Lucia Cojocaru1,2, Luminita Matei1,2, Andreea Cuculici3, Ionut Bulbuc2, Ioan Mircea Coman3,4, Marius Toringhibel1,2
1 Clinic of Cardiology, Emergency Clinical Hospital of Constanta, Romania
2 Faculty of Medicine, „Ovidius” University, Constanta, Romania
3 „Prof. Dr. C.C. Iliescu” Emergency Institute for Cardiovascular Diseases, Bucharest, Romania
4 „Carol Davila” University of Medicine and Pharmacy, Bucharest, Romania
Abstract: Extended survival in cancer patients raises the problem of late cardiotoxicity. Hodgkin lymphoma survivors are at increased risk of therapy-related cardiac complications, risking premature morbidity and death. We report the case of a 43 years old woman that, after successful combined therapy for Hodgkin lymphoma, has developed, during the next fi fteen years, multiple cardiac involvements: pericardial tamponade and constriction, valvular and myocardial dysfunction. Combined diagnostic techniques have been used to clarify the nature of cardiac disease and to exclude local lymphoma recurrence, and specifi c medical and surgical treatment solutions were approached. Healthcare providers must be aware that screening for early detection of cancer treatments cardiotoxicity is mandatory, and is able to ensure better patient outcomes.
Keywords: Hodgkin lymphoma, cardiotoxicity, anthracycline, radiotherapy, cardiovascular risk
BACKGROUND
Cardiovascular disease (CVD) and cancer are the two leading causes of morbidity and mortality worldwide1. Early diagnosis and improvement of treatment have both contributed to increased survival in many cancer patients2. As a result, there are now close to 30 milli-on cancer survivors worldwide, and the numbers are increasing3. Unfortunately, many cancer treatments carry a great risk of complications and, as life expec-tancy grows, the associated morbidity and mortality will also increase.
Hodgkin lymphoma (HL) is now curable in more than 75% cases4. HL survivors are at increased risk of therapy-related complications (especially cardiac disease) that may present years after treatment and may generate premature morbidity and death4,5. This may be the result of cardiotoxicity (direct toxic effect of the cancer treatment on heart structure and func-tion) or may be due to accelerated development of CVD, especially in the presence of traditional cardio-vascular (CV) risk factors5,6.
Healthcare providers must take into account these complications, since screening can improve early detection that may lead to better management with favorable outcomes.
CASE PRESENTATION
We report the case of a 43 years old woman who was fi rst admitted to the Cardiology Clinic of Emergen-cy Clinical Hospital of Constanta in January 2016, for evaluation of dyspnea and fatigue. The symptoms ap-peared at moderate physical exertion and had a slow progression during the last year. She also accused dry cough and negate having angina.
Her past medical history was signifi cant by being diagnosed in July 2000 with stage IIB nodular sclerosis HL. She was treated with combined chemotherapy and radiotherapy (RT). Chemotherapy consisted of two cycles of ABVD (A for Adriamycin – doxorubicin, B for bleomycin, V for vinblastine and D for dacarba-zine) and two cycles of AVD and lomustine. Following chemotherapy, in March and April 2001, extended fi eld RT was given to the mantle fi eld (which inclu-ded lymph node areas in the neck, chest, and arm-pits). Her CV history begins in April 2002 when, at the chest computed tomography (CT) performed for follow- up, a small pericardial effusion was noted to-gether with pulmonary fi brosis. The pericardial fl u-id accumulated gradually until October 2004, when the patient was admitted for cardiac tamponade and treated by emergency pericardiocentesis followed by anterior interphrenic pericardiectomy. The pericardi-al biopsy taken at the time of the surgery was negative for neoplastic involvement. During the prolonged hospitalization period she experienced an episode of deep vein thrombosis of the left lower limb, with favorable outcome under anticoagulant therapy. Af-ter year 2005 she had no cardiologic follow-up until September 2015 when she was referred to our clinic with the suspicion of heart failure (HF). Regarding the patient’s lifestyle, we mention that she is a former oc-casional smoker.
