Adriana Mursa, Alina Dinu, Monica Rosca, Marinela Serban, Carmen Beladan, Cosmin Calin, Nicoleta Ferariu, Bogdan A.Popescu, Carmen Ginghina, Ruxandra Jurcut*
* “Prof. Dr. C.C.Iliescu” IUBCV, UMF “Carol Davila”, Bucharest, Romania
Abstract: Hypertrophic cardiomyopathy (HCM) is known to be the most common cause of sudden cardiac death (SCD) in athletes. Meanwhile, the annual incidence for cardiovascular death in patients with HCM of 1–2%, SCD being the main cause, followed by heart failure and thromboembolism1. The most commonly recorded cause of SCD in patients with HCM is spontaneous ventricular fibrillation (VF), but asystole, AV block and pulseless electrical activity are also described2.
Keywords: hypertrophic cardiomyopathy, risk, sudden death, implantable cardiac defibrillator
Rezumat: Cardiomiopatia hipertrofică (CMH) este cunoscută a fi una din cele mai frecvente cauze de moarte subită (MSC) la atleţi. Mai mult decât atât, incidenţa anuală a mortalităţii cardiovasculare la pacienţii cu CMH este de 1-2%, MSC fiind principala cauză, urmată de insuficienţă cardiacă şi trombembolism. Cea mai frecventă cauză de MSC la pacienţii cu CMH este fibrilaţia ventriculară (FiV), dar sunt descrise de asemenea şi asistola, blocul AV şi activitatea electrică fără puls.
Cuvinte cheie: cardiomiopatie hipertrofică, risc, moarte subită, defibrilator implantabil
INTRODUCTION
Identification of patients who are at a high risk of SCD has always been challenging, and is one of the most debated topics, as demonstrated by the variety of proposed risk stratification guidelines. It is important to establish a correct balance between benefit and risks of the implantation procedure (e.g. lead endocarditis, venous thrombosis or lead fracture), as well as to keep a proper cost-benefit ratio. So far there are no randomized trials and no prospective validation, and the knowledge that we have is based only on observational and retrospective studies.
The aim of this study was to compare eligibility for implantable cardioverter defibrillator (ICD) implantation in the primary prevention of SCD based on presently used guidelines.
MATERIAL AND METHODS
Study population
The study cohort retrospectively included 187 patients diagnosed with HCM, who were admitted in the 3rd Cardiology Department of “C.C Iliescu Institute of Cardiovascular Diseases” between 2004-2014. Patients with personal history of SCD were excluded. HCM was defined as a maximum LV wall thickness ≥15mm evaluated through echocardiography, unexplained solely by loading conditions. We recorded patients age, gender, family history, established risk factors for SCD: age, presence of nonsustained ventricular tachycardia (NSVT) on Holter monitoring, family history of SCD at a young age, history of unexplained syncope, severe left ventricular hypertrophy (LVH), presence and degree of LV outflow obstruction (LVOTO), abnormal systolic blood pressure response to exercise and left atrial diameter.
Other echocardiographic parameters were also recorded from patients files: maximal wall thickness (MWT), left atrial (LA) anteroposterior diameter, localization of LV hypertrophy, presence of LV apical hypertrophy.
Indications for ICD implantation in primary SCD prevention were established based on present guidelines: 2014 ESC guideline on management of HCM (ESC2014), 2011 ACC/AHA guidelines on management of HCM (ACC2011), as well as American College of Cardiology/European Society of Cardiology Clinical Expert Consensus Document on Hypertrophic Cardiomyopathy (ACC/ESC 2003) for historical comparison.
Guidelines derived primary SCD prophylaxis indications
The 2014 guideline was validated by O’Mahoney on 3675 patients and estimates the probability of SCD at 5 years, by analyzing the following RF for SCD: age, maximal WT, maximal LVOT gradient, LA diameter, presence of family history of SCD, NSVT.
Accordingly with the ESC 2014 guideline Class IIA indication for ICD is when the SCD RISK >6%, Class IIB when the SCD RISK is between 4-6% and Class III when the SCD RISK is less than <4%3. The ACC2011 recommended Class IIA indication for ICD when patients had a history of family of first degree relative with SCD, unexplained recent syncope, WT >30 mm, decrease BP with exercise/NSVT associated with other risk factor for SCD (late gadolinium enhancement on MRI, apical aneurysms, LVOTO, genetic mutations), Class IIB indication for ICD when patients presented decrease BP with exercise/NSVT without other RF and Class III when they had no risk factor4.
In the ACC/ESC 2003 guidelines the recommendation for Class IIA was the presence of two RF for SCD, Class IIB the presence of one RF for SCD and Class III absence of any RF for ICD5.
