Vlad Bataila1, Aura Vijiiac1, Lucian Calmac1, Maria Dorobantu1,2
1 Clinical Emergency Hospital, Bucharest, Romania
2 Carol Davila University of Medicine and Pharmacy, Bucharest, Romania
Introduction: Coronary artery disease remains (CAD) a major cause of morbidity and mortality worldwide1. However, the mortality rates of CAD have declined over the past decades, mainly due to improvements in the treatment of acute co-ronary syndromes, therapies for heart failure or revascularization for chronic angina2, but also because of awareness-raising and prevention strategies.
Keywords: coronary artery disease, ischaemia, fractional flow reserve
The high metabolic demands of the myocardium are met through an elaborate vascular network, which includes the large epicardial coronary arteries (over 500 μm in diameter) and smaller vessels – such as ex-tramyocardial prearterioles, arterioles (<100 μm) and capillaries – that form the coronary microcirculation, the main regulator of flow3. The myocardial vascular supply (Figure 1) is complex not only from an anato-mical point of view, but also from a physiological per-spective, with coronary regulatory mechanisms andmicrovascular dysfunction playing an important role in the pathophysiology of ischemic heart disease.
Angina and myocardial ischaemia usually occur be-cause of flow-limiting epicardial fi xed stenosis, but they may also occur in the setting of normal epicardial coronary arteries due to endothelial dysfunction and subsequent microvascular disease4. A coronary angio-gram assesses the extent of coronary disease severity, but its visual interpretation may be subjective and it has acknowledged limitations in intermediate, eccen-tric or diffuse coronary stenosis5, thus making thera-peutic decisions challenging and potentially inaccurate in such clinical scenarios6.
There are several methods for assessing coronary physiology in the catheterization laboratory, which provide evaluation of coronary blood flow, functional significance of a coronary stenosis and/or microvas-cular dysfunction. Guidewire-based measurements of coronary blood pressure, flow velocity, temperature or resistance, integrated in various parameters, allow the assessment of coronary physiology.
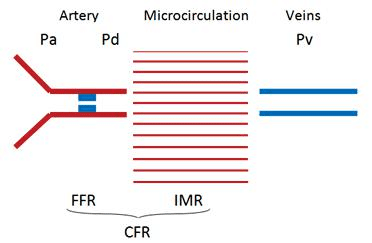
Figure 1. Coronary vascular compartments. Pa – aortic/proximal pres-sure; Pd – distal pressure; Pv – venous pressure; FFR – fractional flow re-serve; IMR – index of myocardial resistence; CFR – coronary flow reserve.
FRACTIONAL FLOW RESERVE
The angiographic aspect of an atherosclerotic lumen does not accurately reflect the physiologic impact on hemodynamics, as the angiographic image is a 2-dimensional representation of a 3-dimensional lesion. The-refore, eccentric lesions, calcifi cations, branch overlap and ostial lesions additionally contribute to the unre-liability of angiographic interpretation7. Studies have shown that angiography is inaccurate in assessing the functional significance of a coronary stenosis when compared with the FFR, not only in the 50% to 70% category, but also in the 70% to 90% angiographic se-verity category8.
Fractional flow reserve (FFR) is a parameter that measures pressure differences across an epicardial stenosis in order to establish if the stenosis is flow-limiting and consequently responsible for myocardial ischaemia. It is defined as the ratio between the ma-ximal achievable blood flow in a diseased vessel and the maximal flow in the hypothetical absence of the stenosis. In a state of maximal hyperaemia, the micro-vascular resistance is minimal and the pressures obtai-ned proximally and distally to a coronary stenosis can be considered proportional to blood flow. Venous pressure is disregarded in clinical practice, so the sim-plified equation for FFR is: FFR=Pd/Pa (Pd=pressure distal to the stenosis, Pa=aortic pressure, recorded at the tip of the guiding catheter). A special coronary 0.014 guidewire with a pressure sensor usually 3 cm from its tip is inserted distally from the target steno-sis. The blood pressure is simultaneously recorded in the aorta and distally, in the target coronary, and an FFR value is provided (Figure 2). Hyperaemia is usually achieved with adenosine, but other drugs may be used (papaverine, nitroprusside, regadenoson), with repro-ducible measurements9.
The theoretical value of FFR in a normal coronary artery is 1, as there should be no pressure drop along a normal vessel. A cutoff value of 0.75 predicts induci-ble ischaemia10,11, but since most studies have used the cutoff of 0.8 for deferral of angioplasty, the current European5 and American12 guidelines for revasculari-zation recommend coronary intervention for stenosis with FFR <0.8 FFR is a reproducible, feasible parame-ter, minimally modified by the baseline status of the patient. However, in patients with sequential lesions, pressure pullback recording during stable hyperaemia is mandatory in order to identify the culprit lesion. FFR is not recommended in the setting of an acute co-ronary syndrome, since the patient’s microcirculation might be severely damaged and thus it might compro-mise the response to adenosine. Trials showed the benefits of using FFR measure-ment to guide revascularization with better outcomes and reduced costs compared to angiography-guided revascularization alone. The first randomized trial of FFR-guided percutaneous coronary intervention (PCI), the DEFER trial, randomly assigned patients with intermediate lesions and FFR >0.75 to PCI (Per-form group) or deferral of PCI (Defer group), whi-le patients with FFR<0.75 underwent PCI (Referen-ce group). The 2 and 5-year follow-up showed that event-free survival did not differ between the Defer and Perform groups, but it was significantly worse in the Reference group13,14. There was no difference between the Defer and Perform group in terms of re-currence of angina. The 15-year follow-up is further proof that the deferral of PCI of functionally nonsig-nificant lesions is safe, while PCI for such lesions does not improve outcome15. The DEFER trial proves that in patients with stable CAD, the most important pro-gnostic factor of a coronary stenosis is its functional significance indicated by a FFR<0.75; in such patients, outcome is worse than in patients with nonsignifi cant stenosis (FFR >0.75), even when treated by PCI.