The physical examination on the admission re-vealed an overweight patient (BMI=29.6 Kg/m2) with a post-sternotomy scar and interscapular hyperpig-mentation (due to radiation dermatitis). She was non-febrile, with a blood oxygen saturation level of 98%, respiratory rate of 16 breaths per minute, blood pres-sure of 115/60 mmHg and a regular heart rate of 100 bpm. The auscultation of the heart revealed a grade 2/6 diastolic murmur at the aortic area and grade 3/6 systolic murmur on the left lower sternal border and apical region. The exam of the peripheral arteries was normal and the signs of systemic or pulmonary con-gestion were absent.
The ECG revealed sinus rhythm with a heart rate of 84 bpm, left anterior fascicular block, complete right bundle branch block, right ventricle hypertrophy, ST-T secondary changes and prolonged corrected QT interval (504 ms) (Figure 1).
Laboratory tests showed normal values of myocar-dial necrosis markers and D-dimer, elevated NT pro-BNP (485 pg/ml), slightly elevated LDL-cholesterol, hyperuricemia, and high values of thyroid stimulating hormone. The complete hemogram, liver function tests, renal function tests and ionogram were in nor-mal limits.
Transthoracic (TTE) and transesophageal (TEE) echocardiography revealed normal cardiac chambers size with mild septal hypertrophy, normal diastolic function, mild left ventricle systolic dysfunction (left ventricle ejection fraction – LVEF of 50%) with slight hypokinesis and paradoxical motion of the interven-tricular septum. The aortic, mitral, tricuspid valves and the remaining pericardium were thickened; there were nodular calcifi cations of the aortic cusps, mi-tral chordae and posterior mitral annulus extended to the basal portion of the posterior mitral lea fl et and an endocardial calcifi cation at the level of left ventri-cular ejection tract. There was aortic sclerosis and moderate to severe aortic insuffi ciency (Figure 2A), moderate mitral insuffi ciency (Figure 2B), severe tri-cuspid insuffi ciency with two regurgitant jets and mild pulmonary hypertension (pulmonary artery systolic pressure of 39 mmHg).
The pulmonary function test revealed a moderate mixed respiratory dysfunction with a 26% decrease in total lung capacity, moderate obstructive ventilatory dysfunction, severe distal obstructive syndrome and a diffusing capacity for carbon monoxide at the lower normal range.
A high-resolution CT and CT pulmonary angiogra-phy showed no signs of pulmonary embolism, confi rmed the presence of pleuro-pulmonary fi brosis and revealed a mass at the level of posterior mediastinum that raised suspicion of lymphoma relapse.
The patient was started on bisoprolol, perindopril (switched to candesartan due to cough), spironolac-tone, furosemide, atorvastatin, aspirin and levothyro-xine, and referred to the Hematology Department to further investigate the mediastinum mass. Due to the high risk of bleeding associated with biopsy, an evalu-ation with positron emission tomography – CT scan was preferred and based on this investigation and on stationary aspect of the mass the lymphoma relapse was excluded. After the discharge the patient was lost to follow-up until November 2017 when she was readmitted to our clinic. The symptoms progressed and she had fatigue and dyspnea at mild effort. The physical exam and ECG revealed the same patholo-gical fi ndings as the previous ones. Laboratory tests showed higher values of NT- proBNP (612 pg/ml), nor-malized lipid profi le and uric acid levels; the complete hemogram, liver function tests, renal function tests, ionogram and myocardial necrosis markers were in normal limits. The pulmonary function test showed similar results to the previous one. The changes at TTE from the previous exam were: mild dilated right chambers, mild left ventricle systolic dysfunction (LVEF of 45%) and mitral and tricuspid Doppler fl ow patterns suggestive of constrictive pericarditis (Figu-re 3). To further elucidate the suspicion of constric-tive pericarditis a CT scan (Figure 4) and a cardiac magnetic resonance imaging (MRI) (Figure 5) were performed, but the results were inconclusive in re-gard to the pericardial involvement, due to extensive mediastinal fi brosis. Because the non-invasive testing was inconclusive she was transferred to “Prof. Dr. C.C. Iliescu” Emergency Institute for Cardiovascular Diseases where she underwent cardiac catheteriza-tion which confi rmed the diagnosis of constrictive pericarditis. The coronarography performed on this occasion revealed normal epicardial coronary arte-ries. The patient was referred for surgery, and given the severity of the associated valvulopaties, the heart team decided in favor of complex intervention with pericardial stripping and concomitant aortic, mitral and tricuspid valve surgery. At the moment she is ad-mitted to “Prof. Dr. C.C. Iliescu” Emergency Institute for Cardiovascular Diseases in
the I Cardiovascular Surgery Clinic recovering after she successfully un-derwent surgery.
