Anca-Maria Popara-Voica1, Ruxandra Jurcut1,2, Adina Croitoru3, Ioana Dinu3, Dragos Alexandru4, Laura Dima2, Bogdan Alexandru Popescu1,2, Carmen Ginghina1,2
1 “Carol Davila” University of Medicine and Pharmacy, Bucharest, Romania
2 “Prof. Dr. C.C. Iliescu” Emergency Institute for Cardiovascular Diseases, Bucharest, Romania
3 Fundeni Clinical Institute, Bucharest, Romania
4 University of Medicine and Pharmacy of Craiova, Romania
Abstract: The use of antiangiogenic therapy has become an important therapeutic tool in oncology, leading to signifi-cantly improved outcomes. But, as its use expanded, it has become increasingly recognized that it also induces a wide spec-trum of toxicities, including cardiovascular, at higher than anticipated rates. We conducted a prospective study including 45 consecutive oncologic patients (31 men, (68.8%), with a mean age of 60.7±9.7 years (men 61.2±7.9, women 59.5±13.1)), with digestive and renal malignant tumors, receiving antiangiogenic therapies (bevacizumab, sunitinib, sorafenib), aiming to characterize the complex cardiovascular response to antiangiogenic agents, focusing mainly on cardiac dysfunction and arte-rial hypertension (HTN) and on the possible link between them. Patients were evaluated before the start of the antiangioge-nic therapy, at one month after they started the treatment, and then at 3-month intervals. The main findings of the present study were that antiangiogenic therapy induces worsening of pre-existing HTN or de novo HTN and also a reduction in all systolic function parameters of both ventricles, in almost half of the studied patients, stressing that it is important to assess both ventricles during cancer therapy. Although antiangiogenic therapy induced HTN may seem an obvious mechanism lea-ding to myocardial dysfunction, it is probably not the primary one, as we did find neither causality nor association between de novo HTN and left ventricular dysfunction. We did fi nd, however, that pre-existing HTN represents a major risk factor for patients receiving antiangiogenic treatment, as it usually gets worse and difficult to control, and correlates statistically with reductions in left ventricular ejection fraction meeting the cardiotoxicity criteria. It also tends to induce more frequent reductions of the global longitudinal strain of both left and right ventricles, but above the statistically signifi cant level. Left ventricular systolic dysfunction induced by antiangiogenic agents should not be regarded as a consequence of HTN, but as rather related to the intrinsic myocardial toxicity of these agents, pre-existing HTN being probably a major permissive/ precipitating factor.
Keywords: antiangiogenic therapy, anti-VEGF agents, bevacizumab, sunitinib, sorafenib, cardiovascular toxicities, arterial hypertension, global longitudinal strain, ejection fraction, myocardial function, speckle tracking echocardiography
INTRODUCTION
As survival of oncologic patients has significantly im-proved, the cardiac toxicity of oncologic treatments has become an increasingly challenging issue, both for oncologists and cardiologists. According to the con-cept of an “angiogenic switch”, the induction of angi-ogenesis is a key step in tumor growth and metastasis and it is mainly controlled by the vascular endothelial growth factor (VEGF)1,2. The VEGF signaling pathway has thus become an important therapeutic target in oncology, and the use of anti-VEGF agents has led to significantly improved outcomes in a variety of advan-ced solid tumors3. But, as the use of antiangiogenic therapy expanded, it has become recognized that it also induces a wide spectrum of toxicities, including cardiovascular complications (arterial hypertension (HTN), arterial and venous thromboembolic events, QTc interval prolongation, left ventricular dysfunction, and myocardial ischemia), at higher than anticipated rates4,5. Moreover, as these agents mainly target the endothelial cells rather than the tumor cells themsel-ves, many of their toxicities are unique from the ones reported with conventional chemotherapy agents.
In this context, knowing the possible cardiac toxi-cities of these agents and understanding their underli-ning mechanisms becomes highly important. As almost all of the existing studies on anti-VEGF therapy were not designed with standardized cardiac endpoints, and only clinically signifi cant cardiovascular events were recorded, the actual incidence of the cardiotoxicity of these agents in unselected cancer patient populations remains yet unknown and highlights the importance of further prospective studies with appropriate cardio-vascular surveillance.
The aim of our study was to characterize the com-plex cardiovascular response to anti-VEGF agents, focusing mainly on cardiac dysfunction and its deter-minants.
METHODS
Study population
During January 2015 and January 2016, we conducted a prospective study including consecutive oncologic patients, with digestive and renal malignant tumors, receiving anti-VEGF therapies (bevacizumab, sunitinib, sorafenib), referred from the Oncology Department of the “Fundeni Clinical Institute” to the Cardiology Department and EuroEchoLab of the “ Prof. Dr. C.C. Iliescu” Institute for Cardiovascular Diseases.
Exclusion criteria for our study were: non-sinus rhythm, more than moderate valvular heart disease, known coronary artery disease, low echogenicity for optimal echo data acquisition. The study protocol was approved by the institutional Research Ethics Board.