The purpose of our study was to compare the necessity for ICD in primary SCD prevention, accordingly to mentioned guidelines, and to analyze what are the differences between their recommendations, and to see which guideline has the most indications for ICD implantation for primary SCD prevention.
RESULTS
Patients characteristics
In our study cohort included 187 patients diagnosed with HCM, from which 45% were males with a mean age of 59.2±14.5 years old. Ninety-five pts (50.8%) had obstructive HCM with high rest LVOT gradient, 36 (19.2%) had latent obstruction, while 28 (14.9%) presented with apical forms. The distribution of associated risk factors is presented in Figure 1. The most frequent associated risk factors are non-sustained ventricular tachycardia (19 patients, 10.1%) and unexplained syncope (15 patients, 8%).
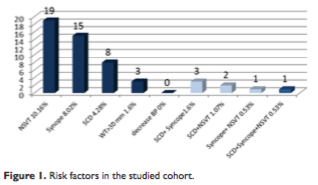
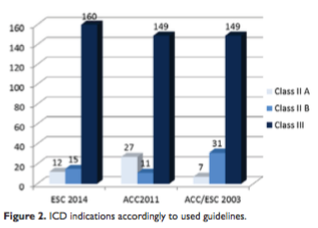
Sudden death risk stratification for primary prophylaxis
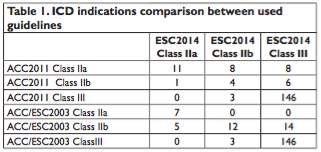
Average HCM Risk-SCD Score (based on ESC2014) of the group was 2.65±2.40% (limits 0.67-18.44%); 12 pts (6.4%) had a high calculated 5-year risk of SCD >6%. Patients with apical hypertrophy had smaller risk for SCD than patients with hypertrophy present in other walls. In comparison with the ESC2014, using the ACC2011 guidelines 27 patients (14.4%) had high risk of SCD and Class IIA indication for ICD. The highest number of patients with no indication for ICD was found in the ESC2014 guidelines (Figure 2, Table 1). We found significant differences between the 3 gui- delines in use, with the largest number of ICD indicati- ons from American guidelines (p < 0.001 for differences between all groups). This difference was mainly driven by an ACC2011 indication of class IIA in patients with ESC2014 HCM Risk-SCD Score <4% due to unexplained syncope (8 pts), SCD family history (5 pts) and LV wall thickness >30 mm (2 pts).
Disagreements were also found between ACC2011 Class IIA indications and ESC2014 Class IIB. They are caused by presence of important RF (syncope, family history of SCD) which recommend a Class IIA indication of ICD in the ACC2011, risk factors that alone do0.001
not qualify for Class IIA indication in the ESC 2014 if the patients has increased age, moderate WT or lack of obstruction.
Furthermore disagreements were found between ACC2011 Class IIA and ESC2014 Class III. The presence of important RF (syncope, SCD, WT >30mm) recommends a Class IIA indication of ICD in the ACC2011, but these risk factors that are not sufficient alone to increase the risk of SCD in the ESC 2014 guideline if the patient has increased age and moderate WT.
DISCUSSIONS
The present study demonstrated the presence of inconsistencies among scientific societies between contemporary guiding the indication of primary SCD prophylaxis in patients with HCM. While the secondary SCD prophylaxis has a clear and consistent class I indication among guidelines6, identifying the patients that qualify for primary SCD prevention is a challenge in order to diminish cardiovascular mortality in these patients, but also to avoid the inappropriate shocks and procedure related complications.
Although most of the ventricular arrhythmias occur in the absence physical exertion and sustained effort induced ventricular tachycardia is rare, patients with HCM are discouraged from intense physical activity, and are advised not to participate in competitive sports especially if they have left ventricular outflow tract obstruction and/or risk factors for SCD7.
The role of antiarrhythmic drugs in patients with HCM is debatable, and there are no randomized, or controlled data to support their use for the prevention of SCD. One of the drugs most studied is amiodarone, which was related with a smaller incidence of SCD in one small observational study, but most of the observational data suggest that amiodarone is unsuccessful in preventing SCD, and so is disopyramide8,9.
Most of the ICD indications in patients with HCM are for primary SCD prevention and are based on the presence of SCD risk factors.
