recently reperfused artery.
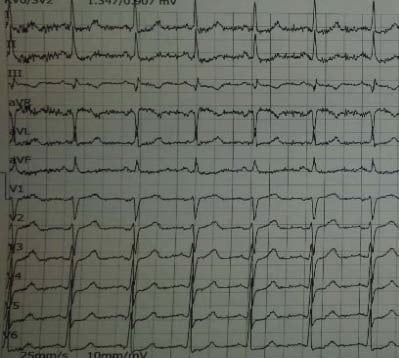
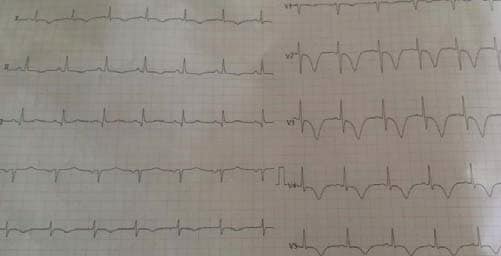
Figure 1. Sinus rhythm, diffuse widespread subendocardial ischaemia.
Figure 2. ECG during chest pain – sinus rhythm, 3 mm ST depression in DI,DII,DIII,aVL,V4-V6, ST elevation in aVR >1 mm.
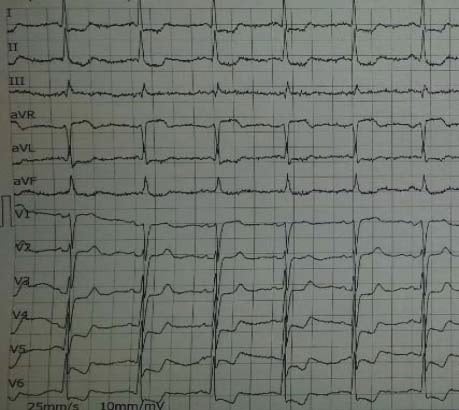
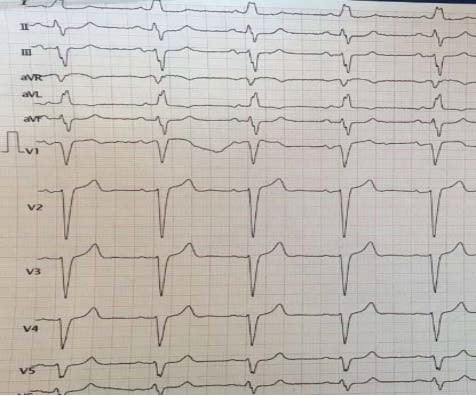
Figure 3. Patient with known diagnosis of angina – Sinus rhythm without ST-T changes in angor free period.
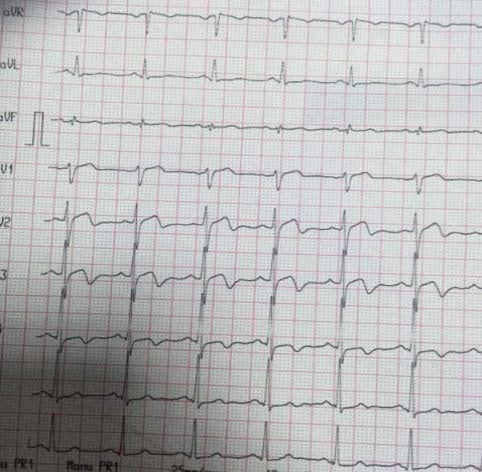
Figure 4. After chest pain- 1.5 mm ST depression in DI,DII,DIII,aVF,V3-V6, 1 mm ST elevation in aVR.
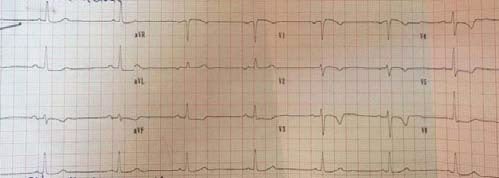
Figure 5. Same patient- Unstable angina- 5 mm max. ST depression in DI,DII,DIII,aVF,V3-V6, >2 mm ST elevation in aVR suggestive for extremely high risk profile.
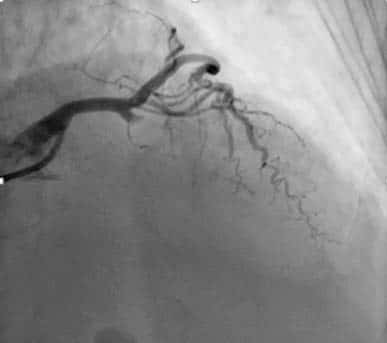
Figure 6. Coronary angiogram. Significant left main, proximal LAD and circumflex artery.
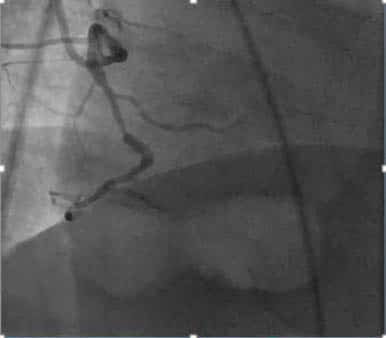
Figure 7. Coronary angiogram, serial significant right coronary artery.