Clinical Data
Patients were evaluated before the start of the an-ti-VEGF therapy (Visit 1), at one month after they started the treatment (Visit 2), and then at 3-month intervals (Visits 3 and 4). The following clinical data were collected: age, gender, history of smoking, HTN (defined as a history of HTN requiring medical the-rapy), diabetes mellitus, and hypercholesterolemia. Functional status was defined according to the New York Heart Association (NYHA) classification.
Echocardiography
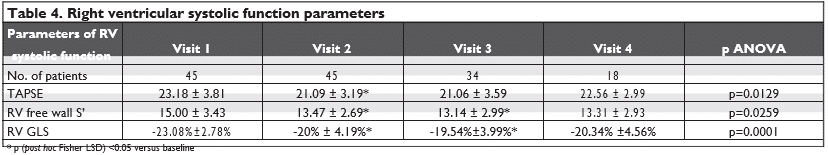
The transthoracic echocardiography examination was performed by the same operator, using a GE Vivid E9 Ultrasound Machine (GE Vingmed Ultrasound AS, Horten, Norway). All 2D and Doppler images were digitally stored as three consecutive cycles, while one cycle was stored for 3D images. All echocardiographic data were analyzed offline using a commercially availa-ble software package (EchoPAC PC version BT12; GE Medical Systems, Milwaukee, WI) by a single observer (AMPV). Transmitral flow velocities were measured from the apical four chamber view using conventional pulsed-wave Doppler imaging. Left ventricular ejecti-on fraction (LVEF) was calculated using the modified biplane Simpson’s method by 2D echocardiography. Tricuspid annular plane systolic excursion (TAPSE) was obtained using an M-mode cursor passed through the tricuspid lateral annulus in a four-chamber view and measuring the amount of longitudinal displace-ment of the annulus at peak-systole. Peak systolic (S’) and peak early diastolic (E’) mitral annular velocities were obtained by pulsed-wave tissue Doppler imaging (TDI) from the apical four-chamber view, using the septal and the lateral sites. Peak systolic velocity of the tricuspid annulus (RV free wall S’) was obtained by pulsed-wave TDI, in the view that achieved paral-lel alignment of the Doppler beam with the RV free wall. Analysis of myocardial global longitudinal strain (GLS) was performed offl ine for both the left ventricle (LV) and right ventricle (RV), by the speckle tracking technique using commercially available software (au-tomated function imaging (AFI)). The apical 4-, 3-, and 2-chamber images of the LV were used for the calcu-lation of the LV GLS (16-segment model) and an apical 4-chamber view of the RV was used for the calculation of RV GLS (6-segment model).
We defined cardiotoxicity as a decrease in the 2D LVEF of >10 percentage points from baseline or to a value <53%6,7. For the LV GLS, we interpreted that a >15 relative percentage reduction from baseline may suggest the risk of cardiotoxicity7. For the RV GLS, we tested the significance of arbitrary cutoffs of >15 and 20 relative percentage reductions from baseline.
Ambulatory blood pressure monitoring
All patients had a 24 hours ambulatory blood pressure (BP) monitoring (ABPM) at Visits 1, 2, 3 and 4. Avera-ge 24-hours, average daytime, and nighttime, as well as maximal BP values, were noted at each visit. We used a value above 130/80 mmHg of the average 24-hours blood pressure, on the 24 hours ABPM, to identify patients having arterial hypertension (HTN). All pati-ents identifi ed as having HTN at any time during the study received antihypertensive treatment with beta-blockers, angiotensin-converting enzyme inhibitors, and/or calcium channel blockers, based on the treating physician options.
Statistical analysis
Statistical analysis was performed using Microsoft Ex-cel (Microsoft Corp., Redmond, WA, USA), together with the XLSTAT add-on for MS Excel (Addinsoft SARL, Paris, France) and IBM SPSS Statistics 20.0 (IBM Corporation, Armonk, NY, USA) for processing the data. We used the Chi-square test ( 2) to assess the infl uence of the factors considered to measure cardiotoxicity over the other factors. It is a statistical test that shows if two factors are independent or not, and was used to interpret incidence tables generated by cross tabulation of the categorical coding of the recor-ded variables. Because the study involved comparisons between numerical data, we used either Student’s t test (for 2 groups) or ANOVA (for 3 or more groups) to compare the mean values of the analyzed parame-ters. If the ANOVA test result was statistically signifi-cant, we continued the analysis with “post hoc” tests, such as Fisher’s LSD tests, to identify pairs of catego-ries which had significant differences. A p value <0.05 was considered significant.
