Lucian Florin Dorobantu1, Paolo Spirito2, Cristina Caldararu1, Maria Alexandrescu1, Ruxandra Jurcut3,4, Bogdan Alexandru Popescu3,4, Ana Fruntelata1, Robert Adam4, Gheorghe Cerin5, Paolo Ferrazzi6
1 Spitalul Monza, Bucharest, Romania
2 Ente Ospedaliero Ospedali Galliera, Genoa, Italy
3 “Prof. Dr. C.C. Iliescu” Emergency Institute of Cardiovascular Diseases, Bucharest, Romania
4 “Carol Davila” University of Medicine and Pharmacy, Bucharest, Romania
5 Clinica S. Gaudenzio, Novara, Italy
6 Policlinco di Monza, Italy
Abstract: Hypertrophic cardiomyopathy (HCM) is a common genetic cardiac disease characterized by a diverse clinical presentation and natural history. Many affected individuals have mild or no symptoms and remain undiagnosed, others develop severe heart failure, and some die suddenly, often at a young age and in the absence of previous symptoms. Left ventricular outflow tract obstruction is the most frequent cause of heart failure. Abolition of outfl ow obstruction and alleviation of symptoms in drug-refractory patients requires invasive intervention. In the 1960s, surgical septal myectomy emerged as the primary strategy to relieve the obstruction. Since the 1990s, alcohol septal ablation has become a frequent alternative to myectomy, because it reduces the obstruction without requiring open-heart surgery. However, in recent years, the enthusiasm for septal ablation has decreased because of its arrhythmogenicity and incomplete abolition of obstruction. On the other hand, during the last 15 years, surgical myectomy has undergone important technical evolution and has become one of the safest open-heart procedures, with an operative mortality of <1% when performed by experienced surgeons at specialized centers. The operation substantially improves quality of life, prolongs survival and is considered by the international HCM guidelines the gold standard for invasive treatment of outflow obstruction. Keywords: HOCM, ventricular obstruction, surgical myectomy
Hypertrophic cardiomyopathy (HCM) is the most common inherited cardiac disease and has been estimated to occur in 1 of every 500 persons, a prevalence based on large study populations of different ethnicity1. These data also imply that many thousands of Romanians are affected by HCM. Such a high prevalence of the disease in the general population is in apparent contradiction with the clinical perception that HCM is relatively uncommon. This inconsistency between scientific evidence and clinical experience indicates that most affected individuals do not develop HCM-related symptoms and remain undiagnosed2. Indeed, the natural history of HCM is extremely heterogeneous. A large proportion of patients remain asymptomatic throughout life, while others develop important symptoms of heart failure, and some die suddenly often at a young age and in the absence of previous symptoms1-5. This strikingly diverse clinical course can be explained by the variety of morphologic expressions and pathophysiologic complexity of HCM, ranging from mild and localized to massive and extensive left ventricular (LV) hypertrophy6, and including diastolic dysfunction, LV outflow tract obstruction, mitral valve regurgitation, arrhythmias and myocardial ischemia7-10. Within this large spectrum of HCM pathophysiologic abnormalities, LV outflow obstruction represents the most frequent cause of heart failure symptoms and poses difficult management decisions1,2,10. Therefore, proper patient management requires a clear understanding of the pathophysiology and natural history of the disease, as well as an up to date knowledge of the recent advances in the treatment of LV outfl ow obstruction. Purpose of the present review is to summarize the most recent approaches to the management of HCM patients with outflow obstruction and new technical advances in HCM surgery, which are also
now available in Romania.
PATHOPHYSIOLOGY OF HEART FAILURE IN HCM
Symptoms of heart failure usually manifest as exertional dyspnea and occur in about 50% of HCM patients evaluated at referral centers1,2,10. In >60% of such patients, symptoms are predominantly caused by obstruction to LV outfl ow, which is most commonly due to systolic anterior motion of the mitral valve against the hypertrophied ventricular septum and is often associated with mitral valve regurgitation2,10 (Figure 1). In HCM, LV outfl ow obstruction is routinely assessed with echocardiography and defi ned as a peak instantaneous LV outflow gradient >30 mmHg2,10. Outflow obstruction is present at rest in about 25% of patients and develops in another 50% of patients using physiologic maneuvers, such as the Valsalva maneuver and
exercise echocardiography11. In those patients with heart failure symptoms and without a signifi cant LV outflow gradient either at rest or induced by the Valsalva maneuver, exercise echocardiography should be performed to identify a significant LV outflow obstruction, not elicited by the Valsalva maneuver, that may be responsible for symptoms during effort2,11-13. In the absence of LV outfl ow obstruction, symptoms of heart failure are due to diastolic dysfunction (with or without atrial fi brillation) in about 30% of HCM patients2,10. In patients with LV diastolic dysfunction who are in sinus rhythm, left atrial dysfunction may contribute to impaired LV filling and heart failure symptoms14. In about 5% of HCM patients, symptoms of heart failure are due to systolic dysfunction (ejection fraction <50%) with LV wall thinning and cavity dilatation2,10,15. This latter functional and morphologic evolution of the disease, generally described as the end-stage phase, is associated with the most advanced symptoms of heart failure within the HCM spectrum and may require heart transplantation15.