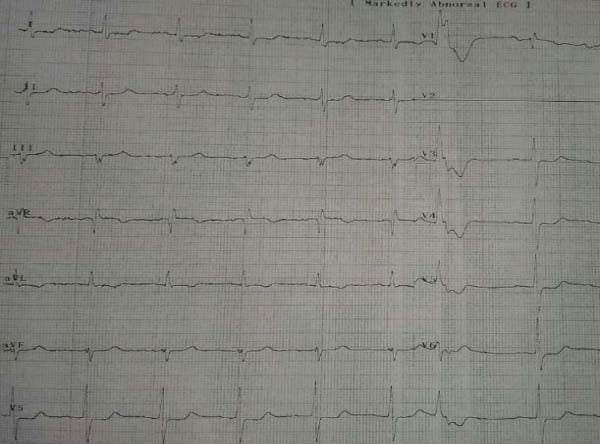
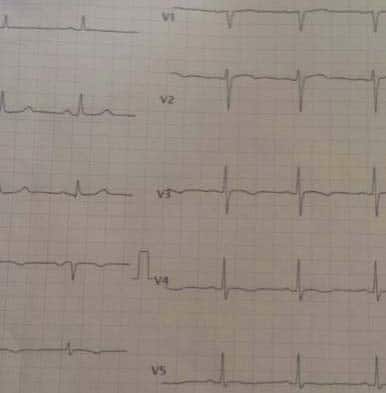
Figure 8. In the left, the ECG recorded immediately after the pain debuted: Poor R wave progression in V1-V3, up sloping ST-segment depression in V3- V6 and de Winter T waves in V2-V6. In the right, the ECG recorded later: QS in V1-V3, Q in V4, ST elevention of 3 mm in V2-V4.
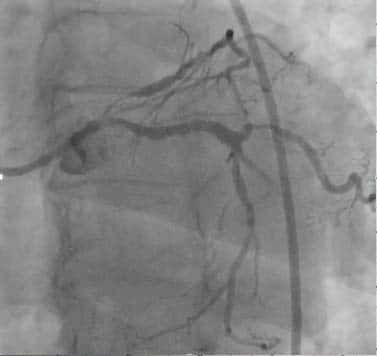
Figure 9. Coronary angiogram, occlusions left anterior descending artery (LAD).
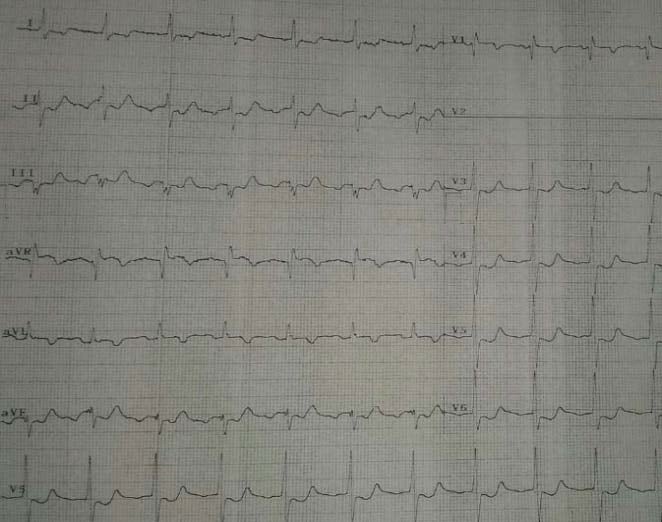
Figure 10. Different from the previous case: preserved precordial R wave
Figure 11. Coronary angiogram – LAD coronary artery subocclusion. progression, biphasic T waves in V2-4.
Figure 12,13. In the left, a non specific flattened T waves in the precordials leads and QT prolongation due to an ischemic stroke. In the right: Widespread deep T wave inversion in the precordials leads (V2-V6) and D1,aVL due to a hemorrhagic stroke.
Figure 14,15. Case of a female patient who presented with intermittent LBBB and in the bottom image we see the “memory” T waves (T wave inversion persisting in V1-V5, DIII, aVF).
Wellens’ syndrome – a pattern of deeply inverted or biphasic T waves in V2-39 developping after the angina attack, with preserved precordial R wave progression, no precordial Q waves (Figure 10), recent history of angina and normal or slightly elevated cardiac markers – which is highly specifi c for a critical stenosis of the left anterior descending artery (Figure 11) must be diligently recognized since these patients usually require early invasive therapy, due to the high risk of LAD occlusion10. Acute cerebrovascular events, especially hemorrhage, may present with diffuse precordial T wave inversions due to small vessel ischemia11. Although some suggest the QT interval is usually prolonged in these cases in opposition to NSTEMI/ACS, in most patients there is no proven way to differentiate between the two entities solely on the ECG (Figure 12,13). T wave inversion persisting after right ventricular pacing, LBBB or WPW are called “memory” T waves, caused by an unique phenomenon of electrical remodeling seen after periods of altered ventricular conduction wherein the T-wave direction during ‘memory’, i.e. during periods after altered depolarization, is similar to that of the QRS complex during periods of abnormal depolarization (Figure 14, 15). Although it appears to be a relatively benign pathophysiologic finding, T-wave inversions (TWI) due to cardiac memory may lead to confusion with NSTEMI/ACS and therefore to unnecessary testing and treatment12.