RESULTS
Study population characteristics
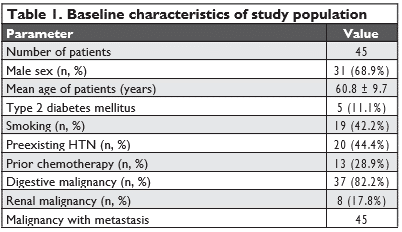
We included in the study 45 patients (31 men, 68.8%), with a mean age of 60.7±9.7 years (men 61.2±7.9, wo-men 59.5±13.1, p=0.58). The baseline characteristics of the patients are presented in Table 1. During the study, 18 patients died due to oncologic causes and 9 patients were lost to follow-up. Overall, 45 patients completed Visits 1 and 2, only 34 patients completed Visit 3 and only 18 patients completed Visit 4.
Left ventricular function parameters
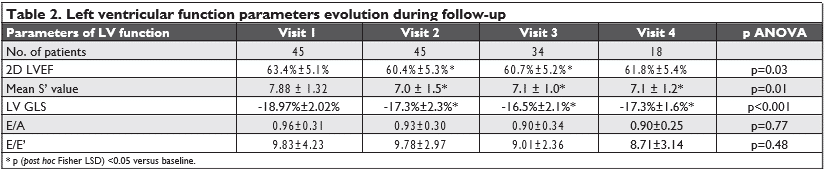
All patients had preserved 2D derived LVEF (≥53%) at the beginning of the anti-VEGF therapy. During the course of antiangiogenic therapy, there was a statisti-cally significant reduction in the parameters that are used to identify left ventricular systolic dysfunction: 2D LVEF, mean LV S’ value and LV GLS (Table 2). We found no statistically significant variations in the para-meters that are used to characterize left ventricular diastolic function: E/A and E/E’ values (Table 2).
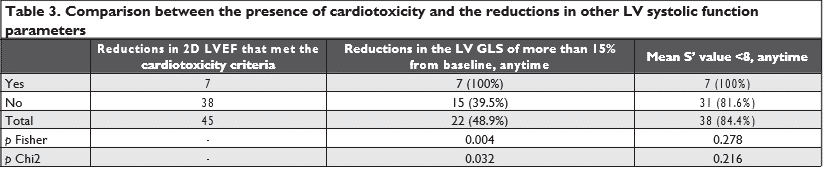
Seven patients (15.5%) presented with cardiotoxicity criteria during follow-up at Visit 2 (2D LVEF dropping from 63.8% ± 8.6% to 54.0% ± 7.2%, p=0.039), but were clinically asymptomatic. A reduction in the LV GLS of more than 15% from baseline was present in 22 patients (48.8%) at different time-points: 10 pati-ents at Visit2; 20 patients at Visit 3 (12 new patients and 8 patients that had a reduction also at Visit 2) and 2 patients at Visit 4 (who had also presented a reduc-tion at Visit 3).
When comparing the reductions in the LV GLS (of more than 15% from baseline) or in the mean S’ value (below 8 cm/s) with the reductions in 2D LVEF (that met the criteria for cardiotoxicity), we found that the reductions in 2D LVEF correlate with the reductions in the LVGLS, but not with the reductions in the mean S’ value (Table 3).
Right ventricular function parameters
Regarding the RV, we noted a statistically significant reduction, mainly until Visit 3, in the parameters of RV systolic function: TAPSE, RV free wall S’, and RV GLS (Table 4). By comparing the percentage reduction (vs baseline) of the LV GLS and RV GLS, we noticed that the reduc-tion of the RV GLS was significantly higher than that for the LV GLS (-13.5±13.6% vs -8.2±9.4%, p=0.03). As this reduction was observed very early (from Visit 2) after the start of the antiangiogenic treatment, we could not analyze if the RV GLS is affected earlier than the LV GLS (Figure 1).
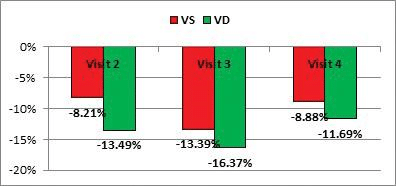
Figure 1. Comparison between the reductions from baseline of RV GLS and LV GL, during the antiangiogenic treatment (p value for Visit 2 =0.036; p value for Visit 3= 0.387; p value for Visit 4 = 0.644).
Arterial hypertension
Using ABPM, 14 patients (31.11%) had pre-existent HTN at baseline (Visit1), and they received antihyper-tensive treatment. After the start of the antiangioge-nic treatment, only 2 of these patients presented op-timally controlled BP values with medical treatment, while the rest of 12 patients (26.67%) presented wor-sening BP levels and needed further titration of the medication. During the antiangiogenic treatment, 13 patients (28.89%), who did not present HTN at baseli-ne, developed de novo HTN and were also started on antihypertensive medication (Table 5).