MEDICAL TREATMENT OF HEART FAILURE ASSOCIATED WITH LV OUTFLOW OBSTRUCTION
In patients with LV outflow obstruction and symptoms of heart failure, medical therapy with beta-blockers represents the first treatment option2. By their negative chronotropic and inotropic effects, these drugs reduce the heart rate, thereby increasing LV diastolic fi lling, and improve the myocardial oxygen supply-demand relationship16. Beta-blockers often alleviate heart failure
symptoms and may reduce the LV outflow gradient during exercise. There is no definitive evidence that these medications reduce the outflow gradient at rest, or prolong survival2. In HCM patients with outflow obstruction who do not tolerate beta-blockers, or whose symptoms are unresponsive to these drugs, verapamil or diltiazem may relieve heart failure by negative chronotropic and inotropic effects similar to those of beta-blockers2,17. However, calcium channel blockers, by reducing peripheral resistance and systemic arterial blood pressure may increase the LV gradient and worsen heart failure symptoms in HCM patients with outflow obstruction. Therefore, these medications should be used with caution and started at low doses1,2,4,5,10. In patients with persisting heart failure symptoms and elevated systolic pulmonary pressure despite the use of beta-blockers and calcium-channel blockers, the addition of diuretics may be effective for symptomatic relief1,2,10. However, diuretics should be used at low dosages in obstructive HCM, because an important decrease in LV filling pressure and diastolic volume may increase the outflow gradient in a disease which is also characterized by diastolic dysfunction and a small enddiastolic cavity2,10.
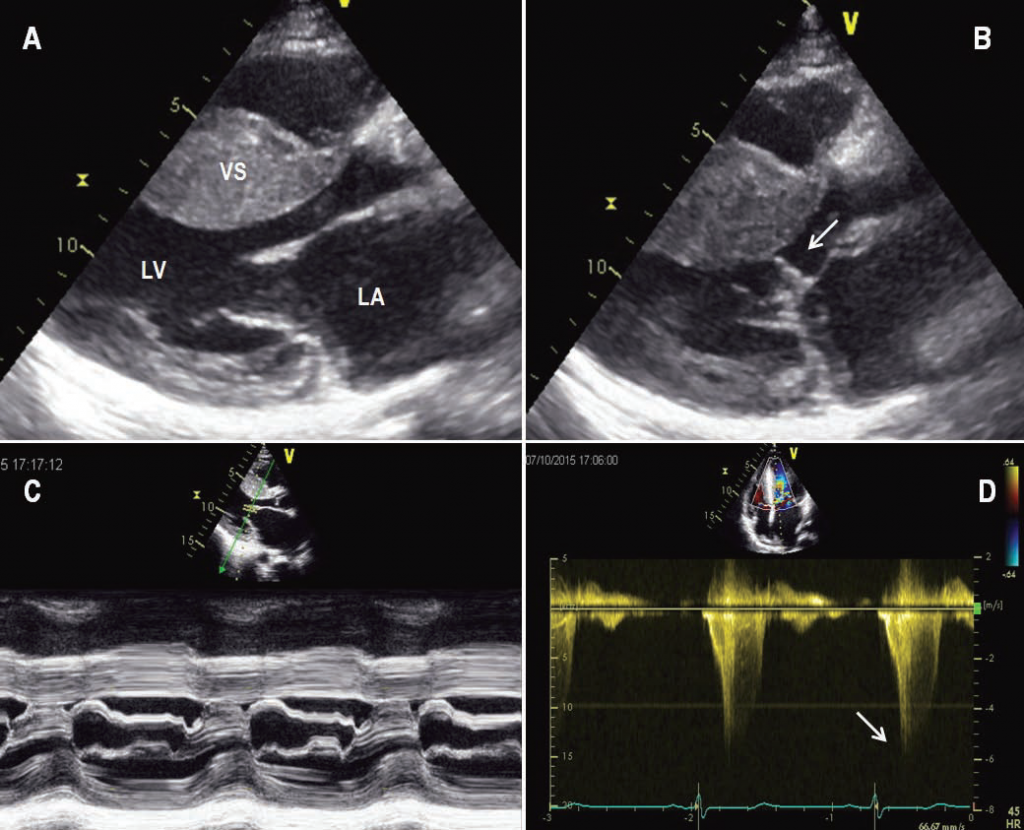
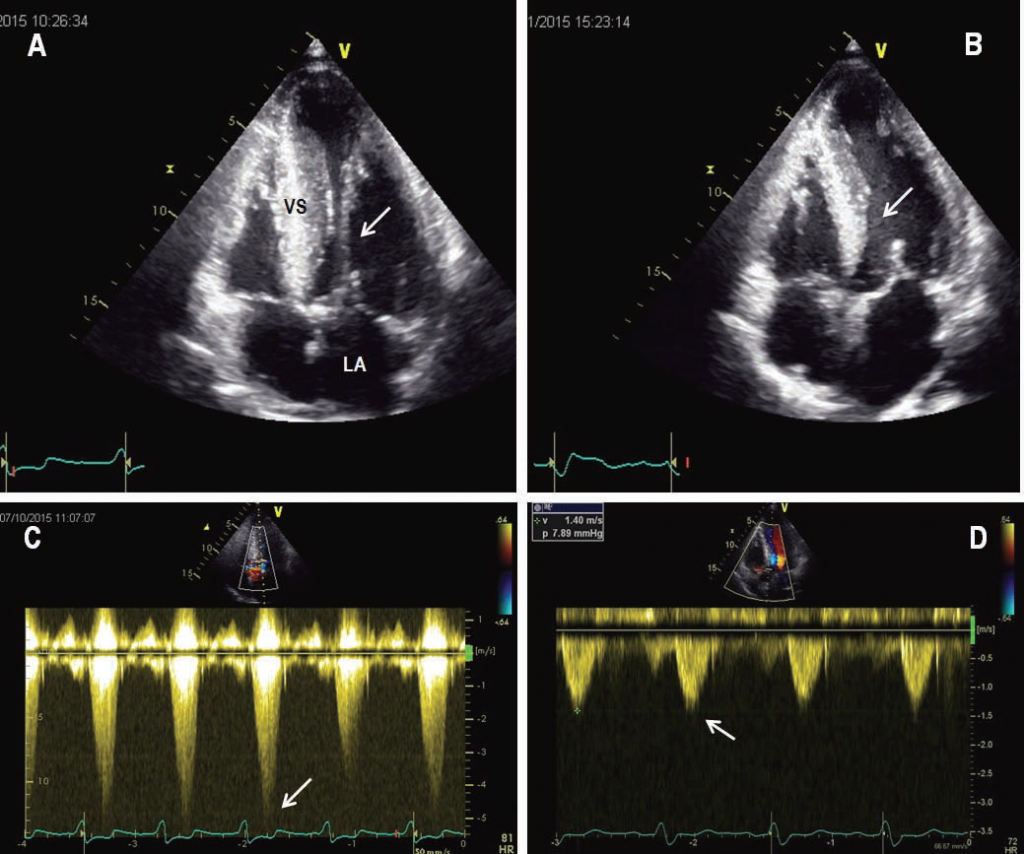
Figure 1. Echocardiogram from a patient with severe symptomatic obstructive HCM. (A) Still frame obtained during diastole shows a marked increase in ventricular septal thickness. (B) Still frame obtained during systole shows systolic anterior motion of the mitral-valve apparatus that causes obstruction of the left ventricular outfl ow tract (arrow). (C) M-mode echocardiogram shows prolonged mitral-septal contact during systole. (D) Doppler echocardiographic recording in the outfl ow tract demonstrates a peak instantaneous 130 mmHg gradient (arrow). LA=left atrium; LV=left ventricle; VS=ventricular septum.
INVASIVE THERAPY OF LV OUTFLOW OBSTRUCTION
Two very different invasive interventions, surgical septal myectomy or catheter-based alcohol septal ablation, can be performed for the purpose of relieving heart failure in HCM patients with LV outflow obstruction and symptoms unresponsive to medical therapy1,2,5,10. Because of the documented long-term results and many decades of experience with surgical septal myectomy,
the American College/American Heart Association Guidelines and the European Society of Cardiology Guidelines have selected septal myectomy as the gold standard treatment (i.e. Class I recommendation) for relief of LV outflow obstruction and heart failure symptoms in HCM patients with marked gradients (>50 mmHg) at rest or with provocation and important symptoms
