Daniela I. Anghelina2, Oana Ionita1, Vlad A. Iliescu1,2, Ovidiu Chioncel1,2
1 ”Carol Davila” University of Medicine and Pharmacy, Bucharest, Romania
2 ”Prof. Dr. C. C. Iliescu” Emergency Institute for Cardiovascular Diseases, Bucharest, Romania
Abstract: Antiphospholipid syndrome (APS) is an autoimmune disease characterized by the presence of thromboembolic complications and/or pregnancy morbidity in the presence of persistently increased titers of antiphospholipid antibodies (the most commonly are lupus anticoagulant, anticardiolipin and anti-beta-2-glycoprotein I antibodies). Venous thrombosis is most commonly lower limb deep vein thrombosis with/without pulmonary embolism and the most frequent site of arterial thrombosis is in the cerebral vasculature (resulting in transient cerebral ischaemia/stroke). Myocardial infarction is less common, although subclinical myocardial ischaemia may be under-recognized. Due to the risk of recurrence of thrombosis, long-term anticoagulant treatment is required.
Keywords: antiphospholipid syndrome, venous and arterial thrombosis, cardioembolic strokes
Rezumat: Sindromul antifosfolipidic este o boală autoimună ce se caracterizează prin existenţa unui nivel plasmatic persistent crescut al anticorpilor antifosfolipidici, care determină complicaţii tromboembolice şi/sau morbiditate obstetricală (cei mai frecvenţi anticorpi sunt anticoagulantul lupic, anticorpii anticardiolipină şi anticorpii anti beta2 glicoproteina I). Trombozele venoase afectează în special sistemul venos profund al membrelor inferioare (cu sau fără apariţia tromboembolismului pulmonar); trombozele arteriale cele mai frecvente sunt la nivelul circulaţiei cerebrale (determină accident ischemic tranzitor sau accident vascular cerebral). Infarctul miocardic are o incidenţă mai mică, dar de cele mai multe ori ischemia miocardică silenţioasă este subdiagnosticată. Tratamentul anticoagulant este indicat pe termen lung din cauza riscului de tromboze recurente.
Cuvinte cheie: sindrom antifosfolipidic, tromboze venoase şi arteriale, accident vascular cerebral cardioembolic.
A 47-year-old man was admitted to our clinic with severe exertional dyspnea, fatigue and lower limb edema (right> left) exacerbated in the last month. The patient’s anamnesis was difficut because he was aphasic and confused.
The medical history revealed: right lower limb deep vein thrombosis (2010); primary antiphospholipid syndromepositive tests for lupus anticoagulant, IgG anticardiolipin and IgG anti-beta-2-glycoprotein I antibodies (2012); ischemic stroke in the left middle cerebral arteries territory (MCA) in 2014, when CT angiography revealed the permeability of MCA and those that form the circle of Willis. Previous ECGs showed a Q wave in the anterior leads. A poor compliance to the anticoagulant treatment was everytime noted.
At the current hospitalization, a complete blood count revealed mild autoimmune hemolytic anemia (Hb=12 g/dl; the direct Coombs test was positive and warm and cold autoantibody were found), moderate autoimmne thrombocytopenia (69.000/mm3), the leukocytes, lymphocytes and eosinophils were in the normal range.
The other laboratory tests showed mild renal dys- function (creatinine=1,38 mg/dl, MDRD eGFR=67 ml/ min/1.73 m2); the levels of brain natriuretic peptide (BNP) and D dimers were increased: BNP=600 pg/ ml (NR < 100 pg/ml), D-dimers=2.7 mcg/ml (NR < 0.7 mcg/ml); the cardiac troponin TnI was negative.
The tests for lupus anticoagulant and anticardiolipin antibodies were positive (the test for anti-beta2-glycoprotein I antibodies was not available). A false positive VDRL has been noted; the antinuclear , anti dsDNA, antiSm, anti SSA and anti SSB antibodies were negative (this was consistent with a primary antiphopholipid syndrome).
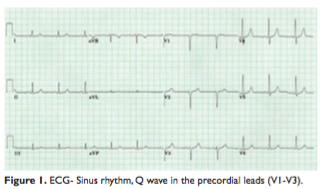
The ECG revealed sinus rhythm with a Q wave in the precordial leads (V1-V3), without any other modificationabnormalities described since 2014 (Figure 1).