Arterial hypertension and ventricular function parameters
When comparing the influence of HTN on the reduc-tion in ventricular function parameters, we noticed that a reduction of LV GLS (of more than 15% from baseline) was more frequently encountered in patients with HTN at baseline, compared with patients who developed de novo HTN or patients with normal BP values. But, when testing the statistical significance of this observation, the result of the p Chi 2 test was 0.096 (above the maximum admitted cutoff of 0.05) (Figure 2). The same observation is true for a reduc-tion of RV GLS (of more than 20% from baseline), but the differences are even more subtle (p Chi 2 test= 0.332 >0.05) (Figure 3). However, for the reductions in the 2D LVEF (decrease of >10 percentage points from baseline, or to a value <53%), the observed diffe-rences are statistically significant, patients with HTN at baseline being more prone to develop cardiotoxi-city (p Chi 2 test=0.030 < 0.05) (Figure 4).
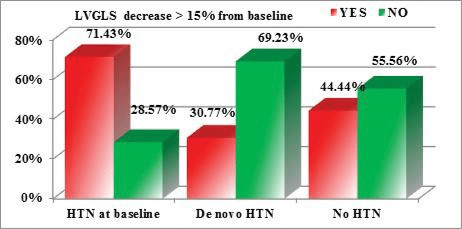
Figure 2. Correlations between the reduction of the LV GLS and the pres-ence/development of HTN (p Chi 2=0.096 >0.05).
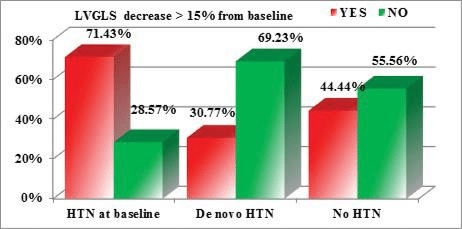
Figure 3. Correlations between the reduction of the RV GLS and the pre-sence/development of HTN (p Chi 2=0.332 >0.05).
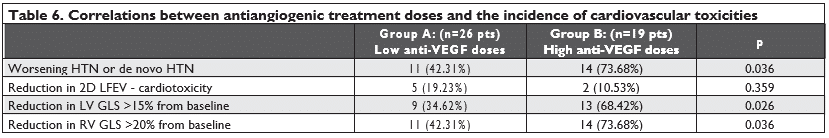
Influence of treatment doses, diabetes mellitus and age on the incidence of HTN and LV/RV systolic dysfunction
We also considered the doses of the anti-VEGF agents and tried to see if there is any correlation with the incidence of toxicities. We divided our patients into two groups:
– group A: patients who received smaller doses of anti-VEGF agents (bevacizumab 5 mg/kg or sora-fenib 400 mg or sunitinib 37.5 mg)
– group B: patients who received higher doses of anti-VEGF agents (bevacizumab 7.5 mg/kg or so-rafenib 800 mg or sunitinib 50 mg).
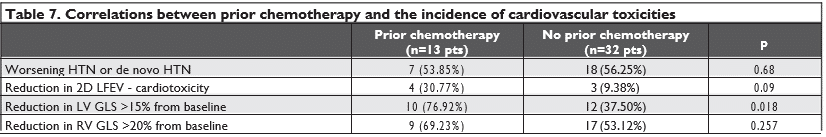
We found a significant statistical difference among the two groups, patients in group B presented a higher incidence of worsening HTN or de novo HTN and a higher incidence of significant reductions in LV GLS (with more than 15% from baseline) and in RVGLS (with more than 20% from baseline). We found no significant statistical difference regarding the reducti-ons in 2D LVEF that met the cardiotoxicity criteria (Table 6). We also found that patients with prior chemothe-rapy presented a higher incidence of significant reduc-tions in LV GLS (with more than 15% from baseline) but not a higher incidence of worsening HTN, de novo HTN, reductions in 2D LVEF (that met the cardiotoxi-city criteria) or reductions in RV GLS (with more than 20% from baseline) (Table 7).
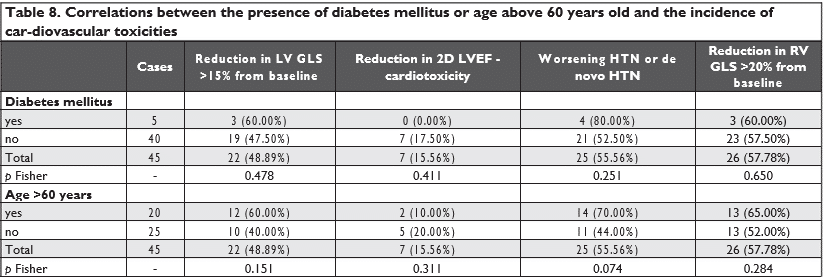
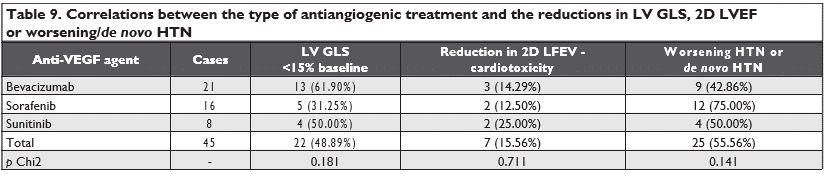
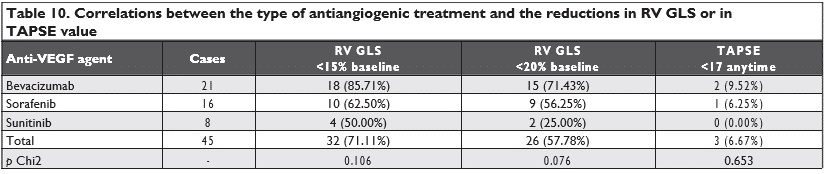
There were no statistically significant correlations between the reductions in LV GLS, RV GLS, 2D LVEF or worsening/de novo HTN and the presence of diabe-tes mellitus or with age above 60 years old (Table 8). Also, there were no differences between the three types of antiangiogenic agents used and the reductions in LV GLS, RV GLS, 2D LVEF, TAPSE or worsening/de novo HTN (Table 9 and Table 10).