Christiaans et al. analyzed several electronic databases and concluded10 that several risk factors are valid: family history of SCD, severe LVH, unexplained syncope, NSVT, and abnormal pressure response to exercise as major risk factors in risk stratification for SCD as recommended in international guidelines. However LVOTO was considered to be possibly associated with SCD and could be included in the overall risk profile of patients with a marked left ventricular outflow gradient under basal conditions. Further research of the combined association between LVOTO and the presence of >1 major risk factors was considered necessary. At present, the most important risk factors for SCD are age, presence of NSVT, family history of SCD at a young age, unexplained syncope, severe left ventricle hypertrophy (LVH), LVOTO, abnormal systolic blood pressure response to exercise and left atrial diameter.
Recently, studies focused not only on the importance of classical risk factors for SCD, but also about new emerging risk factors like fibrosis11 and edema assessed with T2 weighted on cardiac MRI, or the presence of certain mutations12,13. There are studies that have suggested that the presence of myocardial fibrosis, apical aneurysms and the presence of some sarcomeric mutations are risk factors for SCD, and their presence might be important when deciding to implant an ICD in a patient with an intermediate risk of SCD1416. Whether these new risk factors will be included in calculating the risk score for SCD by future guidelines is a matter of debate. For example a study published in 2016 shows that the presence of edema assessed with T2 weighted CMR is associated with a higher estimated 5-year risk of SCD than those without17.
Another important dilemma is what guidelines to use when deciding to properly implant an ICD for primary prevention of SCD, as results appear to differ.
Before the publishing of the ESC 2014 guideline the dilemma was between 2003 ACC/ESC Guidelines versus 2011 ACC Guidelines. The main disagreement between these guidelines is centered on the number of risk factors required before consideration of an implantation of an ICD for primary prevention. Each conventional RF has a low positive predictive value, when taken separately. The initial European approach 2003 ACC/ESC guideline has been to implant an ICD only in the presence of >1 risk factor. In comparison the ACC 2011 guideline recommends ICD implantation patients even with only one RF if the RF are family history of SCD from HCM in a first-degree relative, LV wall thickness ≥30 mm or recent unexplained syncope. This difference in management is in part due to the different results between American and European studies regarding the risk stratification. The American studies have revealed that an important discharge of ICD occur in patients with just one risk factor18.The European studies on the other hand state that if ICDs were inserted in all patients with one risk factor the incidence of device complications would exceed the benefits taking into account that reported inappropriate ICD interventions and complication of 4.8 %/year and 3.4 %/year, accordingly to a meta-analysis of 16 HCM cohorts19.
An observational retrospective study by O’Mahony20 tried to assess the power of the 2003 ACC/ESC Guideline and 2011 ACC Guideline SCD risk stratification algorithms to distinguish high risk patients who might be eligible for an implantable cardioverter defibrillator (ICD) from low risk individuals. He evaluated 1606 HCM patients and assessed the presence of 5 risk factors (RF): NSVT, severe LVH, family history of SCD, unexplained syncope and abnormal blood pressure response to exercise. During a follow-up, SCD/appropriate ICD shock occurred in 3% of patients without RF, 4.8% of patients with 1 RF, 10.8% of patients with 2 RF, 13.7% of patients with 3 RF and 40% of patients with ≥4 RF. The risk of SCD increased with multiple RF (2 RF: HR 2.87, p≤0.001; 3 RF: HR 4.32, p=0.001; ≥4 RF: HR 11. 37, p < 0. 0001) but not with a single RF (HR 1.43 p=0.21). The conclusions of the study was that scd risk increases with the aggregation of RF and that the 2003 ACC/ESC and 2011 ACCF/AHA guidelines distinguish high from low risk individuals with partial control.
In 2014 The ESC adopted a new guideline: ESC 2014 based on the HCM Risk-SCD model, but even this guideline has some limitations because is not validated in pediatric patients (<16 years=”” severe=”” lvh=””>35 mm), and the population used in the root study was Caucasian, which raises the question if the results of the study can be extrapolated to non-Caucasian individuals. Another item that is not quantified by the HCM Risk-SCD model is the effect of latent LVOTO or the effect of LVOTO reduction by alcohol ablation or myomectomy.
The appropriateness of implantable cardioverter defibrillator therapy was evaluated in a recent study from 2015. The authors analyzed the 2014 European Society of Cardiology guidelines, and risk stratification methods of the 2003 American College of Cardiology/ European Society of Cardiology guidelines and 2011 Ame-rican College of Cardiology Foundation/American Heart Association guidelines. They evaluated a cohort of 706 patients with HCM without prior SCD event and followed-up their patients for 7.7±5.3 years, during which SCD occurred in 42 (5.9%). After the statistical analysis of the three guidelines they concluded that best method to estimate the SCD risk in patients with HCM is the HCM Risk-SCD model proposed by the 2014 guideline which improves the risk stratification of patients with HCM for primary prevention of SCD21.