The FAME trial enrolled 1005 patients with multi-vessel disease and randomly assigned them to angio-graphy-guided PCI and FFR-guided PCI. In the former group, all lesions were treated with DES, while in the latter group, PCI was performed only for lesions with FFR <0.80. At 1 year, the FFR-guided PCI group had lower rates of MACE (13.2% vs 18.3%, p=0.02) and lower rates of combined death and MI (7.3% vs 11%, p=0.04) when compared to the angiography-guided PCI group16. The 2-year follow-up showed similar be-nefits of FFR-guided PCI17. The 5-year follow-up show-ed similar rates of MACE between the two groups, but this clinical outcome was achieved with less stents and less resource use in the FFR-guided PCI group18.
The FAME 2 trial compared FFR-guided PCI plus optimal medical therapy (OMT) with OMT alone in patients with functionally significant stenosis (FFR <0.80). The study was stopped prematurely because of the significant difference in the rates of death, MI and urgent revascularization (4.3% in the FFR group vs 12.7% in the OMT group, p<0.001)19,20. FAMOUS-NSTEMI was a prospective, randomi-zed trial that included 350 NSTEMI patients with at least one coronary stenosis >30%, who were assigned randomly to either the FFR-guided group, where FFR <0.80 was an indication for revascularization by PCI or CABG, as appropriate, or the angiography-guided group, where FFR measurements were not disclosed and the decision of revascularization was made accor-ding to visual assessment of the lesion. At 12 months, there were no significant differences in health outco-mes and quality of life between the groups21. However, a recent study showed that deferring PCI in patients with NSTEMI and culprit lesions with FFR >0.75 is associated with significantly worse outcomes22, which brings into question the long-term safety of assessing the physiology of an acute lesion and of using the same threshold as for stable CAD. The FAMOUS-NSTEMI trial proved that FFR measurement in NSTEMI pati-ents is safe and feasible, but further studies are needed to assess the prognostic significance and cost-effecti-veness of a FFR-guided management in such a clinical setting.
A recent multicenter study, PRIMULTI, enrolled 627 patients with STEMI and a significant nonculprit lesion; all culprit lesions were treated, while noncul-prit lesions were randomized to angiography-guided or FFR-guided revascularization. At 1 year, cardiac death and repeated revascularization were lower in the FFR-guided group, while there were no significant differences among hospitalizations for recurrent angi-na23. The preliminary results of a recent similar study showed that in patients with STEMI and FFR-guided complete revascularization of non-culprit lesions the rate of MACE was lower than in patients with STEMI treated for the infarct-related artery only, mainly be-cause of a reduction in repeated revascularization24.
CABG is the current recommendation for patients with complex 3-vessel disease25. The investigators of FAME 3, an ongoing multicenter, randomized trial, hypothesized that the inferiority of PCI in 3-vessel di-sease might be explained by the use of older types of stents and the lack of FFR-guidance of PCI. Therefo-re, the trial will investigate whether FFR-guided PCI with new-generation stents is noninferior to CABG in patients with 3-vessel disease, not including left main stenosis26.
FFR is a well established and essential tool to gui-de the elective revascularization of intermediate lesi-ons, as it provides specific information regarding their functional significance. Revascularization offers a gre-ater absolute benefit for more severe FFR values27. An FFR-guided revascularization significantly reduces MACE and increases freedom from angina when com-pared to an angiographic – based strategy.
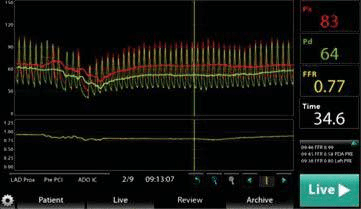
Figure 2. FFR – console screenshot. Red wave: aortic/proximal pressure. Yellow wave: distal pressure, FFR value of 0.77.
INSTANTANEOUS WAVE-FREE RATIO
The instantaneous wave-free ratio (iFR) is a logical follow-up of the FFR. Like FFR, it determines whether an epicardial coronary stenosis leads to a drop in pre-ssure that is signifi cant. The iFR precludes the need for hyperaemia, one of the most common pitfalls of FFR. After an analysis of coronary pressure and velo-city measurements, Sen et al. identified a wave – free period during diastole in which the resistance is both minimal and constant28. This wave-free period starts 25% into diastole and ends 5 ms before the end. It can be used to calculate the ratio between the distal and proximal pressures (iFR = Pd/Pa) without the need of hyperaemia (Figure 3). An iFR measurement is made by using a normal pressure wire connected to a ma-chine with dedicated software. It is a highly reprodu-cible measurement with excellent spatial resolution, used with ease to perform a coronary pullback tracing, in order to distinguish between diffuse atherosclerotic disease and focal stenosis.
The iFR usually has a cut-off value of 0.90, which is used to predict with a diagnostic accuracy of 80% the FFR value of 0.8029. Usually a grey zone of 0.86–0.93 is used in order to establish the need of further hype-raemia measurements and FFR determination30,31. The grey-zone setting provides two thirds of patients with a hyperaemia – free functional measurement while maintaining an excellent correlation with the FFR.