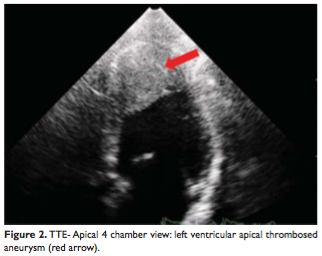
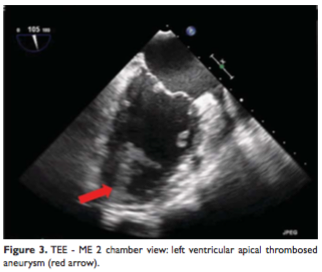
The transthoracic and transesophageal echocardiography showed severe global LV systolic dysfunction (the overall LEEF was estimated at 35%) and a large left ventricular apical aneurysm filled with thrombus. The thrombus was very mobile and friable with a high risk of embolism (Figure 2, 3).
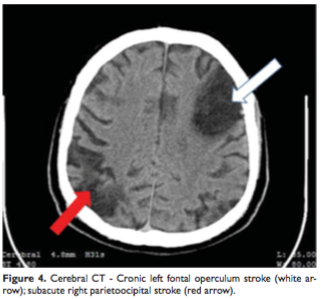
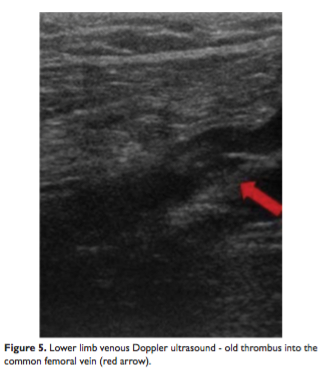
A cerebral computed tomography was performed and a new lesion was found – a subacute right parietoocipital stroke (Figure 4). The lower limbs venous Doppler ultrasound showed an old thrombus into the common femoral vein (Figure 5). The vertebral and extracranial carotid arteries were permeable (Doppler ultrasound).
During hospitalization, the patient received anticoagulant therapy: unfractionated heparin (1000 UI/h infusion, with adjusted doses to maintain a therapeutic range for the aPTT -activated partial thromboplastin timeof 1.5 to 2.5 times; heart failure treatment (betablockers, ACE inhibitors, diuretics) and statins; no antiplatelet therapy was administered due to thrombocytopenia.
In the presence of ECG changes (anterior necrosis) and left ventricular apical aneurysm, a coronary angiography was performed; normal epicardial coronary arteries were found.
The patient was transferred to the cardiovascular surgery department for urgent left ventricular aneurysm resection. At day 2 after admission, he developed global aphasia accompanied by tetraparesis.
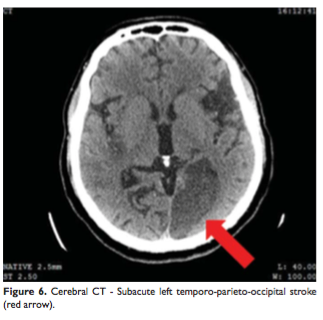
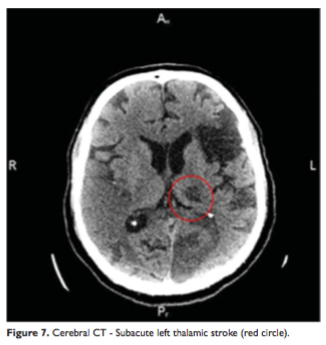
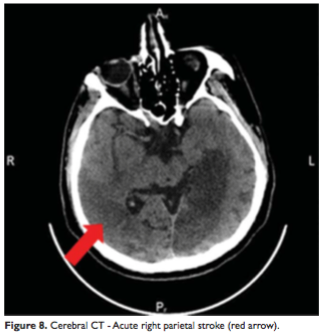
The new cerebral CT scan showed two more subacute ischemic strokes over the left temporo-parieto-occipital and thalamic regions (Figure 6, 7) and an acute one in the right parietal lobe (Figure 8). In condition of a high surgical risk the cardiac surgery was postponed, and the patient was transferred to a neurologic department. After three weeks to transfer patient died.
DISCUSSIONS
Antiphospholipid antibodies are associated with a wide spectrum of cardiac manifestations that include accelerated atherosclerosis, ischemic coronary artery disease, valve abnormalities, intracardiac thrombosis and myocardial microthrombosis.
The correlation between APS and coronary artery disease is not well established. Thrombus formation, in situ or embolic thrombus, with the occlusion of the artery and subsequent ischemia is considered to be the main mechanism involved in acute vascular events in patient with APS („thrombotic risk”). The „atherosclerotic risk” in APS patient, leading to atherosclerosis plaque generation, became more discussed in the medical literature.