However another recent article published in 2015 states different results, sustaining that using the 2014 ESC guideline is not the most appropriate guideline to use. The authors assessed the ESC SCD model for HCM. They calculated the HCM Risk-SCD model and measured outcome against primary prevention ICD. They evaluated 372 patients from two referral centers, 40 (10%) had appropriate ICD interventions. The interesting finding was that 55% of patients who experienced appropriate ICD interventions for VT/VF paradoxically had a low risk scores of less than 4% / year over 5 years, which accordingly to the guideline is a class III indication and does not justify ICD implantation. Just 27% of those patients with appropriate ICD interventions had a high-risk ESC score (>6% risk/year over 5 years), which is considered class II a ICD indication. The authors concluded that the majority of patients who experienced appropriate ICD interventions would not have been considered candidates for prophylactic device therapy and the use of the HCM Risk-SCD model scoring system would lead to many high-risk patients unprotected from arrhythmic SCD22.
So what is the most appropriate guideline to use is still under debate, as guidelines are in a continuous change. One year after the publication of the 2014 ESC guideline, ESC published another guideline for management of patients with ventricular arrhythmias and the prevention of sudden cardiac death, were they modified the current indication of Class III from the ESC 2014, in Class II B. ICD implantation may now be considered in patients with an estimated 5-year risk of SCD less than 4% if they have clinical features and if the lifelong risk of complications suggests a net benefit from ICD therapy. Also this latest SCD 2015 guideline stated that when evaluating the SCD risk of a patient ≥16 years of age without a history of resuscitated VT or VF or spontaneous sustained VT causing syncope or hemodynamic compromise , we should use stratify the risk of SCD with the HCM Risk-SCD calculator, Class IB recommendation23.160.
Limits of the study
The main limitation of this study results from its retrospective character, basing its findings on the data included in the medical files of the patients. However, in many patients the evaluation of sudden death risk was a medical issue, making available the necessary data.
The comparison of the risk assessment methods is not validated by a long-term follow-up of the cohort. However, our study was not powered and designed to validate the risk evaluation methods, but merely to show their present inconsistencies and raise necessary questions. We referred to larger studies with followup from the literature which validated these observations.
CONCLUSIONS
There are significant inconsistencies among guidelines in use for the ICD implantation indication for primary SCD prevention in HCM. ACC/AHA guidelines appear to have the highest yield of implantations, hence economic burden. The 2014 guideline seems to be the optimal recent validated formula to estimate the risk prediction of SCD in HCM patients, however recent studies state that HCM Risk-SCD model scoring system would lead to many high-risk patients unprotected from arrhythmic SCD. When deciding when to properly implant an ICD for primary SCD prevention in patients with HCM, we should take into consideration the current guidelines, but we should not forget clinical judgement, and the fact that guidelines are in a continuous change.
Novel RF for SCD in HCM are emerging, and whether they will be included in future guidelines will be seen.
Formal prospective validation with comparison of current indications is necessary both for establishing optimal treatment and minimizing costs.
Conflict of interest: none declared.
References
1. Elliott PM, Gimeno JR, Thaman R, Shah J, Ward D, Dickie S, Tome Esteban MT, McKenna WJ. Historical trends in reported survival rates in patients with hypertrophic cardiomyopathy. Heart 2006; 92:785–791.
2. Elliott PM, Sharma S, Varnava A, Poloniecki J, Rowland E, McKenna WJ. Survival after cardiac arrest or sustained ventricular tachycardia in patients with hypertrophic cardiomyopathy. J Am Coll Cardiol 1999;33:1596–1601.
3. Elliott PM, Anastasakis A, Borger MA, Borggrefe M, Cecchi F, Charron P, et al. 2014 ESC Guidelines on diagnosis and management of hypertrophic cardiomyopathy. European heart journal. 2014;35(39):273379.
4. Gersh BJ, Maron BJ, Bonow RO, Dearani JA, Fifer MA,Link MS, Naidu S, et al. 2011 ACCF/AHA Guideline for the Diagnosis and Treatment of Hypertrophic Cardiomyopathy, JACC 2011:e212–60
5. Maron BJ, McKenna WJ, Danielson GK, Kappenberger LJ, Kuhn HJ et al, American College of Cardiology/European Society of Cardiology Clinical Expert Consensus Document on Hypertrophic Cardiomyopathy ,European Heart Journal (2003) 24, 1965–1991
6. Elliott PM, Sharma S, Varnava A, Poloniecki J, Rowland E, McKenna WJ. Survival after cardiac arrest or sustained ventricular tachycardia in patients with hypertrophic cardiomyopathy. J Am Coll Cardiol 1999; 33:1596–1601.