Recently, two high volume clinical trials that prove the non-inferiority of the iFR method to the FFR re-garding hard clinical outcomes were published. DEFI-NE – FLAIR was a 2492 patient multicenter, randomi-zed, clinical trial that compared iFR and FFR – guided PCI in patients with intermediate – severity lesions. The composite primary endpoint of all-cause death, nonfatal MI or unplanned revascularization occurred in 6.8% in the iFR group and 7.8% in the FFR group (95% CI 0.68–1.33, P=0.78). In the iFR group, the peri-procedural signs and symptoms were reported in 3.1% (39 patients) as compared to the 30.8% (385 patients) in the FFR group (p<0.001)32.
The iFR – SWEDEHEART STUDY consists of 2037 patients with an indication for a physiologically guided assessment of a coronary lesion randomized to either iFR or FFR guided PCI. The primary endpoint occur-red in 6.7% of the patients in the iFR group and in 6.1% in the FFR group, meeting the noninferiority margin (p = 0.007 for noninferiority)33.
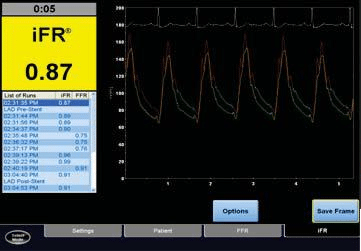
Figure 3. iFR – console screenshot. Re wave: aortic/proximal pressure. Yellow wave: distal pressure; Green wave: wave-free period.
CORONARY FLOW RESERVE
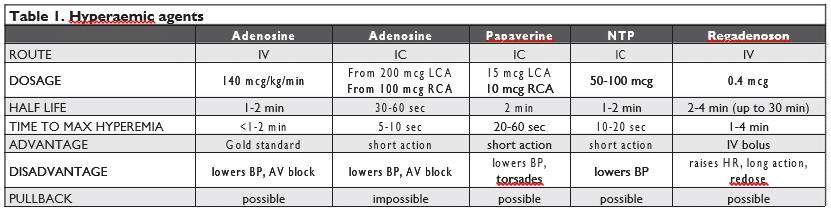
The coronary flow reserve (CFR) expresses the ca-pacity of the entire coronary vascular bed to respond to an increase in oxygen demand with a correspon-ding increase in blood flow through vasodilation. CFR represents the ratio between total coronary blood flow at maximal hyperaemia and at baseline. In normal subjects CFR is usually over 3 and a cutoff value of 2 is generally accepted in clinical practice for the de-tection of inducible ischaemia34. CFR can be assessed non-invasively using transthoracic Doppler echocardi-ography, myocardial contrast echocardiography, PET, cardiac magnetic resonance; each test has its own li-mitations in terms of availability, accuracy, costs and potential risks. Coronary fl ow at rest and under maxi-mal hyperaemia can also be assessed invasively during coronary angiogram, through either Doppler-velocity assessment or thermodilution method35. A special co-ronary 0.014” guidewire with a 12–15 MHz piezoelec-tric ultrasound transducer in its tip is advanced usually until the mid segment of the target coronary artery and oriented away from the vessel wall. A base value is first recorded, and then, the second value during ma-ximal hyperaemia, is obtained. Hyperaemia is usually achieved with adenosine, administered either intrave-nously or by a single intracoronary bolus, but several other drugs (papaverine, nitroglycerin, nitroprusside) can be used (Table 1). The most common side effects associated with CFR measurement are those related to adenosine administration (bradycardia, hypotensi-on, bronchoconstriction, flushing, dyspnea), the drug being contraindicated in patients with persistent bron-chospasm.
The role of CFR measurement lies in the assessment of global coronary vascular function (both epicardial vessels and microcirculation), in unraveling the total burden of myocardial ischaemia36 and in the diagnosis of microvascular angina (in the setting of chest pain with normal coronary angiogram and CFR less than 2). Trials regarding the prognostic value of non-inva-sive CFR have been conducted and they showed that CFR less than 2 was a independent predictor for ma-jor adverse cardiac events (MACE)37,38 and that higher CFR was associated with better outcome in patients with normal perfusion imaging39,40. A non-randomized study with a longer follow-up period (up to 5.4±2.2 years) showed not only that CFR is an independent predictor of MACE, but also that, in patients with nor-mal perfusion, a low CFR allowed further prediction of high annual event rates41. This predictive power was maintained during the first 3 years of follow-up; afterwards, the survival curves converged. A recent study showed that reduced CFR was associated with adverse outcomes independently of the angiographic severity of coronary disease, and that it may affect the outcomes of revascularization: patients with low CFR who underwent coronary artery bypass grafting, but not percutaneous coronary intervention, had event rates similar to those with normal CFR42.
While all these trials were based on non-invasive PET assessment of CFR, one previous study showed that a low invasive CFR (assessed by intracoronary Doppler) was an independent predictor of poor long-term outcome in patients with angina and normal or mildly diseased coronary arteries43.
CFR has several limitations in clinical practice. In-vasive measurement of CFR is expensive, time con-suming and it has potentially serious adverse effects44. An abnormal CFR does not help differentiate between epicardial and microvascular disease45, as this parame-ter assesses the function of the whole coronary vas-cular bed and the cohesive effects of epicardial steno-sis, vessel remodeling, microvascular dysfunction on myocardial perfusion46. Any disturbances in the coro-nary blood flow at rest (such as tachycardia, abnor-mal coronary perfusion pressure, vasoactive drugs or hormones, left ventricular hypertrophy) will alter the ratio of hyperemic to baseline fl ow, potentially leading to a falsely abnormal CFR47.