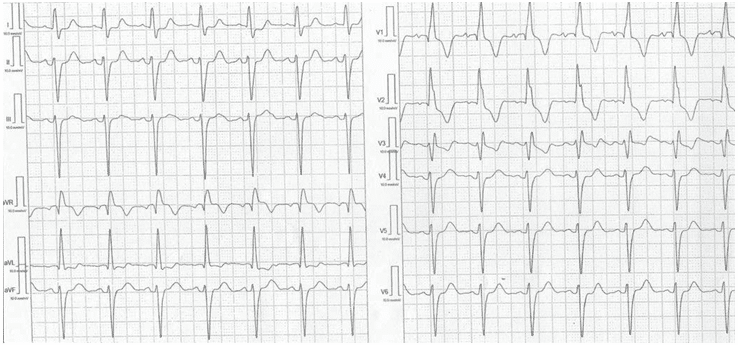
Figure 1. Electrocardiography. Sinus rhythm, 84 bpm, bifascicular block (left anterior fascicular block + complete right bundle branch block), right ventricular hypertrophy (qR pattern in V1), secondary ST-T changes, corrected QT interval = 504 ms.
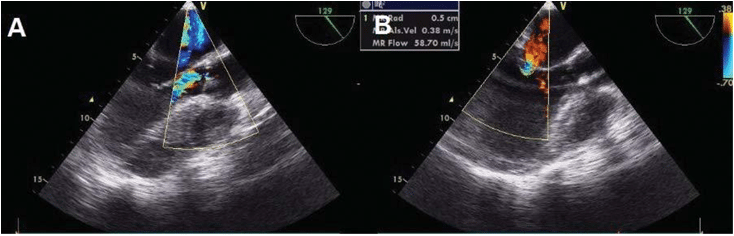
Figure 2. Transesophageal echocardiography. Midesophageal 129-degree view. Color Doppler at the level of aortic valve – eccentric aortic regurgita-tion (AR) with a jet width ratio relative to left ventricular outfl ow tract of 48% (qualitative parameter that can underestimate the severity of AR in eccentric jets) corresponding to moderate AR (panel A). Color Doppler at the level of mitral valve – measure of the proximal isovelocity surface area radius (0.5 cm). This value was used to calculate the effective regurgitant orifi ce area (0.2 cm2) and regurgitant volume (37 ml), quantitative parameters indicative of moderate mitral regurgitation (panel B).
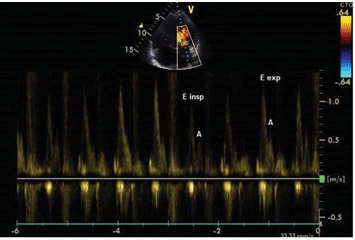
Figure 3. Transthoracic echocardiography. Mitral infl ow pulsed-wave spectral Doppler showing restrictive pattern (E/A ratio >2) and respira-tory variations – a decrease of peak fl ow velocity with >25% (from 1.27 to 0.9 m/s) during inspiration (insp – inspiration, exp – expiration).