unresponsive to medications2,12. Surgical myectomy remains the only option in patients who have outflow obstruction and associated abnormalities of the mitral valve apparatus that require surgical correction12. The Guidelines recommend the preferential use of alcohol septal ablation in elderly patients, or patients with significant comorbidities that would increase surgical
risk2,12. Because HCM is an extremely heterogeneous disease and is relatively uncommon in the general cardiology practice, surgical myectomy and alcohol septal ablation require specific operator and institutional experience. Therefore, in order to obtain successful outcomes with a low complication rates, it is imperative that these interventions are performed by operators
and teams with a large expertise in the evaluation and treatment of patients with HCM2,12. Despite these recommendations, the selection of patients for these two invasive interventions has varied substantially in different geographical areas. In the United States, surgical myectomy is generally performed by highly trained surgeons at HCM referral centers18,19. At the same centers, alcohol septal ablation is performed in a smaller and highly selected number of patients. On the other hand, a number of alcohol septal ablations are performed across the United States by interventional cardiologists at centers with more limited experience with HCM20. The decision of which procedure the patient should undergo is often influenced by the geographic location and HCM experience of the managing cardiologist. In Europe, invasive treatment of LV outflow obstruction has become a sore point in the management of patients with HCM, because in many European countries surgical myectomy has been virtually eradicated and replaced by alcohol septal ablation, including some countries with previous important surgical myectomy programs, such as Germany and Switzerland21. Therefore, in most European countries, HCM candidates to invasive treatment of outfl ow obstruction are left with alcohol septal ablation as the only therapeutic option, independently of their age and clinical presentation18. However, during the last 10 years, some European surgical centers in Rotterdam (the Netherlands)22,23, and Bergamo and Monza (Italy)24,25, have developed major myectomy programs with results that are similar, in terms of operative mortality (<1%) and long-term clinical improvement and survival, to those of some of
the most qualified centers in the United States, such as the Mayo Clinic and Cleveland Clinic18,19. This resurgence of septal myectomy in Europe has been driven by the evidence of frequent failures of alcohol septal ablation in relieving the LV outflow gradient2,23,20 and continuing concern with the potential consequences of the sizable myocardial infarction caused by alcohol ablation10,20. Indeed, the distribution of the septal perforator arteries in individual patients limits the possibility of this catheter-based procedure to target the site of outflow obstruction. Disaffection with septal ablation has also increased as a result of the rapidly growing evidence that abnormalities of the mitral valve leafl ets contribute importantly to LV outflow obstruction in many (probably most) patients with HCM and cannot be resolved with septal alcohol ablation, but only by surgery23,25-29.