INDEX OF MICROVASCULAR RESISTANCE
The scenario of a patient with normal FFR and low CFR is generally attributed to microvascular dysfunc-tion; however, this setting can also be explained by diffuse atherosclerotic disease or altered baseline co-ronary flow. For such cases, parameters evaluating the function of the microcirculation have been developed. The index of microvascular resistance (IMR) is a mea-sure of coronary microvascular function, it was first described in 200348 and it is calculated as the ratio between distal coronary pressure recorded under ma-ximal hyperaemia and coronary blood flow, or, using the simplified equation, as the product between co-ronary pressure and the mean transit time of a 3 ml bolus of saline during hyperaemia. An IMR higher than 25 is considered abnormal, expressing microvascular dysfunction49. Measurement of IMR is specific for the microvasculature, reproducible and independent of haemodynamic variations and myocardial contractility. However, in the setting of associated significant epicar-dial stenosis, collateral blood supply may be substanti-al and IMR should be measured taking into account the coronary wedge pressure and venous pressure50 or it can be easier estimated using the myocardial fractional flow reserve51: IMR=mean proximal coronary pressu-re x mean transit time x (1.34 x FFRmyo – 0.32). Besides having a role in the diagnostic of microvas-cular angina, the IMR is also a predictor of myocardial damage in the setting of an acute STEMI. Two small-sample studies showed that the IMR measured during primary PCI is an independent predictor of infarct size and severity assessed by contrast-enhanced cardiac magnetic resonance at 2 days and at 3 months52 and it is also a predictor of recovery of left ventricular func-tion based on the percent change in wall motion score at 3-month follow-up53. A recent prospective, multi-center study, which enrolled 253 patients, showed that the IMR measured at the time of primary PCI for STEMI predicted long-term clinical outcomes such as death and hospitalization for heart failure, with the cu-toff >40 being associated with a higher rate of cardiac adverse events (hazard ratio [HR]=3.95, p=0.028 for death, HR=2.1, p=0.034 for death or rehospitalization for heart failure)54. The study limitations include the relatively small number of adverse events (11 deaths, 24 hospitalizations) and the lack of data regarding the extent of coronary disease. The main clinical implica-tion of such fi ndings is that the IMR may be a useful method for identifying high-risk patients who would benefit most from novel therapies targeting microvas-cular recovery, such as intracoronary streptokinase or transplant of autologous stem cells. Nevertheless, further studies are needed to establish whether pati-ents with a high IMR need particular therapeutic inter-ventions to improve outcome.
Susceptibility to periprocedural MI is related to pro-cedural aspects or lesion complexity (plaque burden assessed by intravascular ultrasound or thin cap fibro-atheroma assessed by optical coherence tomography), but coronary microvascular dysfunction may also play a role in predisposing a patient to periprocedural MI. In a study of 50 patients undergoing elective PCI for a single lesion of the left anterior descending artery, pre-procedural IMR >27 was associated with a 23-fold risk of developing periprocedural MI (sensitivity=80%, specificity=85%, p=0.003)55, showing that impaired mi-crocirculation before PCI determines susceptibility for periprocedural MI independent of lesion characteris-tics and that such patients might benefit from adjuncti-ve risk-reduction strategies. The main limitation of the study is the inclusion only of left anterior descending artery lesions; further studies are needed to define IMR cutoffs for other territories.
ABSOLUTE CORONARY BLOOD FLOW
The latest method of characterizing the coronary physiology is the measurement of the absolute coronary blood flow and microvascular resistance. It is performed by continuous thermodilution with saline administered via a special microcatheter with four-si-de holes in order to obtain an optimal mixing of the indicator with blood. A pressure-temperature sensor-tipped wire is advanced in the distal vessel. With the aid of a dedicated software, the coronary absolute hyperemic blood flow measured in ml/min is obtai-ned and also the microvascular resistance measured in dyne*s*cm-556,57. The technology is available at the moment only for research.
CONCLUSIONS
Despite the low prevalence of invasive physiologic assessment in clinical practice, comprehensive evalu-ation of both macro- and microvasculature systems enhances the patients’ outcomes. Coronary physiolo-gical measurement overcomes the limitations of coro-nary angiography, as it integrates the atherosclerotic burden with its haemodynamic impact.
For patients with acute coronary syndromes, co-ronary physiology has the potential to improve the treatment of the culprit lesion, but further studies are needed. For stable patients and for acute non-culprit lesions, coronary physiology assessment allows functi-onal quantifi cation of the ischaemic burden of the co-ronary bed and thus guides the therapeutic decision. Physiology of both microvascular disease and diffuse atherosclerosis needs to be further explored, as the coexistence of these entities with focal stenoses may lead to failure of a local mechanical intervention to change the long-term outcome of the disease58.
Standards of coronary physiologic assessment are constantly evolving and they will continue to be explo-red, in order to fully comprehend the mechanisms of endothelial dysfunction, microvascular disease or vul-nerable coronary lesions. Although physiology testing in the catheterization laboratory means supplemen-tal time, costs and potential side effects, it provides a substantial clinical benefit for the patient and thus savings to the health care system. Newer methods, like the iFR and absolute coronary blood flow and mi-crovascular resistance, not only make the physiology assessment safer and quicker, but also open new ways for research. Understanding coronary physiology in patients with CAD complements the anatomical in-formation provided by angiography and assists the physician in decision-making and improving long-term outcomes.
Conflict of interest: none declared.