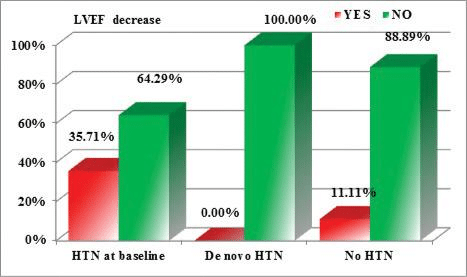
Figure 4. Correlations between the reduction of the 2D LVEF and the presence/development of HTN (p Chi 2=0.030 <0.05).
DISCUSSIONS
The main findings of the present study were that an-tiangiogenic therapy induces de novo HTN or wor-sening of pre-existing HTN and, also, a reduction in the systolic function parameters of both ventricles in almost half of the studied patients, stressing that it is important to assess both ventricles during can-cer therapy. This is in line with the ASE/EACVI Expert Consensus Statement on the multimodality imaging of adult patients receiving cancer therapy recommenda-tions, which highlight the importance of monitoring the left and also the right ventricular function parame-ters during cancer therapy6.
Although de novo HTN induced by anti-VEGF agents may seem an obvious mechanism leading to myocar-dial dysfunction, it is probably not the primary one, as we did find neither causality nor association betwe-en de novo HTN and cardiac dysfunction and, to our knowledge, these were not reported in any trial. We did find, however, that pre-existing HTN represents a major risk factor for patients receiving anti-VEGF treatment, as it usually gets worse and difficult to control, and correlates statistically with reductions in LVEF meeting the cardiotoxicity criteria. It also ten-ds to induce more frequent reductions of LVGLS and RVGLS, but above de statistically significant level.
Myocardial dysfunction induced by antiangiogenic agents should not be regarded only as a consequen-ce of HTN, but also as rather related to the intrinsic myocardial toxicity of these agents, pre-existing HTN being probably a major permissive/precipitating factor.
HYPERTENSION AND ANTIANGIOGENIC DRUGS
Among the cardiovascular toxicities of anti-VEGF agents, HTN is the most frequent, with a reported incidence of 19% to 47%8. Several mechanisms have been linked to the occurrence of VEGF inhibitor-rela-ted HTN: microvascular rarefaction, decreased nitric oxide and prostacyclin production, reduced lymphan-giogenesis, increased endothelin-1 production, incre-ased arterial stiffness and renal dysfunction9. HTN has been reported to be a dose-dependent adverse cardiac effect, with the greatest magnitude occurring rapidly, within hours to days after the beginning of the-rapy, and with a rapid decrease in BP associated with the withdrawal of the anti-VEGF agents9. Risk factors for anti-VEGF-induced HTN remain largely unknown.
In our study, we used 24 hours Holter BP moni-toring as an objective tool to identify patients having HTN. We found that 14 patients (31.11%) had pre-existent HTN before the start of antiangiogenic treat-ment, and they received treatment with beta blockers, angiotensin-converting enzyme inhibitors, and calcium channel blockers. After the start of the antiangioge-nic treatment, only 2 of these patients presented op-timally controlled BP values with medical treatment, while the rest of 12 patients (26.67%) presented wor-sening BP levels and needed further titration of the medication. Moreover, 13 patients (28.89%) presented de novo HTN, probably induced by the antiangiogenic treatment, and were also started on beta blockers, angiotensin-converting enzyme inhibitors, and calcium channel blockers.
Regarding the possible risk factors for HTN induced by the antiangiogenic treatment, we found that patients who received higher doses of anti-VEGF agents (group B) presented a higher incidence of worsening HTN or de novo HTN than patients who received smal-ler doses of anti-VEGF agents (p Chi 2=0.0359<0.05). There was no correlation between prior chemothe-rapy, diabetes mellitus, and age above 60 years old or the type of antiangiogenic medication with the risk of worsening or de novo HTN.