TECHNICAL EVOLUTION OF SURGICAL MYECTOMY
A surgical myectomy for obstructive HCM was first performed in the late 1950s by Cleland and colleagues30. In the 1960s, the operation progressively evolved to a transaortic resection of a small amount of muscle from the proximal ventricular septum, a technique generally described as the Morrow classic myotomy and myectomy31. In the late 1970s, replacement of the mitral valve
with a prosthesis was suggested as an alternative to septal myectomy for the purpose of abolishing the outflow gradient and improve symptoms32. However, the main disadvantage of this procedure is that outflow obstruction is merely replaced with the important risks of limited durability, infection, thromboembolism and anticoagulation that are associated with prosthetic
valves.
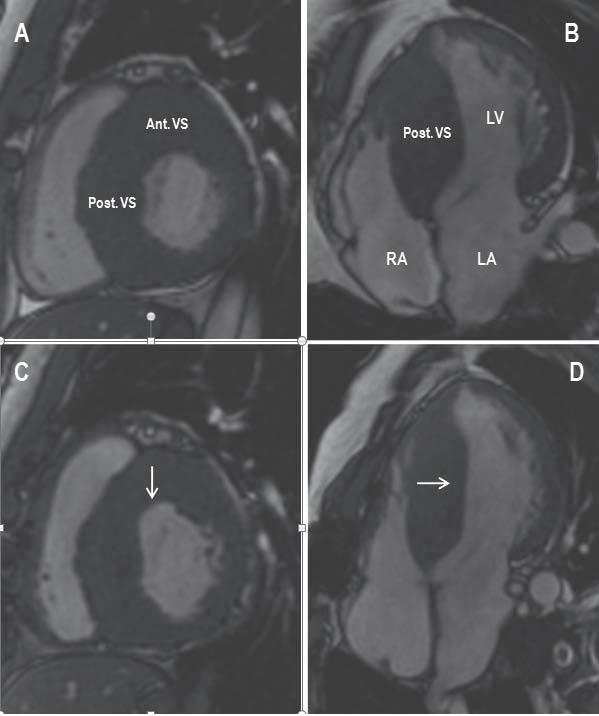
Figure 2. Comparison of preoperative and postoperative cardiovascular magnetic resonance (CMR) images shows the location and extent of the surgical myectomy. (A and B) Preoperative short axis and 4-chamber CMR images show marked hypertrophy of the anterior and posterior ventricular septum. (C and D) Postoperative short axis and 4-chamber CMR images show the area of the myectomy at the level of the anterior ventricular septum (arrow in C) and posterior ventricular septum (arrow in D). Ant.=anterior; LA=left atrium; LV=left ventricle; Post.=posterior; RA=right
atrium; VS=ventricular septum.
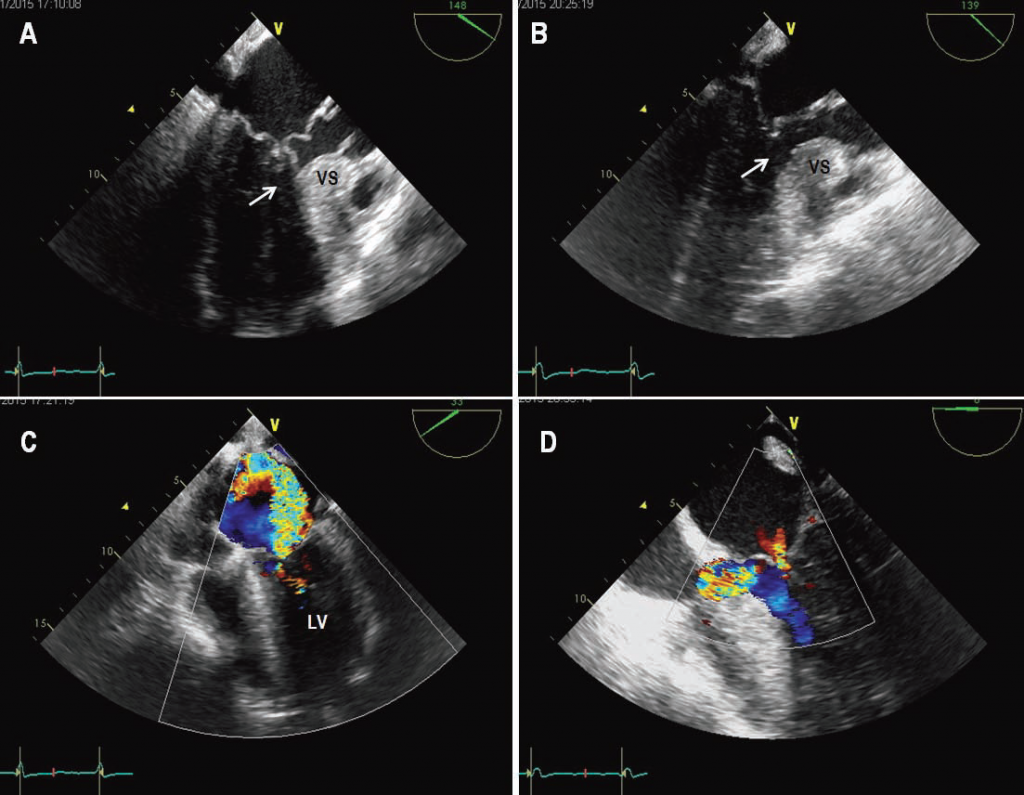
Figure 3. Intraoperative transesophageal echocardiography in a patient with obstructive HCM undergoing surgical myectomy. (A) Before myectomy, transesophageal echocardiographic still frame obtained in systole shows a redundant posterior mitral leaflet, and an anterior mitral leaflet that comes into contact with the anterior ventricular septum, causing left ventricular outfl ow obstruction (arrow); and (B) transesophageal Color Doppler shows severe mitral valve regurgitation with a jet directed towards the lateral wall of the left atrium. (C) In the same patient, after myectomy, transesophageal echocardiographic still frame obtained in systole shows absence of mitral-septal contact and outfl ow obstruction (arrow); and (D) transesophageal Color Doppler
shows only minimal mitral valve regurgitation. LV=left ventricle; VS=ventricular septum.