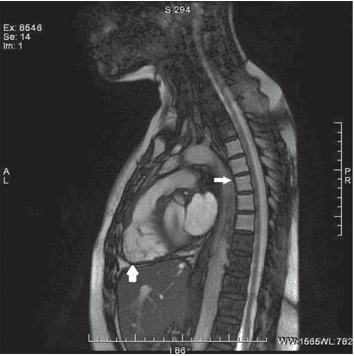
Figure 5. Cardiac MRI FIESTA RVOT showing segmental myocardial thinning at the level of the free wall of the right ventricle, with T1/T2 hyposignal with progressive contrast enhancement, having fi brotic sub-strate (big arrow); the bodies of T2-T6 thoracic vertebrae with hypersig-nal T1/T2 compatible with radiation-induced bone marrow degeneration (small arrow).
DISCUSSIONS
We presented this case to highlight the risk of CV complications that can appear years after fi nishing the treatment for HL and thus, the importance of systematic follow-up of patients who received cardiotoxic therapies.
The long-term survivors of HL must be monito-red periodically not only for disease recurrence but also for long- term complications related to the treat-ment4. Since 2000s most patients are treated with ABVD chemotherapy with or without RT, and those may generate various long-term sequelae (second ma-lignancy, CVD, pulmonary fi brosis, thyroid dysfunc-tion, infertility, muscle atrophy etc)4. From these, CVD is the most common non-malignant cause of death in long- term survivors of HL4. Long-term CV complications are mainly due to doxorubicine- based chemotherapy and RT, with an additive effect when both are used7. HL survivors will experience more than twice the number of CVD, nearly fi ve times the number of more severe CV conditions and, on avera-ge, have one severe, life -threatening, or fatal CV con-dition, when compared to community controls8.
Anthracyclines (eg, doxorubicin) induce cardio-toxicity mainly by oxidative stress, the generation of reactive oxygen species and lipid peroxidation of the cell membrane damaging the cardiomyocites5. This cardiotoxicity may be classifi ed in acute (immediately after infusion), early (within the fi rst year of treat-ment) or late (median of 7 years after treatment) and is mainly related to the total dose used during the treatment4,5,9. A dose of 400 mg/m2 of doxorubicin is associated with a 5% incidence of congestive HF, and higher doses lead to an exponential increase in risk10. Anthracycline induced cardiotoxicity usually consist in a continuous progressive decline in LVEF that is initially asymptomatic5. If this dysfunction is detected early and treated with HF medications, patients usu-ally have a good functional recovery, but if it’s identi-fi ed later, HF is typically diffi cult to treat11.
The incidence of RT- induced cardiotoxicity is di-ffi cult to evaluate but the risk is related to the vol-ume of the heart irradiated and the dose received5,12. Supradiaphragmatic RT, that include portions of the heart, can generate acute or delayed pericardial dis-ease, myocardial ischemia (through the development of severe atherosclerotic and non -atherosclerotic disease), cardiomyopathy (restrictive cardiomyopathy due to myocardial fi brosis), heart failure, valvular abnormalities, or conduction defects (bradycardia, sick sinus syndrome and heart block)3. From those, the most common are valvular disorders, angina pec-toris and myocardial infarction12. The median time to diagnosis is 19 years after treatment and the risk of CVD persists for at least 25 years after the initial treatment12,13. A clinically signifi cant valvular disease can appear in up to 40% of HL survivors and gradually progresses in time12. It can involve the aortic, mitral and tricuspid valves with aortic insuffi ciency being the most prevalent one12. Cardiac valve surgery is challen-ging in these patients due to mediastinal fi brosis, im-paired wound healing and associated coronary artery, myocardial and pericardial disease5. The transcathe-ter valve implantation may be an attractive option in this situation5. Pericardial disease (pericardial effusion with or without tamponade and pericardial constric-tion), rarely occurs nowadays, due to lower doses and modern RT techniques12. In this patients the diagno-sis of constrictive pericarditis is very challenging due to the diffi culty to identify the degree of underlying restrictive cardiomyopathy, so these patients may require multimodal imaging (including echocardiogra-phy, CT, and cardiac MRI) and invasive hemodynamic catheterization to assess elevation in fi lling pressures with diastolic equalization, ventricular interdepen-dence and intrathoracic-intracardiac dissociation14,15.