A „combined” mechanism might involve thrombus generation on a pre-existent plaque, leading to acute events1. The frequency of myocardial infarction in patients with APS is reportedly 4%, and the presence of antiphospholipid antibodies has been associated with myocardial infarction in young patients. A prospective study on a cohort of 4081 healthy middle-aged men found that a high level of anticardiolipin antibodies constituted an independent risk factor with relation to myocardial infarction or cardiac
death, with high prevalence in patients younger than 45 (22%)2.
Intracardiac thrombi are a rare complication of APS. They may occur in all cardiac chambers (but mainly in the right heart) and are a dangerous source of systemic embolization. Although the exact mechanism of intracardiac thrombus formation in antiphospholipid syndrome is unclear, there were some reports of this rare presentation of APS. The studies suggested that the endocardial surface might be an important site for thrombus formation in patients with circulating aPL, because these antibodies, in the presence of other hemostatic defects, will abolish the balance between thrombosis and fibrinolysis, and might change the endocardial surface factors so that clot formation is promoted3. Some authors speculated that an abnormal intracardiac blood flow pattern might contribute to thrombosis4 and other hypothesized that diffuse ventricular dysfunction might predispose to the formation of intracardiac thrombus4. However, mural thrombosis into a normally contracting LV remains most unusual.
The patient had regional LV systolic dysfunction. The presence of this wall motion abnormalities supports diagnosis of myocardial infarction due to in situ coronary thrombosis. This was complicated with a left ventricular apical thrombosed aneurysm. The hypothesis is also supported by the absence of coronary lesions. A paradoxical embolism from venous thromboembolism transit through a PFO (patent foramen ovale) was excluded by transesophageal echocardiography (no atrial septal defect was identified).
Cardiac embolism represent the mechanism for recurrent ischemic strokes. The patient had a clear embolic sourse and no atherosclerotic lesions on the vertebral and extracranial carotid arteries.
Catastrophic APS (CAPS) is the most severe form of APS with multiple organ involvement developing over a short period of time, usually associated with microthrombosis. Definite CAPS is defined as thromboses in three or more organs developing in less than a week, microthrombosis in at least one organ (confirmed by histopathology) and persistent aPL positivity5. However, if a patient has only three out of these four requirements, then the patient is classified as probable CAPS6. Usually it is accompanied by hematologic manifestations (hemolytic anemia, thrombocytopenia).
We do not have arguments to support this diagnosis: during hospitalization, the patient did not developed any new venous or arterial thrombosis of large vessels; we did not performed a biopsy to confirm an eventually renal, cerebral or myocardial thrombotic microangiopathy (the patient had renal, cerebral and cardiac involvement); the absence of schistocytes on the peripheral blood smear (microangiopathic hemolytic anemia was ruled out).
For this patient with primary antiphospholipidic syndrome, the lack of compliance in the anticoagulant therapy had fatal consequences: “in situ” coronary thrombosis complicated with myocardial infarction and left ventricular apical thrombosed aneurysm witch determined recurrent cardioemolic strokes.
Conflict of interests: none declared.
References:
1. S Caraiola, C Jurcuţ, R Jurcuţ, C Tănăsescu. Vascular disease in patients with antiphospholipid syndrome: what do the cardiologists need to know? Romanian Journal of Cardiology 2012;22(4):177–183.
2. Vaarala O, Manttari M, Manninen V, et al. Anti-cardiolipin antibodies and risk of myocardial infarction in a prospective cohort of middleaged men. Circulation 1995;91:23–7.
3. Julio AA, Carmen S. Intracardiac thrombus in antiphospholipid antibody syndrome. J Am Soc Echocardiogr 2000; 13: 873–875.
4. Coppock MA, Safford RE, Danielson GK. Intracardiac thrombosis, phospholipid antibodies, and two-chambered right ventricle. Br Heart J 1988; 60: 455–458.
5. Kaplan SD, Chartash EK, Pizzarello RA, Furie RA. Cardiac manifestations of the antiphospholipid syndrome. Am Heart J 1992; 124: 1331 –1338.
6. Cervera R., Font J., Gomez-Puerta A., Espinosa G., Cucho M., Bucciarelli S., et al. for CAPS Registry Project Group (2005) Validation of the preliminary criteria for the classification of catastrophic antiphospholipid syndrome. Ann Rheum Dis 64: 1205–1209.
 This work is licensed under a
This work is licensed under a