References
1. Mozaffarian D, Benjamin EJ, Go AS, Arnett DK, Blaha MJ, Cushman M, Das SR, de Ferranti S, Després J-P, Fullerton HJ, Howard VJ, Huffman MD, Isasi CR, Jiménez MC, Judd SE, Kissela BM, Lichtman JH, Lisabeth LD, Liu S, Mackey RH, Magid DJ, McGuire DK, Mohler ER III, Moy CS, Muntner P, Mussolino ME, Nasir K, Neumar RW, Nichol G, Palaniappan L, Pandey DK, Reeves MJ, Rodriguez CJ, Ro-samond W, Sorlie PD, Stein J, Towfighi A, Turan TN, Virani SS, Woo D, Yeh RW, Turner MB; on behalf of the American Heart Associati-on Statistics Committee and Stroke Statistics Subcommittee. Heart disease and stroke statistics—2016 update: a report from the Ame-rican Heart Association. Circulation 2016;133(4):e38-360.
2. Gomar FS, Quilis CP, Leischik R, Lucia A. Epidemiology of coronary heart disease and acute coronary syndrome. Annals of Translational Medicine 2016;4(13):256.
3. Fonseca DA, Antunes PE, Cotrim MD (2016). The Morphology, Phy-siology and Pathophysiology of Coronary Microcirculation. In Lena-si H (Ed.), Microcirculation Revisited – From Molecules to Clinical Practice (pp.15-47). Rijeka, InTech.
4. Lee BK, Lim HS, Fearon WF, Yong AS, Yamada R, Tanaka S, Lee DP, Yeung AC, Tremmel JA. Invasive evaluation of patients with angina in the absence of obstructive coronary artery disease. Circulation 2015;131:1054-1060.
5. Windecker S, Kolh P, Alfonso F, Collet JP, Cremer J, Falk V, Filippa-tos G, Hamm C, Head SJ, Juni P, Kappetein AP, Kastrati A, Knuuti J, Landmesser U, Laufer G, Neumann FJ, Richter DJ, Schauerte P, Uva MS, Stefanini GG, Taggart DP, Torracca L, Valgimigli M, Wijns W, Witkowski A; on behalf of the Task Force on Myocardial revas-cularization of the European Society of Cardiology (ESC) and the European Association for Cardio-Thoracic Surgery (EACTS). 2014 ESC/EACTS Guidelines on myocardial revascularization. Eur Heart J 2014;35:2541-2619.
6. Berry, C. Fractional Flow Reserve, Coronary Flow Reserve and the Index of Microvascular Resistance in Clinical Practice. RadcliffeCar-diology.com, February 2014
7. Kern MJ, Samady H. Current Concepts of Integrated Coronary Physiology in the Catheterization Laboratory. J Am Coll Cardiol 2010;55:173-185.
8. Tonino PAL, Fearon WF, De Bruyne B, oldroyd KG, Leesar MA, Ver Lee PN, MacCarthy PA, van’t Veer M, Pijls NH. Angiographic versus Functional Severity of coronary Artery Stenoses in the FAME Study: Fractional Flow Reserve Versus Angiography in Multivessel Evaluati-on. J Am Coll Cardiol 2010;55:2816-2821.
9. Lim WH, Koo BK, Nam CW, Doh JH, Park JJ, Yang HM, Park KW, Kim HS, Takashima H, Waseda K, Amano T, Kato D, Kurita A, Oi M, Toyofuku M, van Nunen L, Pijls NH. Variability of fractional flow reserve according to the methods of hyperemia induction. Catheter Cardiovasc Interv 2015;85:970-976.
10. Pijls NH, Van Gelder B, Van der Voort P, Peels K, Bracke FA, Bonni-er HJ, Gamal MI. Fractional fl ow reserve. A useful index to evaluate the infl uence of an epicardial coronary stenosis on myocardial blood flow. Circulation 1995;92:3183-3193.
11. Fearon WF, Takagi A, Jeremias A, Yeung AC, Joye JD, Cohen DJ, Chou TM, Kern MJ, Yock PG. Use of fractional myocardial flow re-serve to assess the functional significance of intermediate coronary stenoses. Am J Cardiol 2000;86:1013-1014.
12. Levine GN, Bates ER, Balnkenship JC, Bailey SR, Bittl JA, Cercek B, Chambers CE, Ellis SG, Guyton RA, Hollenberg SM, Khot UN, Lange RA, Mauri L, Mehran R, Moussa ID, Mukherjee D, Nallamothu BK, Ting HH. 2011 ACCF/AHA/SCAI Guideline for Percutaneous Co-ronary Intervention: executive summary: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines and the Society for Cardiovascular An-giography and Interventions. Circulation 2011;124:2574-2609.
13. Bech GJ, De Bruyne B, Pijls NH, de Muinck ED, Hoorntje JC, Esca-ned J, Stella PR, Boersma E, Bartunek J, Koolen JJ, Wijns W. Frac-tional fl ow reserve to determine the appropriateness of angioplas-ty in moderate coronary stenosis: a randomized trial. Circulation 2001;103:2928-2934.
14. Pijls NH, van Schaardenburgh O, Manoharan G, Boersma E, Bech JW, van’t Veer M, Bar F, Hoorntje J, Koolen J, Wijns W, de Bruyne B. Percutaneous coronary intervention of functionally nonsignificant stenosis: 5-year follow-up of the DEFER Study. J Am Coll Cardiol 2007;49:2105-2111.
15. Zimmermann FM, Ferrara A, Johnson NP, van Nunen LX, Escaned J, Albertsson P, Erbel R, Legrand V, Gwon HC, Remkes WS, Stella PR, van Schaardenburgh P, Bech GJ, de Bruyne B, Pijls NH. Deferral vs. performance of percutaneous coronary intervention of functionally nonsignificant coronary stenosis: 15-year follow-up of the DEFER trial. Eur Heart J 2015;36:3182-3188.