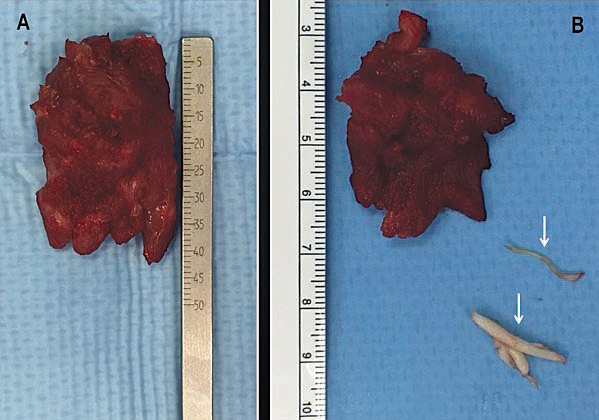
Figure 4. Specimen from an extended septal myectomy operation in two different patients. (A) The excised cardiac muscle specimen measures 4.5 cm in length and 3.0 cm in width, with a weight of 11 grams. (B) The excised cardiac muscle specimen measures 4.0 cm in length and 2.5 cm in width, with a weight of 5 grams. Two secondary chordae resected at the time of the myectomy are also shown (arrows). The cardiac muscle is excised via two incisions in the basal septum from 2 to 3 cm below the aortic valve and then extended distally to the base of the papillary muscles.
Therefore, mitral valve replacement is now reserved for highly selected HCM patients with primary mitral valve abnormalities not amenable to repair2. Over the past three decades, surgical myectomy has evolved from the classic Morrow operation to a more extended septal myectomy, which is guided by preoperative cardiovascular magnetic resonance and intraoperative
transesophageal echocardiography2,18,19,33 (Figures 2 and 3). Under the guidance of these imaging techniques that permit detailed visualization of septal anatomy and mitral valve function, the muscle incision is extended apically well past the 1.5 to 2.5 cm length of the original Morrow myectomy and beyond the area of mitral-septal contact18,19,24,25 (Figure 4). Extending the myectomy beyond the point of mitral-septal contact is of crucial importance to obtain relief of the outflow gradient, because failure to achieve this surgical target is routinely associated with a persistent postoperative gradient33. Fibrous or muscular structures connecting the papillary muscles to the ventricular septum or LV free wall are present in almost all patients with obstructive
HCM. These abnormalities limit the mobility of the papillary muscles and, by displacing the leafl ets into the outflow tract, contribute to systolic anterior motion of the mitral valve against the septum18,19,25,33. Therefore, careful inspection of the subvalvular mitral apparatus and mobilization of the papillary muscles from their attachments to the septum and ventricular free wall
has become an integral part of the extended surgical myectomy. When present, these fibrous or muscular structures are systematically excised and/or dissected free (up to the base of the papillary muscle) in order to increase papillary muscle mobility. Anomalous attachments of the papillary muscle directly into the anterior mitral leaflet are less common but must be resected when the papillary muscle is attached to the body of the leaflet24,25,33 (Figure 5). Anomalous chordal structures or fi brous attachments of the mitral leaflets to the ventricular septum or free wall can also be found in patients with HCM and, whenever present, have to be excised or divided25,33. Cardiovascular magnetic resonance has proved useful in the identification of these multiple abnormalities of the subvalvular mitral apparatus and has gained an important role in the preoperative evaluation of HCM candidates to surgical myectomy34. Most recently, intraoperative transesophageal 3-D echocardiography has been used to obtain detailed reconstruction of the pre and postoperative morphology and function of the mitral valve leaflets in candidates to ventricular septal myectomy and associated mitral valve repair35 (Figure 6). Indeed, the proper use of different imaging modalities is of critical importance for planning the surgical intervention, as well as monitoring the procedure during the operation, and assessing the results36.