7. O’Mahony C, Lambiase PD, Rahman SM, Cardona M, Calcagnino M, Quarta G, Tsovolas K, Al Shaikh S, McKennaW, Elliott P. The relation of ventricular arrhythmia electrophysiological characteristics to cardiac phenotype and circadian patterns in hypertrophic cardiomyopathy. Europace 2012;14:724–733.
8. McKenna WJ, Oakley CM, Krikler DM, Goodwin JF. Improved survival with amiodarone in patients with hypertrophic cardiomyopathy and ventricular tachycardia. Br Heart J 1985;53:412–416.
9. Melacini P, Maron BJ, Bobbo F, Basso C, Tokajuk B, Zucchetto M, Thiene G, Iliceto S. Evidence that pharmacological strategies lack efficacy for the prevention of sudden death in hypertrophic cardiomyopathy. Heart 2007;93:708–710
10. Christians I, van Engelen K, van Langen IM, Birnie E, Bonsel GJ, Elliott PM, Wilde AA Risk stratification for sudden cardiac death in hypertrophic cardiomyopathy: systematic review of clinical risk markers, Europace 2010;431: 313-321
11. Bruder, O. et al. Myocardial scar visualized by cardiovascular magnetic resonance imaging predicts major adverse events in patients with hypertrophic cardiomyopathy.JACC.2010;05-007.
12. Arad M, Monserrat L, Haron-Khun S, et al., Merits and pit falls of genetic testing in a hypertrophic cardiomyopathy clinic, Isr Med Assoc J, 2014;16:707–13.
13. Calore C, De Bortoli M, Romualdi C, et al., A founder MYBPC3 mutation results in HCM with a high risk of sudden death after the fourth decade of life, J Med Genet,2015;52:338–47.
14. Richard P, Charron P, Carrier L, Ledeuil C, Cheav T, Pichereau C, Benaiche A, Isnard R, Dubourg O, Burban M, Gueffet JP, Millaire A, Desnos M, Schwartz K, Hainque B, Komajda M. Hypertrophic cardiomyopathy: distribution of disease genes, spectrum of mutations, and implications for a molecular diagnosis strategy. Circulation 2003;107: 2227–2232.
15. MaronMS, Finley JJ, Bos JM, Hauser TH, Manning WJ, Haas TS, Lesser JR, UdelsonJE, Ackerman MJ, Maron BJ. Prevalence, clinical significance, and natural history of left ventricular apical aneurysms in hypertrophic cardiomyopathy. Circulation 2008; 118:1541–1549.
16. Green JJ, Berger JS, Kramer CM, Salerno M. Prognostic value of late gadolinium enhancement in clinical outcomes for hypertrophic cardiomyopathy. JACC Cardiovasc Imaging 2012;5:370–377.
17. Gommans F, Cramer E, Bakker J, Michels M,et al Myocardial edema assessed with T2-weighted CMR imaging and SCD risk in patients with hypertrophic cardiomyopathy. JACC April 5, 2016: 67, Issue 13
18. Maron BJ, Shen WK, Link MS, et al., Efficacy of implantable cardioverter-defibrillators for the prevention of sudden death in patients with hypertrophic cardiomyopathy, N Engl J Med, 2000;342:365–73.
19. Lin G, Nishimura RA, Gersh BJ, et al., Device complications and inappropriate implantable cardioverter defibrillator shocks in patients with hypertrophic cardiomyopathy,Heart, 2009;95:709–14.
20. O’Mahony C1, Tome-Esteban M, Lambiase PD, Pantazis A, Dickie S, McKenna WJ, Elliott PM. A validation study of the 2003 American College of Cardiology/European Society of Cardiology and 2011 American College
21. Vriesendorp PA, Schinkel AF, Liebregts M, Theuns DA2, van Cleemput J, Ten Cate FJ,et al. Validation of the 2014 European Society of Cardiology guidelines risk prediction model for the primary prevention of sudden cardiac death in hypertrophic cardiomyopathy. Circ Arrhythm Electrophysiol. 2015 Aug;8(4):829-35
22. BJ. Maron, Susan Casey,R. Garberich; EJ. Rowin; M. Maron Assessment of European Society of cardiology sudden cardiac death risk model for Hypertrophic cardiomyopathy.J Am Coll Cardiol. 2015;65,7351097
23. Silvia G. Priori, Blomstrom-Lundqvist C, Mazzant A, Bloma N, Borggrefe M, et al. 2015 ESC Guidelines for the management of patients with ventricular arrhythmias and the prevention of sudden cardiac death European Heart Journal (2015) 36, 2793–2867.
 This work is licensed under a
This work is licensed under a