16. Tonino PA, De Bruyne B, Pijls NH, Siebert U, Ikeno F, van’t Veer M, Klauss V, Manoharan G, Engstrom T, Oldroyd KG, Ver Lee PN, MacCarthy PA, Fearon WF, for the FAME Study Investigators. Frac-tional Flow Reserve versus Angiography for Guiding Percutaneous Coronary Intervention. N Engl J Med 2009;360:213-224.
17. Pijls NH, Fearon WF, Tonino PA, Siebert U, Ikeno F, Bornschein B, van’t Veer M, Klauss V, Manoharan G, Engstrom T, Oldroyd KG, Ver Lee PN, MacCarthy PA, De Bruyne B. Fractional Flow Reserve ver-sus Angiography for Guiding Percutaneous Coronary Intervention in Patients with Multivessel Coronary Artery Disease: 2-year follow-up of the FAME study. J Am Coll Cardiol 2010;56:177-184
18. Van Nunen LX, Zimmermann FM, Tonino PA, Barbato E, Baumbach A, Engstrom T, Klauss V, MacCarthy PA, Manoharan G, Oldroyd KG, Ver Lee PN, van’t Veer M, Fearon WF, De Bruyne B, Pijls NH, for the FAME Study Investigators. Fractional flow reserve versus an-giography for guidance of PCI in patients with multivessel coronary artery disease (FAME): 5-year follow-up of a randomized controlled trial. Lancet 2015;386:1853-1860.
19. De Bruyne B, Pijls NH, Kalesan B, Barbato E, Tonino PA, Piroth Z, Jagic N, Mobius-Winkler S, Rioufol G, Witt N, Kala P, MacCarthy P, Engstrom T, Oldroyd KG, Mavromatis K, Manoharan G, Verlee P, Frobert O, Curzen N, Johnson JB, Juni P, Fearon WF, for the FAME 2 Trial Investigators. Fractional Flow Reserve-Guided PCI versus Medical Therapy in Stable Coronary Disease. N Engl J Med 2012;367:991-1001.
20. De Bruyne B, Fearon WF, Pijls NH, Barbato E, Tonino PA, Piroth Z, Jagic N, Mobius-Winkler S, Rioufol G, Witt N, Kala P, MacCarthy P, Engstrom T, Oldroyd KG, Mavromatis K, Manoharan G, Verlee P, Frobert O, Curzen N, Johnson JB, Limacher A, Nuesch E, Juni P, for the FAME 2 Trial Investigators. Fractional Flow Reserve-Guided PCI for Stable Coronary Disease. N Engl J Med 2014;371:1208-1217.
21. Layland J, Oldroyd KG, Curzen N, Sood A, Balachandran K, Das R, Junejo S, Ahmed N, Lee MMY, Shaukat A, O’Donnell A, Nam J, Briggs A, Henderson R, McConnachie A, Berry C, on behalf of the FAMOUS-NSTEMI investigators. Fractional flow reserve vs. angio-graphy in guiding management to optimize outcomes in non-ST-seg-ment elevation myocardial infarction: the British Heart Foundation FAMOUS-NSTEMI randomized trial. Eur Heart J 2015;36:100-111.
22. Hakeem A, Edupuganti MM, Almomani A, Pothineni NV, Payne J, Abualsuod AM, Bhatti S. Long-Term Prognosis of Deferred Acute Coronary Syndrome Lesions Based on Nonischemic Fractional Flow Reserve. J Am Coll Cardiol 2016;68:1181-1191.
23. Engstrom T, Kelbaek H, Helqvist S, Hofsten DE, Klovgaard L, Holm-vang L, Jorgensen E, Pedersen F, Saunamaki K, Clemmensen P, De Backer O, Ravkilde J, Tilsted HH, Villadsen AB, Aaroe J, Jensen SE, Raungaard B, Kober L, for the DANAMI 3–PRIMULTI Investigators. Complete revascularization versus treatment of the culprit lesion only in patients with ST-segment elevation myocardial infarction and multivessel disease (DANAMI 3-PRIMULTI): an open label, randomi-zed controlled trial. Lancet 2015;386:665-671.
24. Smits PC, Abdel-Wahab M, Neumann FZ, Boxma-de-Klerk, BM, Lunde K, Schotborgh CR, Piroth Z, Horak D, Wlodarczak A, Ong PJ, Hambrecht R, Angeras O, Richardt G, Omerovic E, for the Compa-re-Acute Investigators. Fractional Flow Reserve-Guided Multivessel Angioplasty in Myocardial Infarction. N Engl J Med 2017;376:1234-1244.
25. Serruys PW, Morice MC, Kappetein AP, Colombo A, Holmes DR, Mack MJ, Stahle E, Feldman TE, van den Brand M, Bass EJ, Van Dyck N, Leadley K, Dawkins KD, Mohr FW, for the SYNTAX Investiga-tors. Percutaneous Coronary Intervention versus Coronary Artery Bypass Grafting for Severe Coronary Artery Disease. N Engl J Med 2009;360:961-972.
26. Zimmermann FM, De Bruyne B, Pijls NH, Desai M, Oldroyd KG, Park SJ, Reardon MJ, Wendler O, Woo J, Yeung AC, Fearon WF. Rationale and design of the Fractional Flow Reserve versus Angio-graphy for Multivessel Evaluation (FAME) 3 Trial: A comparison of fractional flow reserve-guided percutaneous coronary intervention and coronary artery bypass grafting surgery in patients with multi-vessel coronary artery disease. Am Heart J 2015;170:619-626.