Figure 5. (A) Preoperative echocardiogram from a patient with obstructive HCM shows an abnormal papillary muscle directly attached to the anterior mitral leaflet, in the absence of chordae, which comes into close proximity of the ventricular septum during systole (arrow). (B) Postoperative echocardiogram. The abnormal papillary muscle has been resected and the area of the septal myectomy (arrow) is clearly visible. There is normal coaptation of the mitral valve leafl ets in systole, without systolic motion of the valve against the ventricular septum. (C) Preoperative Doppler echocardiographic recording in the LV outflow tract shows a peak instantaneous 100 mm Hg gradient (arrow). (D) Postoperative Doppler echocardiographic recording in the LV outflow tract demonstrates absence of a significant intra-ventricular gradient (8 mm Hg) (arrow). LA=left atrium; VS=ventricular septum.
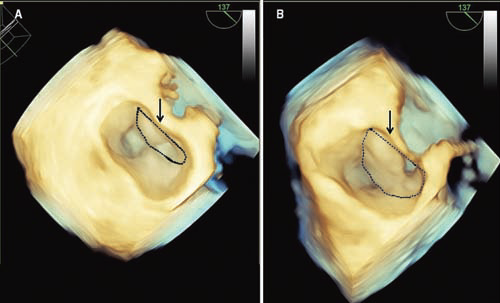
Figure 6. Preoperative and postoperative 3D echocardiographic images of the mitral valve obtained at the time of mitral leaflet coaptation in a patient with obstructive HCM undergoing septal myectomy. (A) Preoperatively, the anterior mitral leaflet (delimited by the dotted area) represents only a minority of the mitral valve surface. (B) Postoperatively, the anterior mitral leaflet (delimited by the dotted area) represents the majority of the mitral valve surface.
In recent years, it has become evident that a wide range of mitral valve abnormalities (most of them secondary to LV remodeling) are present in patients with obstructive HCM, such as elongated or calcified mitral valve leaflets and fibrotic and/or retracted secondary chordae, and play an important role in the pathophysiology of the systolic anterior motion of the mitral valve and outflow obstruction25-29. A variety of surgical approaches have been devised to correct these mitral valve abnormalities, including transaortic extension or plication of the anterior mitral leafl et22,27,28 and, more recently, resection of fibrotic and retracted secondary chordae of the anterior leaflet25. At referral centers for HCM surgery, these advances in the myectomy operation have become a routine component of the surgical procedure and, together with modern myocardial preservation and other surgical techniques, have reduced operative mortality to <1% and improved long-term clinical results and survival10. Indeed, recent studies show that patients with obstructive HCM and severe preoperative heart failure symptoms who undergo the myectomy operation at experienced HCM surgical centers have a long-term outcome substantially more favorable than that of patients with outflow obstruction and no or mild symptoms at initial evaluation, and similar to that of HCM patients without outfl ow obstruction10,18,19,37,38.
FINAL CONSIDERATIONS
Our review of the current management of patients with obstructive HCM underscores the complexity of this disease and the indisputable evidence that LV outflow obstruction leads to severe heart failure symptoms and reduced survival. From the late 1990s, in most European countries percutaneous septal ablation has replaced the myectomy operation as the intervention of choice to abolish the outflow obstruction. However, it has become increasingly evident that, in many HCM patients, septal ablation is incapable of abolishing the pathophysiologic mechanisms responsible for the outflow gradient, and that surgical myectomy is required to guarantee satisfactory results. This clinical evidence has generated an active surgical response and, in recent years, the number of centers of excellence for surgical management of patients with HCM has increased in European countries. Romania is on the way to become one of these countries. Because of this increasing access to surgical myectomy, many more patients with obstructive HCM and severe heart failure symptoms can now aspire to a substantially improved quality of life and an extended life span.
Conflict of interest: None of the authors have conflicts of interest to report. Acknowledgements: We gratefully acknowledge the assistance of George Cerin, Spitalul Monza, Bucharest, in selecting from the informatics archives of the Spitalul Monza the images for the each of the 6 figures included in the manuscript. We also gratefully acknowledge the assistance of Domenico Carratta,
Ente Ospedaliero Ospeli Galliera, Genoa, Italy, in assembling some of the fi gures.
References
1. Maron BJ, Maron MS. Hypertrophic cardiomyopathy. Lancet 2013;381: 242-55.
2. Gersh BJ, Maron BJ, Bonow RO, Dearani JA, Fifer MA, Link MS, Naidu SS, Nishimura RA, Ommen SR, Rakowski H, Seidman CE, Towbin JA, Udelson JE,Yancy CW. 2011 ACCF/AHA guidelines for the diagnosis and treatment of hypertrophic cardiomyopathy: A report of the American College of Cardiology Foundation and America Heart Association Task Force on Practice Guidelines. Circulation 2011;124: 2761-96.
3. P Spirito, F Chiarella, L Carratino, M Berisso, P Bellotti, C Vecchio. N Eng J Med 1989;320:749-55.
4. Spirito P, Seidman CE, McKenna WJ, Maron BJ. N Eng J Med 1997;336: 775-85.
5. Maron BJ, McKenna WJ, Danielson GK, Kappenberger LJ, Kuhn HJ, Seidman CE, Shah PM, Spencer WH 3rd, Spirito P, Ten Cate FJ, Wigle ED. American College of Cardiology/European Society of Cardiology clinical expert consensus document on hypertrophic cardiomyopathy. J Am Coll Cardiol. 2003;42-1687-713.