Our patient experienced complex early (effusive pericarditis with cardiac tamponade) and late CV complications (multiple valvulopaties, mild left ven-tricle – LV – systolic dysfunction, bifascicular block, prolonged QT and constrictive pericarditis) and we believe that extended-fi eld RT was the main cause as the total dose of doxorubicin administered was less than 300 mg/m2.
The HL survivors are also at increase risk of respi-ratory complications, as was the case of our patient. The pulmonary toxicity is mainly generated by ble-omycin and RT. They both can generate pneumoni-tis and pulmonary fi brosis associated with increased mortality3. There are also emerging data that chest RT may be associated with pulmonary hypertension through pulmonary vascular damage, but the con-nection is not as clearly established as the one with pulmonary fi brosis3. The presence of pulmonary fi – brosis in our patient is a confounding factor in esta-blishing the severity of symptoms attributable to the cardiac involvement and negatively impacts survival.
HL survivors must be screened similar to other high-risk populations and offered lifelong surveillance. Patients must undergo an annually exam at their fami-ly doctor with a clinical exam, serum measurements of lipid pro fi les and glucose levels3. A cardiology exam (with ECG and TTE) must be made at the beginning of the treatment, periodically during the treatment and after the completion of treatment (that serves as baseline evaluation for further exams). After this, the patient must be referred to a cardiologist whenever there are symptoms and signs suggestive of CVD, at 1 and 5 years after completion of cancer treatment (if they received ≥300 mg/m2 of doxorubicin or equiva-lent5, or if they developed cardiotoxicity during che-motherapy), and regularly after 5 years from RT (at least every 5 years thereafter, even if asymptomatic). TTE remains the method of choice for the detection of myocardial dysfunction, the two-dimensional (2D) biplane Simpson method being recommended for estimation of ventricular volumes and LVEF (unless three-dimensional – 3D – echocardiography is availa-ble, which is the best echocardiographic method for LVEF measurement)5 . Cancer therapeutics-related cardiac dysfunction is defi ned as a decrease in more than 10% of the LVEF, to a value below 50%5. Additio-nal diagnostic studies can help the diagnosis: contrast echocardiography, 3D echocardiography, 2D and 3D speckle tracking echocardiography, nuclear imaging, MRI, cardiac biomarkers (troponin and natriuretic peptides), noninvasive stress testing or coronary angi-ography3,5,16. Whenever possible, myocardial deforma-tion parameters measured by tissue Doppler imaging or speckle tracking echocardiography should be used to identify early myocardial injury and to anticipate ventricular dysfunction17. A relative percentage reduc-tion in peak systolic global longitudinal strain (GLS) of >15% from baseline is considered abnormal and a mar-ker of early LV subclinical dysfunction5,17. Cardiac MRI is a useful tool if other techniques are non-diagnostic or to confi rm the presence of LV dysfunction if LVEF is borderline5. It also serves to evaluate the pericar-dium (especially after RT) and can detect diffuse myo-cardial fi brosis using T1/T2 mapping and extracellular volume fraction evaluation5 . The cardiac biomarkers may be considered in order to detect early cardiac in-jury, abnormal results being indicative of an increased risk of cardiotoxicity5. New elevation of high-sensiti-vity- cardiac troponin predicts subsequent LV dysfunc-tion with poor prognosis in patients receiving anthra-ciclines5,18. Although the use of natriuretic peptides to detect HF is widely established, in the context of che-motherapy, their role in routine surveillance to defi ne the high- risk patients needs further investigation5. An increase in cardiac biomarkers in patients receiving anthracyclines identifi es those who may benefi t from angiotensin converting enzyme inhibitor (ACEI)5.
Since the CV complications in these patients are frequently complex and need multiple diagnostic techniques that are rarely available in the same hospital, delays in the diagnosis will occur, as in the case of our patient. We want to emphasize the need for centers of excellence with cardio -oncological expertise with readily available adequate diagnostic technologies.