We noticed that de novo HTN induced by antian-giogenic agents responded better to the antihyper-tensive medication, most of the patients achieving BP targets with the initial doses and needed no further titration of the antihypertensive medication, compa-red with pre-existing HTN which tended to worsen and to be difficult to control. Moreover, we found no statistical correlation between de novo HTN and re-ductions in the parameters of ventricular function, but pre-existing HTN correlated significantly with reducti-ons in LVEF meeting the cardiotoxicity criteria (p Chi 2=0.030). It also presented the tendency to induce more frequent reductions of LVGLS and RVGLS, but above the statistically significant level (p Chi 2=0.096 and 0.332 respectively).
We reckon pre-existing HTN as a major risk factor for patients receiving anti-VEGF therapy, and these patients need special attention with aggressive antihypertensive treatment and a very close monitoring during antiangioge-nic treatment.
CARDIOTOXICITY OF ANTIANGIOGENIC DRUGS
Although there are no clear data on the actual inci-dence, pathophysiology, and reversibility of cardiac dysfunction, the potential of anti-VEGF inhibitors to induce cardiac dysfunction is certain10. The mechanism underlying this effect is not well understood. Although anti-VEGF induced de novo HTN may seem an obvious mechanism leading to cardiac dysfunction, it is proba-bly not the primary one, and neither the causality nor association between HTN and HF/ LV dysfunction was reported in any trial9.
In our study, during the antiangiogenic therapy, there was a significant reduction in all parameters of systolic function of both ventricles (LVGLS mean S’ value of LV, 2D LVEF, RV free wall S’ value, TAPSE, RVGLS).
We noticed a reduction in the LVGLS of more than 15% from baseline in 22 patients (48.88%). 7 patients (15.55%) presented a significant reduction in 2D LVEF that met the criteria for defining cardiotoxicity (as a decrease in the 2D LVEF of >10 percentage points from baseline or to a value <53%)6,7, but they were clinically asymptomatic. When comparing the reduc-tions in the LVGLS (of more than 15% from baseline) with the reductions in 2D LVEF (that met the criteria for cardiotoxicity) using the Chi 2 test, we found that the reductions in 2D LVEF correlate with the reducti-ons in the LVGLS (p Chi 2 =0.032<0.05). It is impor-tant to notice that the sensitivity of this test is 100%, meaning that all patients with a decrease in 2DLVEF had, at some point, a decrease in LVGLS (specifi city is 62.16%). Also, the negative predictive value of the Chi 2 test is 100%, which could be interpreted as such: patients that did not have a decrease in LVGLS will not have a decrease of 2DLVEF. But, this cannot be confirmed as, in our study, only 7 patients had a decrease in 2DLVEF, and all of them displayed it on Visit 2, when we had only the baseline value of LVGLS, and not an estimate evolution of LVGLS.
THE RELATION BETWEEN ARTERIAL HYPERTENSION AND CARDIAC DYSFUNCTION
We also tested for correlations between the reducti-on of the ventricular systolic function parameters and HTN. While de novo HTN induced by antiangiogenic treatment proved to be more easily controlled with antihypertensive medication and had no signifi cant impact on cardiac function parameters, pre-existing HTN was identified as a major risk factor for cardi-otoxicity. We noticed that a reduction in LVGLS of more than 15% from baseline was more frequently en-countered in patients with HTN at baseline, compared with patients who developed de novo HTN or patients with normal BP values. But, when testing the statistical signifi cance of this observation, the result of the Chi 2 test was 0.096 (above the maximum admitted cut-off of 0.05). The same observation is true for a reduction of RV GLS (of more than 20% from baseline), but the differences are even more subtle (p Chi 2 test= 0.332 >0.05). However, for the reductions in the LVEF (de-crease of >10 percentage points from baseline, or to a value <53%), the observed differences are statistically significant, patients with HTN as baseline being more prone to develop cardiotoxicity (p Chi 2 test=0.030 <0.05).
Also, patients in group B, who received higher do-ses of anti-VEGF agents, presented a higher incidence of significant reductions in LVGLS (with more than 15% from baseline) than patients who received smal-ler doses of anti-VEGF agents (p Chi 2=0.0165<0.05).
These results may imply that myocardial dysfunction induced by antiangiogenic agents should not be regarded only as a consequence of HTN, but also as rather related to the intrinsic myocardial toxicity of these agents, HTN being probably a major permissive/precipitating factor.
VEGF is critical for capillary density in the myocar-dium and stem-cell differentiation into cardiomyo-cytes9. Also, preclinical studies have shown that the role of VEGF signaling in the heart extends beyond angiogenesis and that it also mediates important com-pensatory responses to stress and injury9,11,12. In animal models with hypertrophied pressure-loaded hearts, VEGF reduced apoptosis and preserved contractile function by the promotion of capillary growth9,11,12. In mice with pressure-overloaded hearts due to trans-verse aortic constriction (TAC), inactivation of en-dogenous VEGF led to decreased capillary density, impaired cardiac hypertrophy and loss of contractile function9,11,13. Thus, inhibiting the VEGF pathway in the setting of HTN contributes to maladaptive hyper-trophy of cardiomyocytes and possibly LV dysfuncti-on9,11,14.