6. Spirito P, Bellone P, Harris KM, Bernabo P, Bruzzi P, Maron BJ. Magnitude of left ventricular hypertrophy and risk of sudden death in hypertrophic cardiomyopathy. N Engl J Med 2000;342:1778-85.
7. WJ Paulus, BH Lorell, WE Craig, J Wynne, JP Murgo, W Grossman. Comparison of the effects of nitroprusside and nifedipine on diastolic properties in patients with hypertrophic cardiomyopathy: altered left ventricular loading or improved muscle inactivation? J Am Coll Cardiol. 1983;2:879–86.
8. Maron MS, Olivotto I, Betocchi S, Casey SA, Lesser JR, Losi MA, Cecchi F, Maron BJ. N Eng J Med 2003;348:295-303.
9. Cannon RO 3rd, Rosing DR, Maron BJ, Leon MB, Bonow RO, Watson RM, Epstein SE. Myocardial ischemia in patients with hypertrophic cardiomyopathy: contribution of inadequate vasodilator reserve and elevated left ventricular filling pressures. Circulation. 1985;71:234–43.
10. Maron BJ, Ommen SR, Semsarian C, Spirito P, Olivotto I, Maron MS. Hypertrophic cardiomyopathy: present and future, with translation into contemporary cardiovascular medicine. J Am Coll Cardiol. 2014;64:83-99.
11. Maron MS, Olivotto I, Zenovich AG, Link MS, Pandian NG, Kuvin JT, Nistri S, Cecchi F, Udelson JE, Maron BJ. Hypertrophic cardiomyopathy is predominantly a disease of left ventricular outflow tract obstruction. Circulation. 2006;114:2232–9.
12. Elliott PM, Anastasakis A, Borger MA, Borggrefe M, Cecchi F, Charron P, Hagege AA, Lafont A, Limongelli G, Mahrholdt H, McKenna WJ, Mogensen J, Nihoyannopoulos P, Nistri S, Pieper PG, Pieske B, Rapezzi C, Rutten FH, Tillmanns C, Watkins H. 2014 ESC Guidelines on diagnosis and management of hypertrophic cardiomyopathy. Eur Heart J 2014;35:2733–79
13. Popescu BA, Rosca M, Schwammenthal E. Dynamic obstruction in hypertrophic cardiomyopathy. Curr Opin Cardiol 2015;30:468-74.
14. Roşca M, Popescu BA, Beladan CC, Călin A, Muraru D, Popa EC, Lancellotti P, Enache R, Coman IM, Jurcuţ R, Ghionea M, Ginghină C. Left atrial dysfunction as a correlate of heart failure symptoms in hypertrophic cardiomyopathy. J Am Soc Echocardiogr 2010;23:1090-98.
15. Harris KM, Spirito P, Maron MS, Zenovich AG, Formisano F, Lesser JR, Mackey-Bojack S, Manning WJ, Udelson JE, Maron BJ. Prevalence, clinical profile, and significance of left ventricular remodeling in the end-stage phase of hypertrophic cardiomyopathy. Circulation 2006;114:216–25.
16. Bourmayan C, Razavi A, Fournier C, Dussaule JC, Baragan J, Gerbaux A, Gay J. Effect of propranolol on left ventricular relaxation in hypertrophic cardiomyopathy: an echographic study. Am Heart J. 1985;109:1311–6.
17. Gilligan DM, Chan WL, Joshi J, Clarke P, Fletcher A, Krikler S, Oakley CM. A double-blind, placebo controlled crossover trial of nadolol and verapamil in mild and moderately symptomatic hypertrophic cardiomyopathy. J Am Coll Cardiol. 1993;21:1672–9.
18. Ommen SR, Maron BJ, Olivotto I, Maron MS, Cecchi F, Betocchi S, Gersh BJ, Ackerman MJ, McCully RB, Dearani JA, Schaff HV, Danielson GK, Tajik AJ, Nishimura RA. Long-term effects of surgical septal myectomy on survival in patients with obstructive hypertrophic cardiomyopathy. J Am Coll Cardiol. 2005;46:470–47
19. Desai MY, Bhonsale A, Smedira NG, Naji P, Thamilarasan M, Lytle BW, Lever HM. Predictors of long-term outcomes in symptomatic hypertrophic obstructive cardiomyopathy patients undergoing surgical relief of left ventricular outflow tract obstruction. Circulation. 2013;128:209-16.
20. Maron BJ, Nishimura RA. Surgical septal myectomy versus alcohol septal ablation. Assessing the status of the controversy in 2014. Circulation 2014;130:1167-24
21. Maron BJ, Yacoub M, Dearani JA. Controversies in cardiovascular medicine. Benefits of surgery in obstructive hypertrophic cardiomyopathy: bring septal myectomy back for European patients. Eur Heart J 2011;32:1055–1058.