To minimize the lost to follow-up, patients sho-uld be encouraged to take a more active role, and this can be achieved by informing them of their in-creased risk of CVD and of the importance of adhe-rence to screening practice, early reporting of signs and symptoms of CVD and risk-reducing behavioral modifi cation4,5 . Cardiac risk factors (hyperlipidemia, hypertension, diabetes mellitus, smoking, obesity, sedentary lifestyle) should be actively screened and aggressively controlled as they increase the risk of CV disease after HL. The LDL-cholesterol targets in HL survivors is 100 mg/dL (or lower) and the recommen-ded therapy is a statin3,4. Some authors recommend for prevention of atherosclerosis in cancer survivors the daily administration of aspirin (at least 81 mg)3. Patients who develop asymptomatic LV dysfunction or HF during or after cancer therapy will benefi t from ACEI or angiotensin II receptor blockers and beta-blockers5,19.
In our patient case, after a severe early complication, a long asymptomatic period followed, with the onset of symptoms 14 years after the cancer therapy. Once the symptoms appeared they progressed despite op-timal medical therapy. This highlights the importance of regular follow-up for early diagnosis and treatment which can have a signifi cant impact on prognosis20. The choice of medical treatment in our patient was in accordance with current practice guidelines for heart failure (an ACEI substituted by an angiotensin II recep-tor blocker due to side effects, a beta-blocker, a loop diuretic and antialdosteronic agent) and prevention of atherosclerotic disease in cancer survivors (aspirin 100 mg/day and statin with a LDL-cholesterol target <100 mg/dL)3-5,21. Surgical treatment was decided for constrictive pericarditis, early pericardiectomy with complete decortication (if technically feasible) being the mainstay of treatment, before severe constriction and myocardial atrophy occur22,23. Although none of the valvular lesions had the criteria to warrant sur-gery by themselves, their association, together with the need for pericardiectomy and the risks associated with a possible further reintervention, prompted the decision for combined surgery, in order to address all issues with a one-time complete operation24. Even in experienced centers, the outcomes of patients with radio-induced heart disease undergoing cardiac surgery are signifi cantly worse than a comparable matched population25. In patients with undergoing valve surgery the most important predictor for pe-rioperative mortality is the presence of constrictive pericarditis, these patients having a 30-day mortality rate of 40%26. Thus, the prognosis of our patient re-mains reserved given the complex heart involvement, the severe fi brosis of the mediastinum that may inter-fere with the surgery (“frozen mediastinum”) and the associated pulmonary disease.
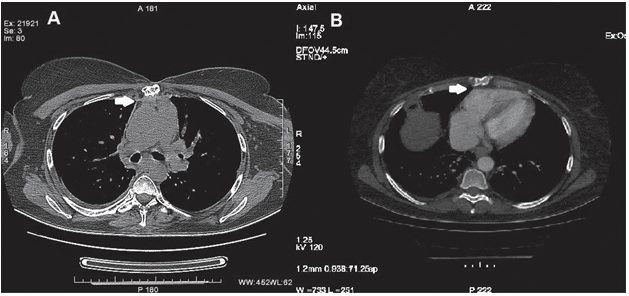
Figure 4. Chest spiral CT scan with radiocontrast agent showing reduction till extinction of the fat from the anterior mediastinum, which is being replaced by high-density strips with minimal contrast stain, indicative of fi brosis (arrow), that cause pleuro-pericardial joining to the anterior thoracic wall (panel A) with minimal retractile effect on the right ventricle (panel B).
CONCLUSIONS
As the life expectancy of HL patients increases, the focus on cardiovascular health becomes a priority. It is imperative to raise awareness of possible CVD among cancer survivors as well as to provide appro-priate follow-up of such patients in clinical practice in order to minimize the burden of late CV morbidity and mortality.
Acknowledgements: We thank all members of the medical team and the patient.