Also, all parameters of RV systolic function (free wall S’; RVGLS and TAPSE) presented reductions during the antiangiogenic treatment, and this may be another possible argument for the intrinsic myocardial toxicity of these agents. Moreover, we found that pa-tients who received higher doses of anti-VEGF agents (group B) presented a higher incidence of significant reductions in RVGLS (with more than 20% from ba-seline) (p=0.036) and that prior chemotherapy had no statistically significant impact on RVGLS reductions. A reduction of TAPSE below 17 mm was encountered in only 3 patients (6.66%). We tested for statistical correlations between the reductions in the RVGLS (using an arbitrary cutoffs of more than 20% from ba-seline) or in the right ventricular free wall S’ value by TDI (below 10 cm/s) with the reductions in TAPSE value below 17 mm and we did not find any signifi-cant correlation. We also compared the percentage reduction of the GLS for the left and right ventricles and noticed that the reduction of the RVGLS was sig-nifi cantly higher than that for the LVGLS, (t Student test p=0.0336 <0.05). It may be tempting to ask if rather the impact of chemotherapy is greater on the RV than on the LV, but it is more plausible to inter-pret this as related to the higher value of the RVGLS compared to the LVGLS and probably to the larger percentage reduction in the RVGLS that may indicate cardiotoxicity. There has been very little focus on the cardiotoxicity of oncologic treatments, in general, on the right ventricle and almost none on the toxicity of antiangiogenic agents. Considering the thinner struc-ture of the RV myocardium, with fewer myofibrils, the RV is probably also susceptible to the cardiotoxic effects of oncologic therapy. Although RV wall moti-on or functional abnormalities have been reported to occur during cancer therapy15-19 and even at the time of cardiotoxicity15, RV dysfunction is not considered in the diagnosis of cardiotoxicity and its incidence and prognostic value in this setting is unknown15. Our re-sults build on the existing literature by demonstrating that right ventricular systolic function parameters are also reduced during antiangiogenic therapy, stressing that it is important to assess both ventricles during cancer therapy. In fact, the ASE Expert Consensus Sta-tement on the multimodality imaging of adult patients receiving cancer therapy recommends to also monito-ring RV function during cancer therapy6. Also, having in view the perspective of using angiogenesis inhibitors in the treatment of pulmonary artery hypertension, we believe that our results will inspire discussion.
Most of the antiangiogenic agents, such as sunitinib and sorafenib, have been purposefully designed to be “multi-targeting” and nonselective, blocking several tyrosine kinase receptors, with broader anticancer efficacy but an increased likelihood of toxicity9. This aspect makes it difficult to identify which targets medi-ate cardiotoxicity9. The mechanisms of myocardial to-xicity induced by anti-VEGF agents should be regarded in relation to the “on-target” and “off-target” toxici-ties that these agents present9. “On-target” toxicity is when the intended targets of anti-VEGF agents are also implicated in normal cardiomyocyte survival, and thus their inhibition leads to myocardial dysfunction9. The “off-target” toxicity occurs when other kinases not intended to be targets of the agents are also inhi-bited9. In our study, there were no signifi cant differen-ces between the three types of antiangiogenic agents used (bevacizumab, sunitinib, and sorafenib) and the reductions in LVGLS, RVGLS, 2DLVEF, TAPSE or worsening/de novo HTN.
STUDY LIMITATIONS
The main study limitations were represented by the limited number of patients in our study, the relative rapid appearance of the cardiac toxicity (most of them at Visit 2), the high mortality and morbidity of oncolo-gic patients receiving this medication (only 18 patients left at Visit 4) and the lack of a long-term follow-up. Due to that, there is a need for further studies in or-der to cover further gaps in knowledge as the possible predictive value of GLS reduction on the consequent reductions in LVEF or TAPSE; if the RV functional im-pairment was independent of or preceded a fall in LV function; if the reported cardiac toxicities are reversi-ble or permanent.
CONCLUSIONS
Our results show the importance of an active cardi-ologic monitoring of oncologic patients receiving an-tiangiogenic therapy. We found that antiangiogenic therapy induces worsening pre-existing HTN or de novo HTN and also a reduction in all systolic functi-on parameters of both ventricles in almost half of the studied patients. Also, myocardial dysfunction induced by antiangiogenic agents should not be regarded only as a consequence of HTN, but also as rather related to the intrinsic myocardial toxicity of these agents, pre-existing HTN being probably a major permissive/ precipitating factor.
Conflict of interest: none declared.
References
1. Folkman J. Role of angiogenesis in tumor growth and metastasis. Se-min. Oncol. 2002; 29 (Suppl 16):15–18.
2. Hanahan D, Folkman J. Patterns and emerging mechanisms of the angiogenic switch during tumorigenesis. Cell 1996; 86:353.