27. Johnson NP, Toth GG, Lai D, Zhu H, Acar G, Agostoni P, Appelman Y, Arslan F, Barbato E, Chen SL, Di Serafino L, Dominguez-Franco AJ, Dupouy P, Esen AM, Esen OB, Hamilos M, Iwasaki K, Jensen LO, Jimenez-Navarro MF, Katritsis DG, Kocaman SA, Koo BK, Lopez-Palop R, Lorin JD, Miller LH, Muller O, Nam CW, Oud N, Puymirat E, Rieber J, Rioufol G, Rodes-Cabau J, Sedlis SP, Takeishi Y, Tonino PAL, Van Belle E, Verna E, Werner GS, Fearon WF, Pijls NH, De Bruyne B, Gould KL. Prognostic Value of Fractional Flow Reserve: Linking Physiologic Severity to Clinical Outcomes. J Am Coll Cardiol 2014;64:1641-1654.
28. Sen S, Escaned J, Malik IS, Mikhail GW, Foale RA, Mila R, Tarkin J, Petraco R, Broyd C, Jabbour R, et al. Development and validation of a new adenosine-independent index of stenosis severity from co-ronary wave-intensity analysis: results of the ADVISE (ADenosine Vasodilator Independent Stenosis Evaluation) study. J Am Coll Car-dio 2012;59:1392–1402.
29. Jeremias A, Maehara A, Généreux P, Asrress KN, Berry C, De Bruy-ne B, Davies JE, Escaned J, Fearon WF, Gould KL, et al. Multicenter core laboratory comparison of the instantaneous wave-free ratio and resting Pd/Pa with fractional flow reserve: the RESOLVE study. J Am Coll Cardiol 2014;63:1253–1261.
30. Petraco R, Park JJ, Sen S, Nijjer SS, Malik IS, Echavarría-Pinto M, Asrress KN, Nam CW, Macías E, Foale RA, et al. Hybrid iFR-FFR decision-making strategy: implications for enhancing universal adop-tion of physiology-guided coronary revascularisation. EuroInterven-tion 2013;8:1157–1165
31. Escaned J, Echavarría-Pinto M, Garcia-Garcia HM, van de Hoef TP, de Vries T, Kaul P, Raveendran G, Altman JD, Kurz HI, Brechtken J, et al. Prospective Assessment of the Diagnostic Accuracy of Instan-taneous Wave-Free Ratio to Assess Coronary Stenosis Relevance: Results of ADVISE II International, Multicenter Study (ADenosine Vasodilator Independent Stenosis Evaluation II) JACC Cardiovasc Interv 2015;8:824–833
32. Davies JE, Sen S, Dehbi H-M, et al. Use of the instantaneous wa-ve-free ratio or fractional flow reserve in PCI. N Engl J Med 2017; DOI:10.1056/NEJMoa1700445
33. Götberg M, Christiansen EH, Gudmundsdottir IJ, et al. Instantaneous wave-free ratio versus fractional flow reserve to guide PCI.N Engl J Med 2017; DOI:10.1056/NEJMoa1616540
34. Kern MJ, Lerman A, Bech JW, De Bruyne B, Eeckhout E, Fearon WF, Higano ST, Lim MJ, Meuwissen M, Piek JJ, Pijls NH, Siebes M, Spaan JA. Physiological assessment of coronary artery disease in the cardi-ac catheterization laboratory: a scientifi c statement from the Ameri-can Heart Association Committee on Diagnostic and Interventional Cardiac Catheterization, Council on Clinical Cardiology. Circulation 2006;114:1321-1341.
35. Barbato E, Aarnoudse W, Aengevaeren WR, Werner G, Klaus V, Bojara W, Herzfeld I, Oldroyd KG, Pijls NHJ, De Bruyne B. Valida-tion of coronary flow reserve measurements by thermodilution in clinical practice. Eur Heart J 2004,25(3):219-223.
36. Ziadi MC. Myocardial flow reserve (MFR) with positron emission tomography (PET)/ computed tomography (CT): clinical impact in diagnosis and prognosis. Cardiovasc Diagn Ther 2017;7(2):206-208.
37. Ziadi MC, deKemp RA, Williams KA, Guo A, Chow BJW, Renaud JM, Ruddy, TD, Sarveswaran N, Tee RE, Beanlands RSB. Impaired Myocardial Flow Reserve on Rubidium-82 Positron Emission Tomo-graphy Imaging Predicts Adverse Outcomes in Patients Assessed for Myocardial Ischemia. J Am Coll Cardiol 2011;58:740-748.
38. Farhad H, Dunet V, Bachelard K, Allenbach G, Kaufmann PA, Prior JO. Added prognostic value of myocardial blood fl ow quantitation in rubidium-82 positron emission tomography imaging. Eur Heart J Cardiovasc Imaging 2013;14:1203-1210.
39. Fukushima K, Javadi MS, Higuchi T, Lautamaki R, Merrill J, Nekolla SG, Bengel FM. Prediction of Short-Term Cardiovascular Events Using Quantifi cation of Global Myocardial Flow Reserve in Pati-ents Referred for Clinical 82Rb PET Perfusion Imaging. J Nucl Med 2011;52:726-732.
40. Murthy VL, Naya M, Foster CR, Hainer J, Gaber M, Di Carli G, Blan-kstein R, Dorbala S, Sitek A, Pencina MJ, Di Carli, MF. Improved Cardiac Risk Assessment With Noninvasive Measures of Coronary Flow Reserve. Circulation 2011;124:2215-2224.
41. Herzog BA, Husmann L, Valenta I, Gaemperli O, Siegrist PT, Tay FM, Burkhard N, Wyss CA, Kaufamann PA. Long-Term Prognostic Value of 13N-Ammonia Myocardial Perfusion Positron Emission Tomogra-phy: Added Value of Coronary Flow Reserve. J Am Coll Cardiol 2009;54:150-156.