Conflict of interest: none declared
Abbreviations:
2D Two-dimensional
3D Three-dimensional
ABVD Adriamycin, Bleomycin, Vinblastine, Dacarbazine ACEI Angiotensin Converting Enzyme Inhibitor
AR Aortic Regurgitation
AVD Adriamycin, Vinblastine, Dacarbazine
BMI Body Mass Index
bpm beats per minute
CT Computed Tomography
CV Cardiovascular
CVD Cardiovascular Disease
ECG Electrocardiogram
GLS Peak Systolic Global Longitudinal Strain
HF Heart Failure
HL Hodgkin Lymphoma
LV Left Ventricle
LVEF Left Ventricle Ejection Fraction
MRI Magnetic Resonance Imaging
RT Radiation Therapy
RVOT Right Ventricle Outfl ow Tract
TEE Transesophageal Echocardiography
TTE Transthoracic Echocardiography
References
1. Masoudkabir F, Sarrafzadegan N, Gotay C, Ignaszewski A, Krahn AD, Davis MK, Franco C, Mani A. Cardiovascular disease and can-cer: Evidence for shared disease pathways and pharmacologic pre-vention. Atherosclerosis 2017; 263: 343-51.
2. Aleman BMP, Moser EC, Nuverc J, Suter TM, Maraldo VM, Specht L, Vrieling C, Darby SC. Cardiovascular disease after cancer ther-apy. EJC Suplements 2014; 12: 18-28.
3. Ky B, Kondapalli L, Lenihan DJ. Cancer survivorship: Cardiovascu-lar and respiratory issues. https://www.uptodate.com (Accessed on February 1, 2018).
4. Ng A, LaCasce A. Overview of the approach to the adult survivor of classical Hodgkin lymphoma. https://www.uptodate.com (Ac-cessed on February 1, 2018).
5. Zamorano JL, Lancellotti P, Muñoz DR, Aboyans V, Asteggiano R, Galderisi M, Habib G, Lenihan DJ, Lip GYH, Lyon AR, Lopez Fernandez T, Mohty D, Piepoli MF, Tamargo J, Torbicki A, Suter TM. ESC Position Paper on cancer treatments and cardiovascular toxicity developed under the auspices of the ESC Committee for Practice Guidelines. EHJ 2016; 37: 2768-801.
6. Armstrong GT, Oeffi nger KC, Chen Y, Kawashima T, Yasui Y, Lei-senring W, Stovall M, Chow EJ, Sklar CA, Mulrooney DA, Mertens AC, Border W, Durand JB, Robison LL, Meacham LR. Modifi able risk factors and major cardiac events among adult survivors of childhood cancer. J Clin Oncol 2013; 31: 3673–80.
7. Myrehaug S, Pintilie M, Tsang R, Mackenzie R, Crump M, Chen Z, Sun A, Hodgson DC. Cardiac morbidity following modern treatment for Hodgkin lymphoma: supra-additive cardiotoxicity of doxorubicin and radiation therapy. Leuk Lymphoma 2008; 49: 1486-93.
8. Bhakta N, Liu Q, Yeo F, Baassiri M, Ehrhardt MJ, Srivastava DK, Metzger ML, Krasin MJ, Ness KK, Hudson MM, Yasui Y, Robison LL. Cumulative burden of cardiovascular morbidity in paediatric, adolescent, and young adult survivors of Hodgkin’s lymphoma: an analysis from the St Jude Lifetime Cohort Study. Lancet Oncol 2016; 17: 1325-34.
9. Steinherz LJ, Steinherz PG, Tan CT, Heller G, Murphy ML. Car-diac toxicity 4 to 20 years after completing anthracycline therapy. JAMA 1991; 266: 1672-7.
10. Swain SM, Whaley FS, Ewer MS. Congestive heart failure in pa-tients treated with doxorubicin: a retrospective analysis of three trials. Cancer 2003; 97: 2869-79.
11. Cardinale D, Colombo A, Lamantia G, Colombo N, Civelli M, De Giacomi G, Rubino M, Veglia F, Fiorentini C, Cipolla CM. Anthra-cycline-induced cardiomyopathy: clinical relevance and response to pharmacologic therapy. J Am Coll Cardiol 2010; 55: 213–20.
12. Marks LB, Constine LS, Adams MJ. Cardiotoxicity of radiation therapy for Hodgkin lymphoma and pediatric malignancies. https:// www.uptodate.com (Accessed February 1, 2018).