3. Folkman J. Tumor angiogenesis: therapeutic implications. N Engl J Med 1971; 285:1182.
4. Ranpura V, Hapani S, Wu S. Treatment-related mortality with beva-cizumab in cancer patients: a meta-analysis. JAMA 2011; 305:487.
5. Schutz FA, Je Y, Richards CJ, Choueiri TK. Meta-analysis of random-ized controlled trials for the incidence and risk of treatment-related mortality in patients with cancer treated with vascular endothelial growth factor tyrosine kinase inhibitors. J Clin Oncol 2012; 30:871.
6. Plana JC, Galderisi M, Barac A, Ewer MS, Ky B, Scherrer-Crosbie M, Ganame J, Sebag IA, Agler DA, Badano LP, Banchs J, Cardinale D, Carver J, Cerqueira M, DeCara JM, Edvardsen T, Flamm SD, Force T, Griffin BP, Jerusalem G, Liu JE, Magalhaes A, Marwick T, Sanchez LY, Sicari R, Villarraga HR, Lancellotti P. Expert consensus for multi-modality imaging evaluation of adult patients during and after cancer therapy: a report from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. Eur Heart J Cardiovasc Imaging 2014;15:1063–1093.
7. Zamorano JL, Lancellotti P, Rodriguez MD, Aboyans V, Asteggia-no R, Galderisi M, Habib G, Lenihan DJ, Lip GY, Lyon AR, Lopez FT, Mohty D, Piepoli MF, Tamargo J, Torbicki A, Suter TM. 2016 ESC Position Paper on cancer treatments and cardiovascular toxic-ity developed under the auspices of the ESC Committee for Practice Guidelines: the Task Force for cancer treatments and cardiovascular toxicity of the European Society of Cardiology (ESC). Eur Heart J 2016;37:2768–2801.
8. Curigliano G, Cardinale D, Dent S, Criscitiello C, Aseyev O, Lenihan D, Cipolla CM. Cardiotoxicity of Anticancer Treatments: Epidemi-ology, Detection, and Management. CA Cancer J Clin 2016;66:309– 325
9. Jurcuţ R, Popară-Voica AM, Şerbănescu GL, Croitoru A, Ginghină O, Ioniţă O, Anghel R, Ginghină C. Cardiovascular effects of antian-giogenic oncological therapies: the fine balance of benefits and risks. Farmacia; 2016; 64:13-23.
10. Groarke JD, Choueiri TK, Slosky D, Cheng S, Moslehi J. Recognizing and managing left ventricular dysfunction associated with therapeu-tic inhibition of the vascular endothelial growth factor signaling path-way. Curr Treat Options Cardiovasc Med. 2014; 16(9):335.
11. Vaklavas C, Lenihan D, Kurzrock R, Tsimberidou AM. Antivascu-lar endothelial growth factor therapies and cardiovascular toxicity: what are the important clinical markers to target? Oncologist. 2010; 15(2):130-41.
12. Zentilin L, Puligadda U, Lionetti V Cardiomyocyte VEGFR-1 acti-vation by VEGF-B induces compensatory hypertrophy and pre-serves cardiac function after myocardial infarction. FASEB J. 2010; 24(5):1467-78.
13. Izumiya Y, Shiojima I, Sato K, Sawyer DB, Colucci WS, Walsh K. Vascular endothelial growth factor blockade promotes the transi-tion from compensatory cardiac hypertrophy to failure in response to pressure overload. Hypertension 2006;47:887–893.
14. Levy BI. Microvascular plasticity and experimental heart failure. Hy-pertension 2006; 47:827–829.
15. Calleja A, Poulin F, Khorolsky C, Shariat M, Bedard PL, Amir E, Ra-kowski H, McDonald M, Delgado D, Thavendiranathan P. Right Ven-tricular Dysfunction in Patients Experiencing Cardiotoxicity during Breast Cancer Therapy. Journal of Oncology; 2015; Volume 2015; 1-10
16. Cottin Y, Touzery C, Coudert B. et al. Diastolic or systolic left and right ventricular impairment at moderate doses of anthracycline? A 1-year follow-up study of women. European Journal of Nuclear Med-icine; 1996; vol. 23; no. 5; 511–516
17. Grover S, Leong DP, Chakrabarty A et al. Left and right ventricular effects of anthracycline and trastuzumab chemotherapy: a prospec-tive study using novel cardiac imaging and biochemical markers. In-ternational Journal of Cardiology; 2013; vol. 168; no. 6; 5465–5467
18. Barendswaard EC, Prpic H, van der Wall EE, Camps JAJ, Keizer HJ, Pauwels EKJ. Right ventricle wall motion abnormalities in patients treated with chemotherapy. Clinical Nuclear Medicine; 1991; vol. 16; no. 7; 513–516
19. Tanindi A, Demirci U, Tacoy G et al. Assessment of right ventricular functions during cancer chemotherapy. European Journal of Echo-cardiography; 2011; vol. 12; no. 11; 834–840.
 This work is licensed under a
This work is licensed under a