42. Taqueti VR, Hachamovitch R, Murthy VL, Naya M, Foster CR, Ha-iner J, Dorbala S, Blankstein R, Di Carli MF. Global Coronary Flow Reserve is Associated With Adverse Cardiovascular Events Indepen-dently of Luminal Angiographic Severity and Modifies the Effect of Early Revascularization. Circulation 2015;131:00-00.
43. Britten MB, Zeiher AM, Schachinger V. Microvascular dysfunction in angiographically normal or mildly diseased coronary arteries pre-dicts adverse cardiovascular long-term outcome. Coron Artery Dis 2004;15(5):259-264
44. Lanza GA, Camici PG, Galiuto L, Niccoli G, Pizzi C, Di Monaco A, Sestito A, Novo S, Piscione F, Tritto I, Ambrosio G, Bugiardini R, Crea F, Marzilli M; on behalf of Gruppo di Studio di Fisiopatolo-gia Coronarica e Microcircolazione, Societa Italiana di cardiologia. Methods to investigate coronary microvascular function in clinical practice. J Cardiovasc Med 2013;14:1-18.
45. Naya M, Murthy VL, Taqueti VR, Foster CR, Klein J, Garber M, Dorbala S, Hainer J, Blankstein R, Resnic F, Di Carli, MF. Preserved Coronary Flow Reserve Effectively Excludes High-Risk Coronary Artery Disease on Angiography. J Nucl Med 2014;55(2):248-255.
46. Gould KL. Does coronary flow trump coronary anatomy? J Am Coll Cardiol Img 2009;2:1009-1023.
47. Diez-Delhoyo F, Gutierrez-Ibanes E, Loughlin G, Sanz-Ruiz R, Vasquez-Alvarez ME, Sarnago-Cebada F, Angulo-Llanos R, Casado-Plasencia A, Elizaga J, Fernandez-Aviles-Diaz F. Coronary physio-logy assessment in the catheterization laboratory. World J Cardiol 2015;7(9):525-538.
48. Fearon WF, Balsam LB, Farouque HMO, Robbins RC, Fitzgerald PJ, Yock PG, Yeung AC. Novel Index for Invasively Assessing the Coro-nary Microcirculation. Circulation 2003;107:3129-3132.
49. Kobayashi Y, Fearon WF. Invasive Coronary Microcirculation Assessment: Current Status of Index of Microcirculatory Resistance. Circ J 2014;78:1021-1028.
50. Yong AS, Ho M, Shah MG, Ng MKC, Fearon WF. Coronary Mi-crocirculatory Resistance is Independent of Epicardial Stenosis. Circ Cardiovasc Interv 2012;5:103-108.
51. Yong AS, Layland J, Fearon WF, Ho M, Shah MG, Daniels D, Whitbo-urn R, MacIsaac A, Kritharides L, Wilson A, Ng MK. Calculation of the Index of Microcirculatory Resistance Without Coronary Wedge Pressure Measurement in the Presence of Epicardial Stenosis. J Am Coll Cardiol Intv 2013;6:53-58.
52. McGeoch R, Watkins S, Berry C, Steedman T, Davie A, Byrne J, Hillis S, Lindsay M, Robb S, Dargie H, Oldroyd K. The Index of Microcircu-latory Resistance Measured Acutely Predicts the Extent and Severity of Myocardial Infarction in Patients With ST-Segment Elevation Myo-cardial Infarction. J Am Coll Cardiol Intv 2010;3:715-722.
53. Fearon WF, Shah M, Ng M, Brinton T, Wilson A, Tremmel JA, Sch-nittger I, Lee DP, Vagelos RH, Fitzgerald PJ, Yock PG, Yeung AC. Predictive Value of the Index of Microcirculatory Resistance in Pa-tients with ST-Segment Elevation Myocardial Infarction. J Am Coll Cardiol 2008;51:560-565.
54. Fearon WF, Low AF, Yong AS, McGEoch R, Berry C, Shah M, Ho MY, Kim HS, Loh JP, Oldroyd KG. Prognostic Value of the Index of Microcirculatory Resistance Measured After Primary Percutaneous Coronary Intervention. Circulation 2013;127:2436-2441.
55. Ng MKC, Yong ASC, Ho M, Shah MG, Chawantanpipat C, O’Connell R, Keech A, Kritharides L, Fearon WF. The Index of Microcirculato-ry Resistance Predicts Myocardial Infarction Related to Percutaneo-us Coronary Intervention. Circ Cardiovasc Interv 2012;5:515-522.
56. Kanaji Y, Murai T, Yonetsu T, Usui E, Araki M, Matsuda J, Hoshino M, Yamaguchi M, Niida T, Hada M, Ichijyo S, Hamaya R, Kanno Y, Isobe M, Kakuta T. Effect of elective percutaneous coronary inter-vention on hyperhemic absolute coronary blood fl ow volume and microvascular resistance. Circ Cardiovasc Interv 2017;10:e005073. doi: 10.1161/ CIRCINTERVENTIONS.117.005073
57. Brahim Harbaoui, Olivier Muller. Quantification of Absolute Co-ronary Blood Flow and Microvascular Resistance. Circ Cardio-vasc Interv 2017;10:e005916.DOI: 10.1161/CIRCINTERVENTI-ONS.117.005916
58. Matsuda J, Murai T, Kanaji Y, Usui E, Araki M, Niida T, Ichijyo S, Hamaya R, Lee T, Yonetsu T, Isobe M, Kakuta T. Prevalence and Cli-nical Significance of Discordant Changes in Fractional and Coronary Flow Reserve After Elective Percutaneous Coronary Intervention. J Am Heart Assoc 2016;5:e004400.
 This work is licensed under a
This work is licensed under a