22. van der Lee C, Koffl ard MJ, van Herwerden LA, Vletter WV, ten Cate FJ. Sustained improvement after combined anterior mitral leafl et extension and myectomy in hypertrophic obstructive cardiomyopathy. Circulation 2003;108:2088-92.
23. ten Cate FJ, Soliman OI, Michels M, Theuns DA, de Jong PL, Geleijnse ML, Serruys PW. Long-term outcome of alcohol septal ablation in patients with obstructive hypertrophic cardiomyopathy: a word of caution. Circ Heart Fail. 2010;3(3):362-369.
24. Iacovoni A, Spirito P, Simon C, Iascone M, Di Dedda G, De Filippo P, Pentiricci S, Boni L, Senni M, Gavazzi A, Ferrazzi P. A contemporary European experience with surgical septal myectomy in hypertrophic cardiomyopathy. Eur Heart J. 2012;33:2080-7.
25. Ferrazzi P, Spirito P, Iacovoni A, Calabrese A, Migliorati K, Simon C, Pentiricci S, Poggio D, Grillo M, Amigoni P, Iascone M, Mortara A, Maron BJ, Senni M, Bruzzi P. Transaortic chordal cutting: mitral valve repair for obstructive hypertrophic cardiomyopathy with mild septal hypertrophy. J Am Coll Cardiol. 2015;66:1687-99.
26. Smedira NG, Lytle BW, Lever HM, Rajeswaran J, Krishnaswamy G, Kaple RK, Dolney DO, Blackstone EH. Current effectiveness and risks of isolated septal myectomy for hypertrophic obstructive cardiomyopathy. Ann Thorac Surg 2008;85:127-33.
27. Balaram SK, Ross RE, Sherrid MV, Schwartz GS, Hillel Z, Winson G, Swistel DG. Role of mitral valve plication in the management of hypertrophic cardiomyopathy. Ann Thorac Surg. 2012;94:1990-7.
28. Vriesendorp PA, Schinkel AF, Soliman OI, Koffl ard MJ, de Jong PL, van Herwerden LA, Ten Cate FJ, Michels M. Long-term benefit of myectomy and anterior mitral leaflet extension in obstructive hypertrophic cardiomyopathy. Am J Cardiol 2015;115:670-5
29. Sherrid MV, Balaram S, MD, Kim B, Axel L, Swistel DG. The mitral valve in obstructive hypertrophic cardiomyopathy. A test in context. J Am Coll Cardiol 2016;67:1846-58.
30. Goodwin JF, Hollman A, Cleland WP, Teare D. Obstructive cardiomyopathy simulating aortic stenosis. Br Heart J. 1960;22:403–414.
31. Morrow AG, Fogarty TJ, Hannah H 3rd, Braunwald E. Operative treatment in idiopathic hypertrophic subacute stenosis. Techniques and the results of preoperative and postoperative clinical and hemodynamic assessments. Circulation. 1968;37:589–596
32. Cooley DA, Wukasch DC, Leachman RD. Mitral valve replacement for idiopathic hypertrophic subaortic stenosis. Results in 27 patients. J Cardiovasc Surg 1976;17:380–7.
33. Dearani JA, Ommen SR, Gersh BJ, Schaff HV, Danielson GK. Surgery insight: septal myectomy for obstructive hypertrophic cardiomyopathy: the Mayo Clinic experience. Nat Clin Pract Cardiovasc Med. 2007;4:503–512.
34. Maron MS, Olivotto I, Harrigan C, Appelbaum E, Gibson CM, Lesser JR, Haas TS, Udelson JE, Manning WJ, Maron BJ. Mitral valve abnormalities identified by cardiovascularmagnetic resonance represents a primary phenotypic expression of hypertrophic cardiomyopathy. Circulation 2011;124:40–7.
35. Yang DH, Kang JW, Kim N, PhD; Song JK, Lee JW, Lim TH, MD. Myocardial 3-D printing for septal myectomy guidance in a patient with obstructive hypertrophic cardiomyopathy. Circulation 2015;132:300-301.
36. Cardim N, Galderisi M, Edvardsen T, Plein S, Popescu BA, D’Andrea A, Bruder O, Cosyns B, Davin L, Donal E, Freitas A, Habib G, Kitsiou A, Petersen SE, Schroeder S, Lancellotti P, Camici P, Dulgheru R, Hagendorff A, Lombardi M, Muraru D, Sicari R. Role of multimodality cardiac imaging in the management of patients with hypertrophic cardiomyopathy: an expert consensus of the European Association of Cardiovascular Imaging Endorsed by the Saudi Heart Association. Eur Heart J Cardiovasc Imaging 2015;16:280-280hh. doi:10.1093/ehjci/jeu291
37. Ong KC, Geske JB, Hebl VB, Nishimura RA, Schaff HV, Ackerman MJ, Klarich KW, Siontis KC, Coutinho T, Dearani JA, Ommen SR, Gersh BJ. Pulmonary hypertension is associated with worse survival in hypertrophic cardiomyopathy. Eur Heart J Cardiovasc Imaging 2016 17: 604-610
38. Spirito P, Ferrazzi P. Pulmonary hypertension in hypertrophic cardiomyopathy: a forgotten marker in the identifi cation of candidates to surgical myectomy? Eur Heart J Cardiovasc Imaging 2016 17: 611-612.
 This work is licensed under a
This work is licensed under a