CONCLUSIONS
ECG is the mainstay of diagnosing NSTEMI which is a true medical emergency and making the correct diagnosis promptly is life-saving. If there is a clinical high index of MI and the ECG is not diagnostic, serial ECGs at 5-10 min intervals are needed. We should keep in mind the multiple ECG patterns of NSTEMI and always interpret them in clinical context. The ECG is the first
clinical tool that allowed assessment of myocardial ischaemia and despite multiple paradigm shifts in the management of ACS, it continues to be the pre-eminent test directing therapeutic management and prognostic stratification.
Conflict of interest: none declared.
References:
1. Third Universal Definition of Myocardial Infarction Kristian Thygesen, Joseph S. Alpert, Allan S. Jaffe, Maarten L. Simoons, Bernard R. Chaitman, and Harvey D. White: the Writing Group on behalf of the Joint ESC/ACCF/AHA/WHF Task Force for the Universal Definition of Myocardial Infarction
2. Supply/Demand Type 2 Myocardial Infarction – Yader Sandoval, MD*; Stephen W. Smith, MD†; Sarah E. Thordsen, MD†; Fred S. Apple, PhD – J Am Coll Cardiol. 2014;63(20):2079-2087. doi:10.1016/j.jacc. 2014.02.541
3. 2015 ESC Guidelines for the management of acute coronary syndromes in patients presenting without persistent ST-segment elevation- Authors/Task Force Members: Marco Roffi * (Chairperson) (Switzerland), Carlo Patrono* (Co-Chairperson) (Italy), Jean-Philippe Collet† (France), Christian Mueller† (Switzerland), Marco Valgimigli† (The Netherlands), Felicita Andreotti (Italy), Jeroen J. Bax (The Netherlands), Michael A. Borger (Germany), Carlos Brotons (Spain), Derek P. Chew (Australia), Baris Gencer (Switzerland), Gerd Hasenfuss (Germany), Keld Kjeldsen (Denmark), Patrizio Lancellotti (Belgium), Ulf Landmesser (Germany), Julinda Mehilli (Germany), Debabrata Mukherjee (USA), Robert F. Storey (UK), and Stephan Windecker (Switzerland)
4. Newby L.K., Jesse R.L., Babb J.D., et al; ACCF 2012 expert consensus document on practical guidelines considerations in the interpretation of troponin elevations: a report of the American College of Cardiology Foundation task force on Clinical Expert Consensus Documents. J Am Coll Cardiol.2012;60:2427-2463.CrossRef | PubMed
5. ACC/AHA guidelines for the management of patients with ST-elevation myocardial infarction–executive summary. A report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Writing Committee to revise the 1999 guidelines for the management of patients with acute myocardial infarction). Antman EM, Anbe DT, Armstrong PW, Bates ER, Green LA, Hand M, Hochman JS, Krumholz HM, Kushner FG, Lamas GA, Mullany CJ, Ornato JP, Pearle DL, Sloan MA, Smith SC Jr; American College of Cardiology; American Heart Association; Canadian Cardiovascular Society.
6. Electrocardiographic classification of acute coronary syndromes: a review by a committee of the International Society for Holter and Non-Invasive Electrocardiology. Nikus K1, Pahlm O, Wagner G, Birnbaum Y, Cinca J, Clemmensen P, Eskola M, Fiol M, Goldwasser D, Gorgels A, Sclarovsky S, Stern S, Wellens H, Zareba W, de Luna AB.
7. Significance of lead aVR in acute coronary syndrome- Akira Tamura ; World J Cardiol. 2014 Jul 26; 6(7): 630–637.
8. Verouden, NJ et al. Persistent precordial “hyperacute” T-waves signify proximal left anterior descending artery occlusion. Heart. 2009 Oct;95(20):1701-6.
9. Mattu A. (2012, April 3). Must know Wellens’ Syndrome mimic. Emergency ECG Video of the Week. Retrieved November 23, 2013. http://ekgumem.tumblr.com/post/20440438902/must-know-wellenssyndrome-mimic-episode.
10. Rhinehardt J, Brady WJ, Perron AD, Mattu A. Electrocardiographic manifestations of Wellens’ syndrome. Am J Emerg Med. 2002 Nov; 20(7):638-43.
11. Electrocardiographic abnormalities in acute cerebrovascular events in patients with/without cardiovascular disease- Mansoureh Togha, Alireza Sharifpour, Haleh Ashraf, Mansour Moghadam, and Mohammad Ali Sahraian
12. Rosenbaum MB, Blanco HH, Elizari MV, Lazzari JO, Davidenko JM. Electrotonic modulation of the T wave and cardiac memory. The American journal of cardiology 1982;50:213-22.
 This work is licensed under a
This work is licensed under a