13. Aleman BM, van den Belt-Dusebout AW, De Bruin ML, van ‘t Veer MB, Baaijens MH, de Boer JP, Hart AA, Klokman WJ, Kuenen MA, Ouwens GM, Bartelink H, van Leeuwen FE. Late cardiotoxicity af-ter treatment for Hodgkin lymphoma. Blood 2007; 109: 1878-86.
14. Geske JB, Anavekar NS, Nishimura RA, Oh JK, Gersh BJ. Differ-entiation of constriction and restriction: complex cardiovascular hemodynamics. J Am Coll Cardiol 2016; 68: 2329-47.
15. Geske JB, Reddy Y. Pathophysiology and diagnosis of constric-tive pericarditis. http://www.acc.org/latest-in-cardiology/arti-cles/2017/03 /13/15/10/pathophysiology-and-diagnosis-of-constric-tive-pericarditis (Accessed on January 28, 2018).
16. Cherata DA, Badano LP, Carstea D, Muraru D. Role of new echo-cardiographic techniques in the detection of cancer treatment-re-lated cardiac dysfunction. Current status and further perspectives. Romanian Journal of Cardiology 2016; 26: 411-22.
17. Popară-Voica AM, Călin A, Popescu BA, Mitrică AM, Anghel R, Jurcuţ R, Ginghină C. Cardiac dysfunction of antineoplastic agents in breast cancer patients. Romaninan Journal of Cardiology 2014; 24: 271-7.
18. Bisoc A, Radoi M. Hs-cTnT plasma level and depression of ST seg-ment at exercise stress test in patients with anthracycline-induced cardiomyopathy. Romanian Journal of Cardiology 2014; 24: 157-62.
19. Copur MS, Obermiller A. An algorithm for the management of hypertension in the setting of vascular endothelial growth factor signaling inhibition. Clin Colorectal Cancer 2011; 10: 151–6.
20. Simion M, Radu R, Popară A, Mihăilă M, Zarma R, Apetrei E. Di-agnostic and therapeutic challenges in a case of cardio-oncology. Romanian Journal of Cardiology 2014; 24: 178-82
21. Ponikowski P, Voors AA, Anker SD, Bueno H, Cleland JGF, Coats AJS, Falk V, González-Juanatey JR, Harjola VP, Jankowska EA, Jes-sup M, Linde C, Nihoyannopoulos P, Parissis JT, Pieske B, Riley JP, Rosano GMC, Ruilope LM, Ruschitzka F, Rutten FH, van der Meer P. ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure. EHJ 2016; 37: 2129-200.
22. Adler Y, Charron P, Imazio M, Badano L, Baro G, Bogaert J, Bru-cato A, Gueret P, Klingel K, Lionis G, Maisch B, Mayosi B, Pavie A, Ristic AD, Tenas MS, Seferovic P, Swedberg K, Tomkowski W. ESC Guidelines for the diagnosis and management of pericardial diseases. EHJ 2015; 36: 2921–64.
23. Depboylu BC, Mootoosamy P, Vistarini N, Testuz A, El-Hamamsy I, Cikirikcioglu M. Surgical Treatment of Constrictive Pericarditis. Texas Heart Institute Journal 2017; 44: 101-6.
24. Baumgartner H, Falk V, Bax JJ, De Bonis M, Hamm C, Holm PJ, Iung B, Lancellotti P, Lansac E, Rodriguez Muñoz D, Rosenhek R, Sjögren J, Tornos Mas P, Vahanian A, Walther T, Wendler O, Win-decker S, Zamorano JL. ESC/EACTS Guidelines for the manage-ment of valvular heart disease. EHJ 2017; 38: 2739–91.
25. Wu W, Masri A, Popovic ZB, Smedira NG, Lytle BW, Marwick TH, Griffi n BP, Desai MY. Long-term survival of patients with radiation heart disease undergoing cardiac surgery: a cohort study. Circula-tion 2013; 127: 1476-85.
26. Finch W, Shamsa K, Lee MS. Cardiovascular complications of ra-diation exposure. Rev Cardiovasc Med 2014; 15: 232-44.
 This work is licensed under a
This work is licensed under a